Abstract
Background:
Group III metabotropic glutamate receptors (mGlu4, mGlu7, mGlu8) display differential brain distribution, which suggests different behavioral functions. However, comparison across the available animal studies remains methodologically hazardous and controversial. The present report directly compares knockouts for each group III receptor subtype using a single behavioral test battery and multivariate analysis.
Methods:
The behavioral phenotypes of C57BL/6J mice lacking mGlu4, mGlu7, or mGlu8 and their respective littermates were examined using a multimetric test battery, which included elements of neuromotor performance, exploratory behavior, and learning and memory. Multivariate statistical methods were used to identify subtype-specific behavioral profiles and variables that distinguished between these mouse lines.
Results:
It generally appears that mGlu7 plays a significant role in hippocampus-dependent spatial learning and in some fear-related behaviors, whereas mGlu4 is most clearly involved in startle and motivational processes. Excepting its influence on body weight, the effect of mGlu8 deletion on behavior appears more subtle than that of the other group III receptors. These receptors have been proposed as potential drug targets for a variety of psychopathological conditions.
Conclusion:
On the basis of these controlled comparisons, we presently conclude that the different group III receptors indeed have quite distinct behavioral functions.
Keywords: Metabotropic glutamate receptors, knockout mice, behavioral phenotyping, behavioral test battery
Introduction
Metabotropic glutamate (mGlu) receptors, belonging to the G-protein–coupled receptor family, are thought to mediate slow, modulatory signals, whereas ionotropic glutamate receptors mediate fast synaptic responses (Pin and Duvoisin, 1995; Schoepp, 2001). The 8 mGlu receptor subtypes identified so far (mGlu1–mGlu8) have been segregated into 3 receptor groups, according to sequence homology, pharmacology, and signal transduction mechanisms (Pin and Duvoisin, 1995). Many authors emphasized the putative importance of these various mGlu receptors in brain physiology and pathophysiology but deplored their largely unexploited potential as therapeutic drug targets (Swanson et al., 2005; Récasens et al., 2007; Niswender and Conn, 2010).
Differential brain distribution in presynaptic receptors belonging to group III (mGlu4, mGlu6, mGlu7, mGlu8) suggests functional dissociation between these receptors (Wu et al., 1998; Dobi et al., 2013). The functions of mGlu6 shall not be further discussed here, because of its restriction to the inner layer of the retina (Nakajima et al., 1993). In contrast, mGlu7 appears to be mainly expressed in telencephalic areas (neocortex, hippocampus, etc.), whereas mGlu4 and -8 are relatively more prominent in lower brain areas (see Table 1 for an overview of expression levels of group III receptors throughout the rodent brain). Consistent with its high expression in cerebellar granule cells (Tanabe et al., 1993; Kinoshita et al., 1996; Mateos et al., 1998), mGlu4 has been implemented in motor learning (Pekhletski et al., 1996; Davis et al., 2012). The prominent telencephalic distribution of mGlu7 (Kinoshita et al., 1998) is definitely consistent with its putative role in learning and memory, anxiety, and depression-related behaviors (Cryan et al., 2003; Callaerts-Vegh et al., 2006; Palucha et al., 2007; Fendt et al., 2013). Finally, mGlu8 has been found mainly in olfactory bulb, olfactory tubercle, and mammillary bodies (Duvoisin et al., 1995), but reports about its precise behavioral function remain inconclusive (Gerlai et al., 2002; Duvoisin et al., 2005; Fendt et al., 2010; Davis et al., 2013).
Table 1.
Brain Distribution of mGlu4, -7, and -8 in Laboratory Rodents
| Subtype | Olfactory Bulb | Neocortex | Striatum | Hippocampus | Thalamus | Amygdala | Cerebellum | Brainstem | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | CA1 | CA3 | DG | |||||||||
| mGlu4 | IH: | -a
+/-c,k |
+c, k | +/-c | -r
+a |
- r
+a |
+ a,r | - a
+c |
+/-c | +++a, c,f | ||
| IS: | +++ t | -u
- -m |
-u
++ l |
-u | -t | -t | +/-u
+++l, t |
+++m,t, u | ||||
| mGlu7 | IH: | +g, j,n
++v |
++g | -g | ++s | ++g,r | ++g
+++r |
++r
+++g |
+/-g | +g | - -g | |
| IS: | ++h, j, n | ++h,j, m, n
+++b |
+h
++j, +++b, l |
++h, n
+++b |
++j | +++j | +++h,j | +j,h
++l, n +++b |
+h | -h
+/-j |
+h | |
| mGlu8 | IH: | ++v | -r
+e |
+r ++e | +r
++e |
+ o | + p | |||||
| IS: | ++b
+++d,q |
+b,q | ++ t | - -d
+q |
+b, l
+++q |
+b | - -d
+q |
|||||
Intensity of immunoreactivity: +++ most intense, ++ intense, + moderate, +/- moderate to weak, - weak, - - negative; IH: immunohistochemistry; IS: in situ hybridization.
a(*) Bradley et al. (1999).
bCorti et al. (1998).
c(*) Corti et al. (2002).
d*Duvoisin et al. (1995).
eFerraguti et al. (2005).
f*Kinoshita et al. (1996).
g(*) Kinoshita et al. (1998).
hKinzie et al. (1995).
iKinzie et al.(1997).
jKosinski et al. (1999).
kKuramoto et al. (2007).
lMessenger et al. (2002).
mOhishi et al. (1995).
nOkamoto et al. (1994).
oPalazzo et al. (2011).
pPamidimukkala et al. (2002),
qSaugstad et al. (1997).
rShigemoto et al. (1997).
sSomogyi et al. (2003).
tTanabe et al. (1993).
uTesta et al. (1994.
vWada et al. (1998).
* data in mice; (*) similar data in rat and mice.
Unfortunately, none of the previously published reports directly compared the functional features of the 3 group III receptor subtypes. Each receptor subtype has been examined separately in these reports, using different background strains, protocols, variables, etc. Comparing behavioral outcomes between studies, models, and protocols is notoriously difficult and controversial (Crabbe et al., 1999). Therefore, the goal of the present study was to compare directly between mGlu4, mGlu7, and mGlu8 knockout mice, backcrossed to the same genetic background, and subjected to the same multimetric test battery (Goddyn et al., 2006). The behavioral test battery included a broad range of neuromotor, exploratory, and cognitive tasks. Relevant multivariate statistical techniques were used to compare the different behavioral profiles and identify discriminating variables (Leighty et al., 2004).
Methods
Animals
Female mGlu4, mGlu7, and mGlu8 knockout mice (mGlu4-/-, mGlu7-/-, mGlu8-/-) were generated and backcrossed to C57BL/6J background as described previously (Duvoisin et al., 2005). Age-matched mGlu4, mGlu7, and mGlu8 wild-type littermates were used as controls (mGlu4+/+, mGlu7+/+, mGlu8+/+), and all genotypes were confirmed using PCR-based methods as described. All in all, 108 mice were examined (mGlu4-/- n=22, mGlu7-/- n=14, mGlu8-/- n=17, wild-type mice n=55; wild-type mice were pooled, since multivariance analysis revealed no statistical difference between the 3 wild-type groups). Mice were bred at the Janssen Pharmaceutica facilities (Beerse, Belgium) and transferred to Leuven University at the age of approximately 12 weeks. Mixed genotype groups were kept in standard animal cages in temperature- and humidity-controlled rooms (12-h-light/-dark cycle, 22°C). Food and water were available ad libitum, unless stated otherwise. Behavioral experiments were conducted during the light phase of the activity cycle. All protocols have been reviewed and approved by the Animal Experiments Committee of KU Leuven in accordance with the European Community Council Directive (86/609/EEC).
Behavioral Test Battery
Neuromotor Performance and Prepulse Inhibition
Mice were first tested in neuromotor tests, because alterations in general cage activity, motor coordination, and grip strength could confound performance in other behavioral tasks. To measure circadian cage activity, mice were placed individually in standard transparent cages (26.7 cm×20.7cm) located between 3 infrared photo beams. For 23 hours, activity was measured by a laboratory-built activity logger and expressed as beam crossings for each 30-minute interval. Grip strength was measured using a T-shaped bar connected to a digital dynamometer (Ugo Basile, Comerio, Italy). Mice were placed on the apparatus so that they spontaneously grabbed the bar and were gently pulled backwards until they released the bar. Maximal strength (in mN) was recorded 10 times and averaged per animal.
Motor coordination and equilibrium were tested on an accelerating rotarod (MED Associates Inc., St. Albans, VT). Mice were first trained at constant speed (4 rounds/min, 2 minutes) before starting with 4 test trials (inter-trial interval, 10 minutes). During these trials, mice had to balance on the rotating rod that accelerated from 4 to 40 rounds/min in 5 minutes. Time until they dropped from the rod was recorded up to a maximum of 5 minutes.
Prepulse inhibition (PPI) was assessed in a sound attenuating cubicle with a load cell platform (MED Associates Inc) as described before. Mice were placed in a small animal holder that restricted movement and placed on the platform. After a 5-minute acclimation period, 5 initial startle pulses were delivered (115 dB, 5kHz, 40ms). Subsequently, 10 trial blocks were presented. Each block consisted of startle pulse alone (SP: 115 dB; 5kHz; 40ms), 3 SPs preceded by prepulses (SPPP; 70, 75, 80 dB; 5kHz, 20ms), and 3 prepulses (PP) alone. Within each block, trial types (SP, SPPP, or PP) were administered at random with an inter-trial interval of on average 15 seconds. Startle reactivity was recorded during 200ms from stimulus onset in all SP trials (SP or SPPP) and PP trials. A 200-ms interval just before stimulus onset was recorded as baseline measurement (“null” interval). During the trial, a constant background noise was delivered (white noise, 50 dB). Acoustic startle response (ASR), that is, reactivity to the SP alone, and percentage PPI were recorded. PPI was calculated for each PP intensity from peak values according to the following formula: %PPI = [1 – (startle peak at SP after PP) / (ASR)]*100.
Exploration
Open field (OF) and social exploration (SE) were examined using a 50 cm×50cm arena. Animals were dark adapted for 30 minutes and placed in a corner of the arena. After 1 minute of exploration, movements of the mice were recorded for 10 minutes using EthoVision video tracking equipment and software (Noldus, Wageningen, The Netherlands). Total path length, rearing frequency, corner crossings, center entries, and percentage path length in the center were recorded. In the SE test, 2 female mice were placed in a centrally located cage enabling visual, olfactory, and limited physical contact. In the elevated plus maze test (EPM), the arena consisted of a plus-shaped maze with 2 open and 2 closed arms (5cm wide). Mice were placed at the center of the maze and were allowed to explore freely for 10 minutes (after 1 minute of adaptation). Five infrared beams (4 for arm entries and 1 for open-arm dwell), connected to a computerized activity logger, recorded exploratory activity. Total number of arm entries (ie, beam crossings in open and closed arms), percentage of open arm entries, and open-arm dwell (ie, percentage of time per minute spent in the open arms) were measured.
Learning and Memory
Learning and memory abilities were examined in 3 tasks. Single trial passive avoidance (PA) learning was examined in a step-through box with a shock grid. The box consisted of an illuminated compartment and a dark compartment separated by a guillotine door. After a 30-minute dark adaptation period, animals were placed in the light part, and after 5 seconds the sliding door to the dark compartment was opened. Latency to enter the dark compartment was measured. On entry of the dark compartment, the door was closed and a 2-second foot shock (0.2 mA) was delivered by a constant current shocker (MED Associates Inc). Twenty-four hours later, mice were again placed in the light box, and latency to enter the dark compartment was measured.
Spatial learning capacity was examined in the standard hidden platform version of the Morris water maze (MWM) as previously described (Goddyn et al., 2006). Briefly, a circular pool (diameter 150cm, depth 32.5cm) filled with water (26°C, opacified with nonoxic white paint) to a depth of 16cm, contained a circular hidden platform (15cm diameter). Mice were trained for 10 days (4 trials/d; ITI of 15 minutes) to find the hidden escape platform, starting randomly from each of 4 starting positions. Mice that failed to find the hidden platform within 2 minutes were gently guided to the platform, where they remained for 15 seconds before being returned to their home cage. Escape latency, path length, swim velocity, and time spent near the wall (thigmothaxis) were recorded with EthoVision video tracking equipment and software (Noldus, Wageningen, The Netherlands). Probe trials were conducted after 5 training days. During these probe trials, the platform was removed from the pool, and the search pattern of the mice was recorded for 100 seconds. Time spent in each quadrant, path length, latency of first entrance in the target quadrant, and mean distance to the former platform location were calculated.
Finally, contextual fear conditioning (CFC) was based on a protocol used by Paradee et al. (1999). On the first day, animals were placed in the StartFear cage (Panlab, Spain) with black walls and a grid floor. Animals were allowed to acclimate to the box for 5 minutes and were then returned to their home cage. On the second day, after 2 minutes of exploration (baseline), a 30-second tone was delivered co-terminating with a 2-second shock (0.3 mA). After another minute of exploration, another tone-shock pairing was delivered followed by 1 minute of exploration. Twenty-four hours later, animals were returned to the testing chamber for 5 minutes of exploration (context trial). After 90 minutes in their home cage, animals were placed in a white paper box inside the StartFear cage (different context) for 6 minutes. After the 3 minutes (preCS trial), the tone was delivered for 3 minutes (CS trial). During each trial, freezing behavior was recorded by a sensitive Weight Transducer system (Panlab, Spain). The percentage of freezing was calculated per trial.
Statistics
Data are presented as mean and SEM. Differences between mean values were determined using both parametric (1-way ANOVA with Tukey tests for posthoc comparison) and nonparametric (Kruskal-Wallis) tests. Within-subject trials were compared using repeated-measures ANOVA (RM-ANOVA) with Greenhouse-Geisser correction in case of sphericity violation. Outliers were defined by total beam crossings in the circadian activity task. Mice with values lower or higher than 1.5 times the interquartile range below or above the 25th or 75th percentile, respectively, were excluded from all analyses.
Correlation analysis was used to examine the relationships between the different behavioral measures. Pearson correlations were calculated for within and between task variables. The complete list of behavioral variables is provided in Table 2. Discriminant function analysis (direct and stepwise DFA) was used to examine which variables contributed significantly to differences between genotypes. Therefore, only knockout mice were included in this analysis. In direct DFA, all variables are included at once and one can examine the significance of discriminability between genotypes. The stepwise forward approach begins with no variables in the model and, based on statistical criteria, variables (one at the time) that contribute significantly to differences between the groups are selected. Direct entry and stepwise DFA were executed using: (1) all variables, (2) only neuromotor variables, (3) only exploratory variables, and (4) only cognitive measures. All analyses were conducted using SPSS 19.0 statistical package (SPSS Inc, Chicago IL) at α = 0.05.
Table 2.
List of Behavioral Variables Recorded in mGlu4, mGlu7, and mGlu8 Knockout and Wild-Type Mice
| Behavioral Task | Variables | Abbreviation |
|---|---|---|
| Cage activity | Total beam crossings | A-TOT |
| Beam crossings during first half hour | A_30 | |
| Grip | Mean | GRIP |
| Rotarod | Total time on rod | ROT |
| PPI task | Startle | ASR |
| %PPI with prepulse of 75dB | PPI_75 | |
| Open field | Path length | OF_PL |
| Rearing | OF_R | |
| Percentage path length in centre | OF_PLc | |
| Latency of first centre entry | OF_LAT | |
| Social exploration | Path length | SE_PL |
| Rearing | SE_R | |
| Percentage path length in centre | SE_PLc | |
| Latency of first centre entry | SE_LAT | |
| Elevated plus maze | Total beam crossings | EP_TOT |
| Open arm dwell | EP_OAD | |
| Passive avoidance | Test-training latency | PA |
| Morris water maze | Total path length day 1 | MW_T1PL |
| Difference in PL between first and fifth day | MW_PL | |
| Average velocity week 1 | MW_VEL1 | |
| Time in target probe 1 | MW_TAR1 | |
| Contextual fear conditioning | Percentage freezing shock | CF_SH |
| Percentage freezing context | CF_CXT | |
| Percentage freezing cue | CF_CUE |
Abbreviations:
Results
Neuromotor Performance and PPI
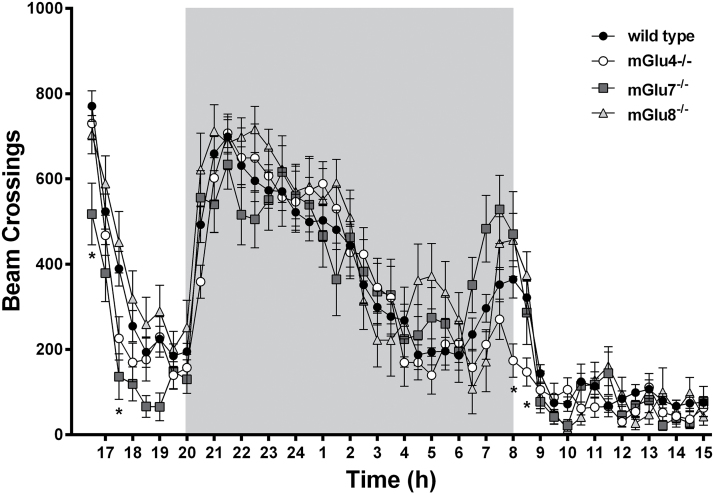
Cage activity was measured by the number of beam crossings. Boxplots of total beam crossings identified 2 mGlu8-/- mice as outliers, which were removed from further analysis. One-way RM-ANOVA (Greenhouse-Geisser correction) with genotype as between-subjects and time as within-subjects variable revealed a significant main effect of time [F(14,1417)=66.54; p<.001], and a significant time by genotype interaction [F(42;1417)=1.68; P=.005], but no significant main effect of genotype [F(3,102)=1.02] on circadian activity. Posthoc comparisons indicated that, at the first hour of the test, mGlu7-/- mice made fewer beam crossings than wild-type animals (16.30: P=.004, 17.00: P=.014). At the start of the second light phase, mGlu4-/- mice made significantly fewer beam crossings (Figure 1).
Figure 1.
Circadian activity in wild-type, mGlu4-/-, mGlu7-/-, and mGlu8-/- mice (dark phase from 8 pm to 8 am, grey block). No gross alterations in behavioral activity could be observed. However, during the first hour, mGlu7-/- mice (dark grey squares) were less active than wild-type animals (filled circles), while during the start of the second light phase mGlu4-/- mice (open circles) were less active. Data are represented as mean ± SEM. Asterisk indicates significant difference from the wild-type group: *P<.05.
No significant differences were observed between subtype-specific knockout and wild-type mice in the grip strength and rotarod tasks [F(3,102)=2.56; F(3,102)=0.58]. One-way ANOVA on weight revealed a significant main effect [F(3,102)=15.14; P<.001]. Posthoc pairwise comparisons showed that mGlu7-/- mice weighed less (M=19.45) than all other subtype-specific knockout groups and wild-type mice (all P-values <.001). On the other hand, mGlu8-/- (M=25.07) weighed somewhat more than wild-type mice (M=23.03; P=.016).
To assess afferent functions and sensorimotor gating, we recorded ASR and PPI in the different genotypes (recordings in one mGlu8-/- animal were discarded for technical reasons). One-way ANOVA examined the effect of genotype on ASR. A significant effect of genotype [F(3,101)=5.36; P=.002] indicated that mGlu4-/- mice showed less ASR than all other subtypes (mGlu7-/-: P=.011, mGlu8-/-: P=.017) and wild-type animals (P=.003; Figure 2A). Repeated-measures ANOVA on percentage PPI showed an effect of genotype [F(3,101)=5.29; P<.02] and pulse intensity [F(2,202)=12.405; P<.001]. Posthoc tests indicated that percentage PPI was significantly lower in mGlu4-/- mice than in mGlu7-/- and wild-type animals (Figure 2B). Because of a possible influence of the initial difference in ASR on percentage PPI (%PPI calculation is partially based on ASR data), correlation analysis and RM-ANOVA with ASR as covariate were executed. Correlation analyses demonstrated significant linear relationships between ASR and percentage PPI in mGlu4-/- mice (70dB: r=0.52, P=.013; 75B: r=0.49; P=.021; 80dB: r=0.54; P=.01) but not in the other groups of animals. In addition, the main effect of genotype on percentage PPI was still significant in repeated-measures ANCOVA with ASR as covariate [F(3,100)=4.16; P=.008]. mGlu4-/- mice had a significantly lower percentage PPI than mGlu7-/- mice (P=.01) and wild-type mice (P=.02). These 2 additional analyses indicate that the decreased percentage PPI in mGlu4-/- mice might reflect a real decrease rather than only a decreased ASR.
Figure 2.
ASR and PPI measures in wild-type and mGlu knockout mice. (A) A clear decrease in acoustic startle reactivity (ASR) is observed in mGlu4-/- mice (white bar). (B) For all 3 prepulse intensities, % PPI (prepulse inhibition) was lower in mGlu4-/- mice (white bars) than in wild-type animals (black bars). Data are represented as mean (A) and estimated marginal mean (B) ± SEM. Asterisks indicate significant difference with wild-type group, *P<.05, **P<.01.
Exploration
Anxiety-related behavior can be assessed in alterations of exploration pattern in OF, SE, and EPM. In these tests, no gross behavioral differences were observed between knockout and wild-type mice (Table 3). Notably, mGlu7-/- mice showed less rearing behavior in both the OF [F(3,102)=3.48; P=.019] and SE [F(3,102)=6.02; P=.001]. While mGlu4-/- mice had a shorter path length in the SE [F(3,102)=3.79; P=.013].
Table 3.
Exploratory Behavior in mGlu4, mGlu7, and mGlu8 Knockout and Wild-Type Mice
| Task | Variable | Wild-Type | mGlu4 -/- | mGlu7 -/- | mGlu8 -/- |
|---|---|---|---|---|---|
| OF | Path length (in cm) | 3969(129) | 3567(136) | 3823(251) | 4064(209) |
| Rearing | 32.1(2.9) | 40(6) | 16.2(2.3) | 29.7(2.6) | |
| % path length centre | 25.4(0.9) | 25.4(1.4) | 23.8(2.2) | 23.0(1.5) | |
| SE | Path length (in cm) | 4093.36(125) | 3349(224)* | 3815(298) | 4225(190) |
| Rearing | 37.6(2.3) | 32(4) | 21(4)** | 45.4(2.7) | |
| % path length centre | 49.3(2.4) | 44(4) | 40(6) | 52.1(2.4) | |
| EPM | Total beam crossings | 135(3) | 135(6) | 134(8) | 143(6) |
| Open arm dwell % | 18.7(1.0) | 21.9(1.3) | 19.9(1.5) | 17.3(2.5) |
Abbreviations: EPM, elevated plus maze; OF, open field; SE, social exploration. Data are means (SEM). Asterisks indicate significant difference with wild-type animals: *P<.05; **P<.01.
Learning and Memory
Learning and memory capacity was investigated in PA, MWM, and CFC, 3 well-known paradigms to assess cognitive abilities in rodents. In PA learning, no effect of genotype could be observed on the latency to enter the dark compartment on the second day (wild-type: 195.15±15.51 seconds; mGlu4-/-: 166.55±27.72 seconds; mGlu7-/-: 181.21±31.89 seconds; mGlu8-/-:224.47±26.16 seconds).
In the MWM, all mice learned to locate the hidden platform during the first week of acquisition training, reflected by a prominent decrease in escape latency and path length (Latency: F(2.82;284.87)=81.89; P<.001; path length: F(3.04;306.71)=96.66; P<.001) (Figure 3). However, RM-ANOVA on path length revealed a significant main effect of genotype (with no significant interaction), and additional posthoc comparisons indicated a significant delay in acquiring the exact platform location in mGlu7-/- mice [F(3,101)=4.063; P=.009; posthoc: mGlu7-/- vs wild-type: P=.044, mGlu7-/- vs mGlu4-/-: P=.005] (Figure 3B). mGlu4-/- mice displayed a significantly shorter latency to reach the target platform [F(3,101)=3.47; P=.019; posthoc: mGlu4-/- vs wild-type: P=.045, mGlu4-/- vs mGlu7-/-: P=.02]. Correspondingly, mGlu4-/- mice showed a significantly increased swimming velocity [F(3,101)=8.52; P<.001] (Figure 3C).
Figure 3.
Comparison of spatial learning between wild-type and mGlu group III knockout mice in the Morris water maze during the first acquisition week. (A) Escape latency data demonstrate that all mice learned to locate the platform by the end of week 1. However, mGlu4-/-(open circles) reached the platform significantly faster than wild-type (black dots) and mGlu7-/- (dark grey squares) animals. (B) mGlu7-/- mice (dark grey squares) swam a longer distance to reach the platform compared to wild-type (filled circles) and mGlu4-/- (open circles) mice. Path length is considered to be a more cognitive measure. (C) Velocity data show a clear increase in swimming speed in mGlu4-/- mice (white bar). Data are presented as mean ± SEM, ***P<.001 compared with wild-type animals.
During the second week of acquisition training, no differences could be observed between genotypes on path length and latency. However, mGlu4-/- mice still swam faster than wild-type animals [F(3,101)=2.78; P=.045; posthoc: mGlu4-/- vs wild-type: P=.048].
At the end of each trial week, a probe trial was performed to measure the spatial accuracy of the mice. Already in the first probe trial, all mice showed a clear preference for the target quadrant in comparison with the other quadrants, indicated by a main effect of quadrant [F(2,212)=46.62; P<.001] with no significant interaction [F(6,212)=0.958] or main effect of genotype [F(3,101)=1.22]. Similar to acquisition trials, a main effect of velocity was observed [F(3,101)=4.47; P=.005]. More specifically, mGlu4-/- mice swam significantly faster than wild-type mice (P=.003) and mGlu8-/- mice (P=.044).
In CFC, mice had to learn the association between a cue (tone) and context (test cage), and an aversive event (shock). RM-ANOVA revealed a main effect of genotype [F(3,102)=4.08; P=.009] and trial [F(3,325)=179.41; P<.001] but no significant interaction-effect [F(10,325)=0.94]. In general, mGlu7-/- mice showed an overall decrease in freezing behavior (mGlu7-/- vs mGlu4-/-: P=.005) (Figure 4).
Figure 4.
Contextual fear conditioning in mGlu group III knockout and wild-type mice. All mice learned to associate the context (increased freezing in “context” phase) and tone (increased freezing in CS phase) to the shock. Over all phases, mGlu7-/- mice (dark grey bars) display less freezing. ** P<.01 compared with mGlu4-/- mice. Data are presented as mean ± SEM.
Correlations between Behavioral Variables
Correlation analyses revealed intra- and inter-task correlations. Overall, high correlations were found between neuromotor measures, between exploratory measures, and between cognitive measures, strengthening the face-validity of grouping these tasks (as previously shown in Caeyenberghs et al., 2006). Analyzing receptor-deficient and wild-type animals separately, different correlation patterns were observed. First, no significant correlations with weight were observed in mGlu7 and mGlu8 knockout mice. In mGlu4-/- mice, a significant negative correlation between weight and rearing in the OF (OF_R) was observed (r=-0.43; P=.044). In wild-type animals, weight significantly correlates positively with grip strength and inversely with freezing to context in the contextual fear task.
Intra- and inter-task correlations of OF and SE are strongest in wild-type and mGlu4 knockout mice. Data of mGlu7 knockout mice demonstrate only intra-task correlation of SE, while in mGlu8 knockout mice, the pattern is more scattered. In cognitive tasks, wild-type and mGlu7 knockout mice displayed high intra-task correlations in the CFC task. In mGlu4 and mGlu8 knockout mice, only shock and context freezing data correlated significantly. Wild-type, mGlu4, and mGlu8 knockout mice show intra-task correlations between MWM performance variables, whereas none of these measures correlated significantly in mGlu7 knockout mice. A highly significant negative correlation (r=-0.47; P=.005) between velocity in week 1 (MW_VEL1) and path length during the first trial block was observed in mGlu4-/- mice. In mGlu7-/- mice, high positive correlations were observed between intra-task measures in the CFC task, while freezing to tone correlated negatively with total beam crossings in the cage activity (r=-0.67; P=.009) and EPM task (r=-0.56; P=.039).
DFA
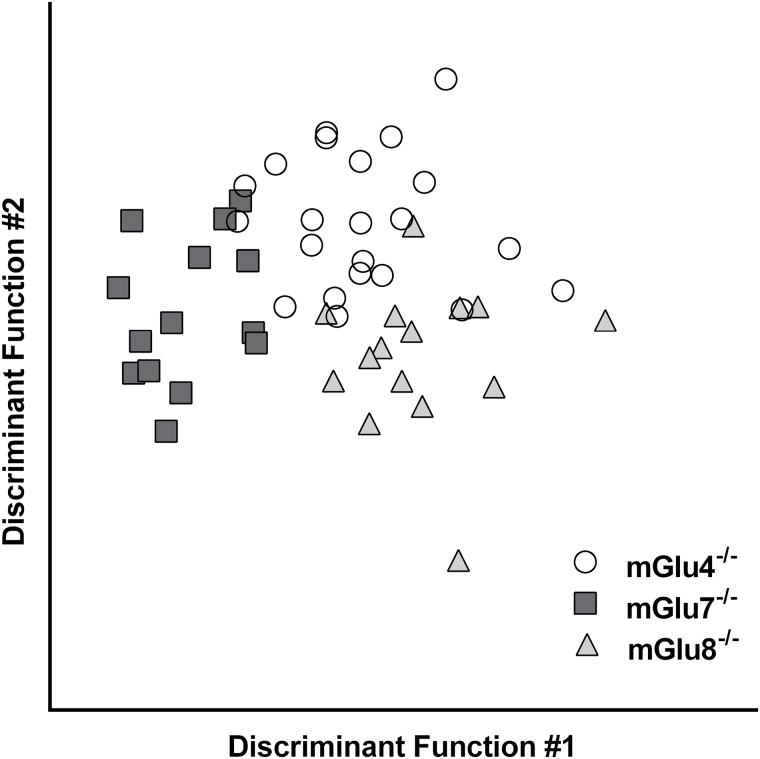
Results of DFA are summarized in Table 4. The 3 group III receptor subgroups could be discriminated when all behavioral measures were included in the model (direct entry method). Using the stepwise method, body weight, OF_R, swimming velocity in MWM (MW_VEL1), and freezing to tone during CFC provided maximal discriminability (84.3% of cases were classified correctly based on these measures). The DFA resulted in 2 significant canonical functions accounting for 71.3% (Eigenvalue=1.83) and 28.7% of variance (Eigenvalue=0.74), respectively (Figure 5). Weight was most strongly correlated with the first function, while OF_R, CFC_CS, and MWM_VEL1 were more strongly correlated with the second function.
Table 4.
DFA on Behavioral Variables of mGlu4, mGlu7, and mGlu8 Knockout and Wild-Type Mice
| Stepwise Forward Method | |||
|---|---|---|---|
| Included Variables | Direct Entry Method | Significance | Variables Retained |
| All variables | P =.030 | P < .001 | Weight |
| MWM_VEL1 | |||
| CFC_CUE | |||
| OF_R | |||
| Neuromotor | N.S. | P=.002 | GRIP |
| ASR | |||
| Exploratory | P=.042 | P < .001 | OF_R |
| SE_R | |||
| Cognitive | N.S. | P < .001 | MWM_VEL1 |
| MWM_PL | |||
Abbreviations: ASR, acoustic startle response; CFC_CUE, contextual fear conditioning, percentage freezing cue; MWM_PL: Morris water maze, difference in path length between day 1 and day 5; MWM_VEL1, Morris water maze average velocity week 1; N.S., not significant; OF_R, open field rearing; SE_R: social exploration rearing.
Figure 5.
Scatterplot using discriminant scores clearly illustrates the distinct behavioral profiles of the 3 mGlu knockout groups (mGlu4-/-: open circles; mGlu7-/-: dark grey squares; mGlu8-/-: light grey triangles). This plot depicts discriminant scores of the 2 discriminant functions derived from behavioral and body weight measures (Table 2). Body weight was more strongly correlated with discriminant function 1, while open filed rearing (OF_R), swimming velocity in Morris water maze (MWM_VEL1), and freezing to tone in the contextual fear conditioning paradigm (CFC_CS) were more strongly correlated with function 2.
The 3 groups could not be discriminated when all 6 neuromotor variables were entered simultaneously, but maximal dissociation was achieved with mean grip strength and ASR using the stepwise method. When all exploratory variables were included in the direct entry model, the 3 receptor-deficient groups could be discriminated. The stepwise method showed that OF_R and SE task (SE_R) were important discriminators. Lastly, the stepwise method revealed significant discriminability between the 3 genotypes using cognitive measures. Velocity and difference in path length between first and last day of MWM performance provided maximal discriminability.
Discussion
Subtype-specific knockout mice have been generated, amongst other things, to investigate the behavioral functions of group III metabotropic receptors and their potential as drug targets for the treatment of neuropathological and mood disorders (Cryan et al., 2003; Goddyn et al., 2008; Fendt et al., 2013; Iscru et al., 2013). However, few of these studies actually analyzed a broader range of behaviors, and none of them compared different group III knockouts directly in a single behavioral battery. Therefore, we tested mGlu4, mGlu7, and mGlu8 knockout mice simultaneously on multiple behavioral tasks, and multivariate statistical techniques were used to uncover subtype-specific behavioral profiles. Their differential brain distribution suggested already that mGlu4, mGlu7, and mGlu8 receptors may serve different behavioral functions, which is definitely confirmed by the present study showing significant functional dissociation between subtype-deficient mice in PPI, MWM, and CFC. Notably, mGlu8-/- mice did not show any behavioral alteration in comparison with wild-type animals throughout the entire test battery. They were significantly heavier than the other mice, and indeed substantial weight gain has been reported previously in mGlu8 knockout mice (Duvoisin et al., 2005; Davis et al., 2013).
In the acoustic response tasks, mGlu4-/- mice showed decreased ASR and PPI, whereas these measures were unaltered in mGlu7 and mGlu8 knockout mice. Notably, covariance analysis indicated that ASR decrease is not responsible for the subsequently observed decrease in percentage PPI. This is in accordance with a study by Paylor and Crawley (1997), which demonstrated that sensorimotor gating (as measured by percentage PPI) and acoustic startle are in fact independent functions. The mGlu4 receptor appears to be expressed in glutamatergic and GABAergic synapses in brain structures that are involved in acoustic startle functions. Startle reactivity is mainly a brainstem reflex that is modulated by cortico-striato-pallido-pontine circuitry (Swerdlow et al., 2000). Weber et al. (2002) hypothesized that glutamate release from auditory afferents in nucleus reticularis pontis caudalis may be inhibited by presynaptically located group III mGlu receptors (still, the actual presence of group III receptors in this structure remains to be demonstrated). Even more significantly, mGlu4 receptors are expressed in basal ganglia and hippocampus, which are both intricately involved in startle-related functions (Shoemaker et al., 2005). Alterations in presynaptic inhibition of GABA and/or glutamate release in these key structures could definitely account for the decrease in startle reactivity (and even PPI) presently observed in mGlu4-/- mice. Similar results have been observed after NMDA infusion in rat ventral hippocampus (Zhang, 2001). Since mGlu4 receptors are known to inhibit presynaptic glutamate release in hippocampus (Phillips et al., n.d.), mGlu4 deletion may have altered ASR and PPI by affecting hippocampal NMDA receptor activity. It should also be noted that disrupted ASR and PPI have been found in a variety of neuropsychiatric disorders, such as schizophrenia and Huntington disease (Swerdlow et al., 1995, 2008). Swanson et al. (2005) specifically mentioned mGlu7 and mGlu8 as promising drug targets for anxiety and depression disorders. In several studies, mGlu7 knockout mice showed an anxiolytic-like phenotype (Cryan et al., 2003; Callaerts-Vegh et al., 2006), whereas mGlu8 knockout mice showed an anxiogenic-like phenotype (Linden et al., 2002; Duvoisin et al., 2005; Robbins et al., 2007). However, in the present study, EPM performance (a prototypic anxiety test for mice) did not discriminate mGlu7- or mGlu8-deficient mice from the other mouse lines. Other authors have also failed to observe increased anxiety in mGlu8 knockout mice (Fendt et al., 2010; Davis et al., 2013), but the lack of EPM alterations in mGlu7 knockout mice was unexpected. Mice in the present study were bred in an SPF facility and transported to the behavioral laboratory at 12 weeks of age, which has been shown to affect EPM behavior (Mineur and Crusio, 2009) and which is different from the other studies that used on-site bred mice. However, mGlu7 knockout mice did display reduced exploratory rearing (in OF and SE), as well as an overall decrease in freezing during the CFC task in agreement with a previous report (Masugi et al., 1999). Freezing to both context and cue are comparable with freezing levels during the shock trial, indicating that mGlu7 knockout mice show an impairment in fear-induced freezing while still able to recall the fear response associated with conditioning context and cue. Masugi et al. (1999) argued that this can be attributed to a lack of mGlu7 receptors in the amygdala.
Cognitive alterations were hypothesized to occur in all 3 mouse strains on the basis of the brain expression patterns of these receptor subtypes (Callaerts-Vegh et al., 2006; Fendt et al., 2010). In agreement with our previous observations (Callaerts-Vegh et al., 2006) and mGlu7’s prominent telencephalic distribution (Table 1), mGlu7-/- mice indeed showed impaired MWM learning, which is generally considered to be strongly hippocampus dependent (D’Hooge and Deyn, 2001; Goddyn et al., 2006). Surprisingly, none of the other mouse lines displayed cognitive defects, but mGlu4-/- mice swam considerably faster than the other mice in this task, which could not be reduced to some kind of general restlessness or hyperactivity. Possibly mGlu4-/- mice might be more motivated to reach the platform, since the motivational aspects of learning are indeed expressed by reward approach velocity (Lubbers et al., 2007). Significantly, mGlu4 receptors do occur in nucleus accumbens (Corti et al., 2002), an important area for reward learning and motivation (Robbins and Everitt, 1996). Nucleus accumbens is the interface between limbic and motor systems and translates motivation into action (Mogenson et al., 1980).
To analyze the differences between the studied mouse lines more in detail, the set of behavioral variables was subjected to correlation analysis and DFA. Correlation analyses revealed intra- and inter-task correlations between behavioral measures, whereas DFA, more specifically the stepwise-forward method, indicated that the 3 different knockout groups could be reliably discriminated by some of the behavioral variables. These analyses decisively confirm that the different group III receptors do play distinct behavioral roles. DFA has only been used in a few rodent studies but has been proven successful to distinguish between behavioral profiles of different mouse strains (Leighty et al., 2004; Caeyenberghs et al., 2006). DFA conclusively discriminated between the knockout groups examined here. We used both direct and stepwise-forward methods on the set of behavioral variables (and body weight). Direct entry did not identify any discriminating variable, whereas the stepwise method did (ie, body weight, swimming velocity in MWM, freezing to tone during CFC, and OF_R significantly dissociated the knockout groups).
Various intra-task correlations were found between OF, SE, MWM, and CFC measures, indicating that variables within these tests might measure the same underlying trait. When correlations were calculated per wild-type or knockout group, different correlation patterns were observed. In wild-type mice, strong intra-task correlations were observed for almost each task, whereas in knockout mice, only the SE task showed significant intra-task correlations. In mGlu4-/- mice, velocity in the MWM correlated negatively with path length in the first block of MWM learning, but not with any other activity-related variable (confirms that the altered swimming velocity is not due to general hyperactivity). Conversely, negative correlations between freezing to tone (during CFC) and IR beam crossings (during cage activity and EPM exploration) in mGlu7-/- mice suggests that reduced freezing can be (at least partially) explained by hyperactivity in these animals.
Although several authors already suggested different behavioral functions between group III metabotropic receptors, comparison across the different reports remained methodologically hazardous and controversial. Therefore, the present study directly compared knockouts for each group III receptor subtype using a single behavioral test battery and multivariate methods. On the basis of this controlled comparison, we are presently able to conclude that the different group III receptors indeed have quite distinct behavioral functions. By and large, it appears that mGlu7 plays a significant role in spatial learning and in some fear-related behaviors, whereas mGlu4 is most clearly involved in startle and motivational processes. Excepting its influence on body weight, the effect of mGlu8 deletion on behavior appears more subtle than that of the other group III receptors. Importantly, these subtle effects might be due to potential developmental compensatory changes. Raber and Duvoisin (2015) argue that other receptors (eg, mGlu4) might compensate for the lack of this receptor subtype. This plausible compensation for receptor subtype-specific deficits is not only limited to mGlu8 receptor but is applicable to all 3 subtypes. To fully understand the differential role of group III receptor subtypes, studies using either conditional single- or double knockouts or RNA interference techniques (O’Connor et al 2013) might provide an alternative approach.
Statement of Interest
None.
Acknowledgments
We thank Dr Robert Duvoisin, Oregon Health & Science University, Department of Physiology and Pharmacology, Portland, OR, for sending us the original breeding pairs. We also thank Johnson & Johnson Pharmaceutical Research and Development, Beerse, Belgium, for maintaining the breeding colony. This work was supported by Fonds Wetenschappelijk Onderzoek - Vlaanderen (PhD fellowship to H.G.) and the KU Leuven-OR Research Fund (GOA research program with R.D. as main promoter).
References
- Bradley SR, Standaert DG, Rhodes KJ, Rees HD, Testa CM, Levey AI, Conn PJ. (1999) Immunohistochemical localization of subtype 4a metabotropic glutamate receptors in the rat and mouse basal ganglia. J Comp Neurol 407:33–46. [PubMed] [Google Scholar]
- Caeyenberghs K, Balschun D, Roces DP, Schwake M, Saftig P, D’Hooge R. (2006) Multivariate neurocognitive and emotional profile of a mannosidosis murine model for therapy assessment. Neurobiol Dis 23:422–432. [DOI] [PubMed] [Google Scholar]
- Callaerts-Vegh Z, Beckers T, Ball SM, Baeyens F, Callaerts PF, Cryan JF, Molnar E, D’Hooge R. (2006) Concomitant deficits in working memory and fear extinction are functionally dissociated from reduced anxiety in metabotropic glutamate receptor 7-deficient mice. J Neurosci 26:6573–6582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corti C, Restituito S, Rimland JM, Brabet I, Corsi M, Pin JP, Ferraguti F. (1998) Cloning and characterization of alternative mRNA forms for the rat metabotropic glutamate receptors mGluR7 and mGluR8. Eur J Neurosci 10:3629–3641. [DOI] [PubMed] [Google Scholar]
- Corti C, Aldegheri L, Somogyi P, Ferraguti F. (2002) Distribution and synaptic localisation of the metabotropic glutamate receptor 4 (mGluR4) in the rodent CNS. Neuroscience 110:403–420. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Wahlsten D, Dudek BC. (1999) Genetics of mouse behavior: interactions with laboratory environment. Science 284:1670–1672. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Kelly PH, Neijt HC, Sansig G, Flor PJ, van Der Putten H. (2003) Antidepressant and anxiolytic-like effects in mice lacking the group III metabotropic glutamate receptor mGluR7. Eur J Neurosci 17:2409–2417. [DOI] [PubMed] [Google Scholar]
- Davis MJ, Haley T, Duvoisin RM, Raber J. (2012) Measures of anxiety, sensorimotor function, and memory in male and female mGluR4−/− mice. Behav Brain Res 229:21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MJ, Duvoisin RM, Raber J. (2013) Related functions of mGlu4 and mGlu8. Pharmacol Biochem Behav 111:11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Hooge R, Deyn PP De. (2001) Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev 36:60–90. [DOI] [PubMed] [Google Scholar]
- Dobi A, Sartori SB, Busti D, Van der Putten H, Singewald N, Shigemoto R, Ferraguti F. (2013) Neural substrates for the distinct effects of presynaptic group III metabotropic glutamate receptors on extinction of contextual fear conditioning in mice. Neuropharmacology 66:274–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvoisin R, Zhang C, Ramonell K. (1995) A novel metabotropic glutamate receptor expressed in the retina and olfactory bulb. J Neurosci 15:3075–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvoisin RM, Zhang C, Pfankuch TF, O’Connor H, Gayet-Primo J, Quraishi S, Raber J. (2005) Increased measures of anxiety and weight gain in mice lacking the group III metabotropic glutamate receptor mGluR8. Eur J Neurosci 22:425–436. [DOI] [PubMed] [Google Scholar]
- Fendt M, Bürki H, Imobersteg S, van der Putten H, McAllister K, Leslie JC, Shaw D, Hölscher C. (2010) The effect of mGlu8 deficiency in animal models of psychiatric diseases. Genes Brain Behav 9:33–44. [DOI] [PubMed] [Google Scholar]
- Fendt M, Imobersteg S, Peterlik D, Chaperon F, Mattes C, Wittmann C, Olpe H-R, Mosbacher J, Vranesic I, van der Putten H, McAllister KH, Flor PJ, Gee CE. (2013) Differential roles of mGlu(7) and mGlu(8) in amygdala-dependent behavior and physiology. Neuropharmacology 72:215–223. [DOI] [PubMed] [Google Scholar]
- Ferraguti F, Klausberger T, Cobden P, Baude A, Roberts JDB, Szucs P, Kinoshita A, Shigemoto R, Somogyi P, Dalezios Y. (2005). Metabotropic glutamate receptor 8-expressing nerve terminals target subsets of GABAergic neurons in the hippocampus. J Neurosci 25:10520–10536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlai R, Adams B, Fitch T, Chaney S, Baez M. (2002) Performance deficits of mGluR8 knockout mice in learning tasks: the effects of null mutation and the background genotype. Neuropharmacology 43:235–249. [DOI] [PubMed] [Google Scholar]
- Goddyn H, Leo S, Meert T, D’Hooge R. (2006) Differences in behavioral test battery performance between mice with hippocampal and cerebellar lesions. Behav Brain Res 173:138–147. [DOI] [PubMed] [Google Scholar]
- Goddyn H, Callaerts-Vegh Z, Stroobants S, Dirikx T, Vansteenwegen D, Hermans D, van der Putten H, D’Hooge R. (2008) Deficits in acquisition and extinction of conditioned responses in mGluR7 knockout mice. Neurobiol Learn Mem 90:103–111. [DOI] [PubMed] [Google Scholar]
- Iscru E, Goddyn H, Ahmed T, Callaerts-Vegh Z, D’Hooge R, Balschun D. (2013) Improved spatial learning is associated with increased hippocampal but not prefrontal long-term potentiation in mGluR4 knockout mice. Genes, Brain Behav 12:615–625. [DOI] [PubMed] [Google Scholar]
- Kinoshita A, Ohishi H, Nomura S, Shigemoto R, Nakanishi S, Mizuno N. (1996) Presynaptic localization of a metabotropic glutamate receptor, mGluR4a, in the cerebellar cortex: a light and electron microscope study in the rat. Neurosci Lett 207:199–202. [DOI] [PubMed] [Google Scholar]
- Kinoshita A, Shigemoto R, Ohishi H, van der Putten H, Mizuno N. (1998) Immunohistochemical localization of metabotropic glutamate receptors, mGluR7a and mGluR7b, in the central nervous system of the adult rat and mouse: a light and electron microscopic study. J Comp Neurol 393:332–352. [PubMed] [Google Scholar]
- Kinzie JM, Saugstad JA, Westbrook GL, Segerson TP. (1995) Distribution of metabotropic glutamate receptor 7 messenger RNA in the developing and adult rat brain. Neurosci 69:167–176. [DOI] [PubMed] [Google Scholar]
- Kinzie JM, Shinohara MM, van den Pol AN, Westbrook GL, Segerson TP. (1997) Immunolocalization of metabotropic glutamate receptor 7 in the rat olfactory bulb. J Comp Neurol 385:372–384. [PubMed] [Google Scholar]
- Kosinski CM, Bradley SR, Conn PJ, Levey AI, Landwehrmeyer GB, Penney JB, Jr., Young AB, Standaert DG. (1999) Localization of metabotropic glutamate receptor 7 mRNA and mGluR7a protein in the rat basal ganglia. J Comp Neurol 415:266–284. [PubMed] [Google Scholar]
- Kuramoto E, Fujiyama F, Unzai T, Nakamura K, Hioki H, Furuta T, Shigemoto R, Ferraguti F, Kaneko T. (2007) Metabotropic glutamate receptor 4-immunopositive terminals of medium-sized spiny neurons selectively form synapses with cholinergic interneurons in the rat neostriatum. J Comp Neurol 500:908–922. [DOI] [PubMed] [Google Scholar]
- Leighty RE, Nilsson LNG, Potter H, Costa DA, Low MA, Bales KR, Paul SM, Arendash GW. (2004) Use of multimetric statistical analysis to characterize and discriminate between the performance of four Alzheimer’s transgenic mouse lines differing in Abeta deposition. Behav Brain Res 153:107–121. [DOI] [PubMed] [Google Scholar]
- Linden A-M, Johnson B, Peters S, Shannon H, Tian M, Wang Y, Yu J, Köster A, Baez M, Schoepp D (2002) Increased anxiety-related behavior in mice deficient for metabotropic glutamate 8 (mGlu8) receptor. Neuropharmacology 43:251–259. [DOI] [PubMed] [Google Scholar]
- Lubbers ME, van den Bos R, Spruijt BM. (2007) Mu opioid receptor knockout mice in the Morris Water Maze: a learning or motivation deficit? Behav Brain Res 180:107–111. [DOI] [PubMed] [Google Scholar]
- Masugi M, Yokoi M, Shigemoto R, Muguruma K, Watanabe Y, Sansig G, van der Putten H, Nakanishi S. (1999) Metabotropic glutamate receptor subtype 7 ablation causes deficit in fear response and conditioned taste aversion. J Neurosci 19:955–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateos JM, Azkue J, Sarría R, Kuhn R, Grandes P, Knöpfel T. (1998) Localization of the mGlu4a metabotropic glutamate receptor in rat cerebellar cortex. Histochem Cell Biol 109:135–139. [DOI] [PubMed] [Google Scholar]
- Messenger MJ, Dawson LG, Duty S. (2002) Changes in metabotropic glutamate receptor 1–8 gene expression in the rodent basal ganglia motor loop following lesion of the nigrostriatal tract. Neuropharmacology 43:261–271. [DOI] [PubMed] [Google Scholar]
- Mineur YS, Crusio WE. (2009) Behavioral effects of ventilated micro-environment housing in three inbred mouse strains. Physiol Behav 97:334–340. [DOI] [PubMed] [Google Scholar]
- Mogenson G, Jones D, Yim C. (1980) From motivation to action: Functional interface between the limbic system and the motor system. Prog Neurobiol 14:69–97. [DOI] [PubMed] [Google Scholar]
- Nakajima Y, Iwakabe H, Akazawa C, Nawa H, Shigemoto R, Mizuno N, Nakanishi S. (1993) Molecular characterization of a novel retinal metabotropic glutamate receptor mGluR6 with a high agonist selectivity for L-2-amino-4- phosphonobutyrate. J Biol Chem 268:11868–11873. [PubMed] [Google Scholar]
- Niswender CM, Conn PJ. (2010) Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol 50:295–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor RM, Thakker DR, Schmutz M, van der Putten H, Hoyer D, Flor PJ, Cryan JF. (2013) Adult siRNA-induced knockdown of mGlu7 receptors reduces anxiety in the mouse. Neuropharmacology 72:66–73. [DOI] [PubMed] [Google Scholar]
- Ohishi H, Akazawa C, Shigemoto R, Nakanishi S, Mizuno N. (1995) Distributions of the mRNAs for L-2-amino-4-phosphonobutyrate-sensitive metabotropic glutamate receptors, mGluR4 and mGluR7, in the rat brain. J Comp Neurol 360:555–570. [DOI] [PubMed] [Google Scholar]
- Okamoto N, Hori S, Akazawa C, Hayashi Y, Shigemoto R, Mizuno N, Nakanishi S. (1994) Molecular characterization of a new metabotropic glutamate receptor mGluR7 coupled to inhibitory cyclic AMP signal transduction. J Biol Chem 269:1231–1236. [PubMed] [Google Scholar]
- Palazzo E, Marabese I, Soukupova M, Luongo L, Boccella S, Giordano C, de Novellis V, Rossi F, Maione S. (2011) Metabotropic glutamate receptor subtype 8 in the amygdala modulates thermal threshold, neurotransmitter release, and rostral ventromedial medulla cell activity in inflammatory pain. J Neurosci 31:4687–4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palucha A, Klak K, Branski P, van der Putten H, Flor PJ, Pilc A. (2007) Activation of the mGlu7 receptor elicits antidepressant-like effects in mice. Psychopharmacology (Berl) 194:555–562. [DOI] [PubMed] [Google Scholar]
- Pamidimukkala J, Hoang CJ, Hay M. (2002) Expression of metabotropic glutamate receptor 8 in autonomic cell groups of the medulla oblongata of the rat. Brain Res 957:162–173. [DOI] [PubMed] [Google Scholar]
- Paradee W, Melikian HE, Rasmussen DL, Kenneson A, Conn PJ, Warren ST. (1999) Fragile X mouse: strain effects of knockout phenotype and evidence suggesting deficient amygdala function. Neuroscience 94:185–192. [DOI] [PubMed] [Google Scholar]
- Paylor R, Crawley JN. (1997) Inbred strain differences in prepulse inhibition of the mouse startle response. Psychopharmacology (Berl) 132:169–180. [DOI] [PubMed] [Google Scholar]
- Pekhletski R, Gerlai R, Overstreet LS, Huang X-P, Agopyan N, Slater NT, Abramow-Newerly W, Roder JC, Hampson DR. (1996) Impaired cerebellar synaptic plasticity and motor performance in mice lacking the mGluR4 subtype of metabotropic glutamate receptor. J Neurosci 16:6364–6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips T, Makoff A, Brown S, Rees S, Emson P. (1997) Localization of mGluR4 protein in the rat cerebral cortex and hippocampus. Neuroreport 8:3349–3354. [DOI] [PubMed] [Google Scholar]
- Pin J-PP, Duvoisin R. (1995) The metabotropic glutamate receptors: structure and functions. Neuropharmacology 34:1–26. [DOI] [PubMed] [Google Scholar]
- Raber J, Duvoisin RM. (2015). Novel metabotropic glutamate receptor 4 and glutamate receptor 8 therapeutics for the treatment of anxiety. Expert Opin Investig Drugs, 24:519–528. [DOI] [PubMed] [Google Scholar]
- Récasens M, Guiramand J, Aimar R, Abdulkarim A, Barbanel G. (2007) Metabotropic glutamate receptors as drug targets. Curr Drug Targets 8:651–681. [DOI] [PubMed] [Google Scholar]
- Robbins MJ, Starr KR, Honey A, Soffin EM, Rourke C, Jones GA, Kelly FM, Strum J, Melarange RA, Harris AJ, Rocheville M, Rupniak T, Murdock PR, Jones DNC, Kew JNC, Maycox PR. (2007) Evaluation of the mGlu8 receptor as a putative therapeutic target in schizophrenia. Brain Res 1152:215–227. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. (1996) Neurobehavioral mechanisms of reward and motivation. Curr Opin Neurobiol 6:228–236. [DOI] [PubMed] [Google Scholar]
- Saugstad JA, Kinzie JM, Shinohara MM, Segerson TP, Westbrook GL. (1997) Cloning and expression of rat metabotropic glutamate receptor 8 reveals a distinct pharmacological profile. Mol Pharmacol 51:119–125. [DOI] [PubMed] [Google Scholar]
- Schoepp DD. (2001) Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J Pharmacol Exp Ther 299:12–20. [PubMed] [Google Scholar]
- Shigemoto R, Kinoshita A, Wada E, Nomura S, Ohishi H, Takada M, Flor PJ, Neki A, Abe T, Nakanishi S, Mizuno N. (1997) Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. J Neurosci 17:7503–7522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker JM, Saint Marie RL, Bongiovanni MJ, Neary AC, Tochen LS, Swerdlow NR. (2005) Prefrontal D1 and ventral hippocampal N-methyl-D-aspartate regulation of startle gating in rats. Neuroscience 135:385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi P, Dalezios Y, Luján R, Roberts JDB, Watanabe M, Shigemoto R. (2003) High level of mGluR7 in the presynaptic active zones of select populations of GABAergic terminals innervating interneurons in the rat hippocampus. Eur J Neurosci 17:2503–2520. [DOI] [PubMed] [Google Scholar]
- Swanson CJ, Bures M, Johnson MP, Linden A-M, Monn J a, Schoepp DD. (2005) Metabotropic glutamate receptors as novel targets for anxiety and stress disorders. Nat Rev Drug Discov 4:131–144. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Paulsen J, Braff DL, Butters N, Geyer MA, Swenson MR. (1995) Impaired prepulse inhibition of acoustic and tactile startle response in patients with Huntington’s disease. J Neurol Neurosurg Psychiatry 58:192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Braff DL, Geyer M a. (2000) Animal models of deficient sensorimotor gating: what we know, what we think we know, and what we hope to know soon. Behav Pharmacol 11:185–204. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Weber M, Qu Y, Light GA, Braff DL. (2008) Realistic expectations of prepulse inhibition in translational models for schizophrenia research. Psychopharmacology (Berl) 199:331–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe Y, Nomura A, Masu M, Shigemoto R, Mizuno N, Nakanishi S. (1993) Signal transduction, pharmacological properties, and expression patterns of two rat metabotropic glutamate receptors, mGluR3 and mGluR4. J Neurosci 13:1372–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa CM, Standaert DG, Young AB, Penney JB., Jr (1994) Metabotropic glutamate receptor mRNA expression in the basal ganglia of the rat. J Neurosci 14:3005–3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada E, Shigemoto R, Kinoshita A, Ohishi H, Mizuno N. (1998) Metabotropic glutamate receptor subtypes in axon terminals of projection fibers from the main and accessory olfactory bulbs: a light and electron microscopic immunohistochemical study in the rat. J Comp Neurol 393: 493–504. [PubMed] [Google Scholar]
- Weber M, Schnitzler H-U, Schmid S. (2002) Synaptic plasticity in the acoustic startle pathway: the neuronal basis for short-term habituation? Eur J Neurosci 16:1325–1332. [DOI] [PubMed] [Google Scholar]
- Wu S, Wright R a., Rockey PK, Burgett SG, Arnold JS, Rosteck PR, Johnson BG, Schoepp DD, Belagaje RM. (1998) Group III human metabotropic glutamate receptors 4, 7 and 8: Molecular cloning, functional expression, and comparison of pharmacological properties in RGT cells. Mol Brain Res 53:88–97. [DOI] [PubMed] [Google Scholar]
- Zhang W. (2001) The ventral hippocampus and fear conditioning in rats: different anterograde amnesias of fear after infusion of N-methyl-?-aspartate or its noncompetitive antagonist MK-801 into the ventral hippocampus. Behav Brain Res 126:159–174. [DOI] [PubMed] [Google Scholar]