Abstract
Background:
Previous meta-analyses of atypical antipsychotics for depression were limited by few trials with direct comparisons between two treatments. We performed a network meta-analysis, which integrates direct and indirect evidence from randomized controlled trials (RCTs), to investigate the comparative efficacy and tolerability of adjunctive atypical antipsychotics for treatment-resistant depression (TRD).
Methods:
Systematic searches resulted in 18 RCTs (total n = 4422) of seven different types and different dosages of atypical antipsychotics and a placebo that were included in the review.
Results:
All standard-dose atypical antipsychotics were significantly more efficacious than placebo in the efficacy (standardized mean differences [SMDs] ranged from -0.27 to -0.43). There were no significant differences between these drugs. Low-dose atypical antipsychotics were not significantly more efficacious than the placebo. In terms of tolerability, all standard-dose atypical antipsychotics, apart from risperidone, had significantly more side-effect discontinuations than placebo (odds ratios [ORs] ranged from 2.72 to 6.40). In terms of acceptability, only quetiapine (mean 250–350mg daily) had significantly more all-cause discontinuation than placebo (OR = 1.89). In terms of quality of life/functioning, standard-dose risperidone and standard-dose aripiprazole were more beneficial than placebo (SMD = -0.38; SMD = -0.26, respectively), and standard-dose risperidone was superior to quetiapine (mean 250–350mg daily).
Conclusions:
All standard-dose atypical antipsychotics for the adjunctive treatment of TRD are efficacious in reducing depressive symptoms. Risperidone and aripiprazole also showed benefits in improving the quality of life of patients. Atypical antipsychotics should be prescribed with caution due to abundant evidence of side effects.
Keywords: Atypical antipsychotics, network meta-analysis, systematic review, treatment-resistant depression
Introduction
Major depression is among the most impairing, common, and costly mental disorders. Approximately 5–12% of males and 9–26% of females will suffer from at least one episode of depression over their lifetime, and about 50% of patients will experience a second depressive episode (Crown et al., 2002; Kessler et al., 2003; Finley, 2009). Although there were several available treatments for depression over the past two decades, a substantial number of patients either did not respond adequately to these drugs or were unable to tolerate their adverse effects (Berlim and Turecki, 2007; Shelton et al., 2010). These patients are broadly defined as having treatment-resistant depression (TRD). Recent clinical trials indicated that only approximately half of depressed patients with initial antidepressant monotherapy showed a favorable treatment response, and only about one-third achieved remission of symptoms (Trivedi et al., 2006); thus, there is need for additional treatment strategies for those patients with TRD. One common alternative approach to the treatment of patients with TRD is augmentation strategies for those who failed to respond to the initial antidepressant (Vieta and Colom, 2011).
The use of atypical antipsychotics has rapidly increased worldwide in the last decade. In 2007 and 2008, there were an estimated 3.7 million patients in per year in the US who were prescribed an atypical antipsychotic medication for depression (Alexander et al., 2011). Currently, three atypical antipsychotic drugs—aripiprazole, quetiapine, and olanzapine—have received approval from the US Food and Drug Administration (FDA) as adjunctive therapies for adult TRD (Hicks et al., 2010). However, clinicians remain unclear as to how to select the optimal atypical antipsychotic for TRD. The mechanisms of atypical antipsychotics differ in their selectivity for 5-HT2 receptors and/or dopamine D2 receptors and in their effects on different brain regions (Blier and Szabo, 2005; Guo et al., 2012a, 2012b), and prominent pharmacologic effects may differ between dosage levels.
The evidence for the efficacy of adjunctive atypical antipsychotic therapy for TRD has been investigated in several previous traditional meta-analyses (Papakostas et al., 2007; Nelson and Papakostas, 2009; Komossa et al., 2010; Spielmans et al., 2013). However, these findings of previous meta-analyses were derived from the combined data of different atypical antipsychotics, and cannot provide hierarchical evidence on the efficacy and tolerability of atypical antipsychotics for depression due to a very limited number of trials with direct comparisons between two active agents. More importantly, the question of the superiority of a given dosage in efficacy and tolerability of atypical antipsychotics has not previously been assessed in the comprehensive setting of a systematic review and meta-analysis.
To address the above concerns, we employed a network meta-analysis, which is a new methodological approach that allows the integration of direct evidence (from studies directly comparing interventions) with indirect evidence (information about two treatments derived via a common comparator, e.g. placebo) from multiple treatment comparisons to estimate the interrelations across all treatments (Salanti et al., 2008). Previously, we conducted a network meta-analysis of comparative efficacy, acceptability, and tolerability of all augmentation agents in treatment-resistant depression in this way (Zhou et al., 2014), but did not investigate the issue of different doses of atypical antipsychotics in the previous study. Further, this methodological approach has previously been used to assess the efficacy of several mental disorders, such as major depression (Cipriani et al., 2009), bipolar disorder (Cipriani et al., 2011), and schizophrenia (Leucht et al., 2013). The aim of this network meta-analysis of randomized controlled trials (RCTs) is to provide comprehensive evidence on the efficacy, tolerability, acceptability, and quality of life of all atypical antipsychotic in the augmentation treatment of patients with TRD.
Methods
This study was conducted according to the guidelines from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (Moher et al., 2009). The protocol has been registered on PROSPERO (CRD42014009666) and published in Systematic Reviews (Liu et al., 2014).
Data Sources and Searches
Seven electronic databases (PubMed, Embase, the Cochrane Library, Web of Science, CINAHL, LiLACS, and PsycINFO) were searched for publications from 1970 up to November 2013 (updated to January 31, 2014) with targeted Medical Subject Headings and text words. Several clinical trial registry agencies, pharmaceutical company websites, and FDA reports were also reviewed. Supplementary Table 1 details the systematic search terms and strategy. There were no search restrictions placed based on language, publication year, or publication type. Additional studies were searched in the reference lists of all identified publications, including relevant meta-analyses and systematic reviews. All relevant authors and principal manufacturers were contacted to supplement incomplete reports of the original papers or to provide new data from unpublished studies.
Study Selection
Two independent reviewers (Drs Qin and Liu) selected studies for inclusion, with divergences in judgment resolved by consensus. They scanned citations at the title/abstract level and then retrieved short-listed studies in full text. Potentially relevant articles were reviewed in full to ensure that they satisfied all the following criteria: (1) RCTs, including cross-over trials and cluster-randomized trials; (2) adult patients (aged more than 18 years) diagnosed with a current episode of major depressive disorder according to standard diagnostic interviews; (3) patients who had an inadequate response to at least one course of conventional antidepressant treatment prior to enrollment in the study; (4) usage of an adjunctive atypical antipsychotic medication; (5) comparator against a different type or different dosage of the adjunctive atypical antipsychotic or against an adjunctive placebo; and (6) one or more outcome(s) of depressive symptoms in acute treatment. Standard dose was defined as equal to or more than the defined daily dose by the FDA-approved indications, and low dose was defined as less than half the defined dose by the FDA-approved indications (Psychopharmacology Institute, 2013)
To reduce inconsistency among trials in this review, RCTs were excluded if trials: (i) included patients with bipolar depression; (ii) co-administered a psychotherapeutic intervention; or (iii) were for relapse prevention or maintenance treatment.
Outcome Measures
We assessed efficacy with continuous measures and categorical measures, respectively. Because previous studies reported that categorical measures may inflate treatment differences relative to the mean change in the continuous scale (Kirsch and Moncrieff, 2007), we chose continuous measures of depressive symptom severity as the primary outcome for efficacy. The primary outcome for efficacy was mean change scores in depressive symptoms, as measured by the mean change score of depression rating scales from baseline to endpoint. The secondary outcome for efficacy was response and remission in depressive symptoms, as estimated by the proportion of patients who achieved a decrease of a certain percentage or moved below the threshold in depression rating scores (Frank et al., 1991).
When a study reported multiple depression rating scales, the Montgomery and Asberg Depression Rating Scale (Montgomery and Asberg, 1979) was used as the primary measure, as it is the more commonly-used measure of depressive symptoms, followed by the Hamilton Depression Rating Scale (Hamilton, 1960) or other rating scales. Acute phase was defined as 4 to 12 weeks. For trials with multiple durations of acute treatment, the eight-week outcomes were used. Results from intention-to-treat analyses were preferred over results from completer analyses.
Tolerability (side-effect discontinuation) was defined as the proportion of patients who dropped treatment during the study due to side effects. The acceptability (all-cause discontinuation) was defined as the proportion of patients who dropped treatment during the study for any reason, which was previously reported to encompass efficacy and tolerability (Cipriani et al., 2009, 2011).
We also examined continuous measures of quality of life and functional improvement (QoL/functioning; Healy, 2000; Bech, 2005), including the Quality of Life Enjoyment and Satisfaction Questionnaire (Q-LES-Q; Endicott et al., 1993), the Short Form 36 Health Survey (SF-36; Ware and Sherbourne, 1992), and the Sheehan Disability Scale (SDS; Sheehan et al., 1996). When data were reported on more than one measure, we first chose data from the Q-LES-Q, then the SDS, and finally the SF-36.
Data Extraction and Quality Assessment
Two independent reviewers (Drs Liu and Zhou) abstracted the data and assessed study quality with good inter-rater agreement (κ statistic = 0.87, 0.88, respectively). The reviewers independently extracted the key study parameters using a standardized data abstraction form and assessed the risk of bias according to the Cochrane Handbook (Higgins and Green, 2011). Disagreements between reviewers were resolved by consensus.
Data Synthesis and Analysis
Bayesian network meta-analyses were performed to compare the relative outcomes of different types and dosages of atypical antipsychotic agents. The pooled estimates of standardized mean differences (SMD) with 95% credible intervals (CrIs) were calculated for continuous outcomes, and odds ratios (OR) with 95% CrIs were calculated for categorical outcomes. The SMD is the difference of means between the two groups divided by the pooled standard deviation (SDs) of the measurements; a negative SMD value indicates greater symptomatic relief (Attari et al., 2006). In the presence of minimally informative priors, CrIs can be interpreted similarly to confidence intervals, and conventional levels of statistical significance at a two-sided p < 0.05 can be assumed if 95% CrIs do not include 0 (Salanti et al., 2008). If means and SDs were not provided, we calculated them from p values or other statistical indices as described elsewhere (Berard et al., 2006).
The pooled estimates were obtained using the Markov Chains Monte Carlo method. Two Markov chains were run simultaneously with different arbitrarily-chosen initial values. To ensure convergence, trace plots and the Brooks-Gelman-Rubin statistics were assessed (Brooks and Gelman, 1998). Convergence was found to be adequate after running 50 000 samples for both chains. These samples were then discarded as “burn-in” and posterior summaries were based on 100 000 subsequent simulations. The node splitting method was used to calculate the inconsistency of the model, which separated evidence on a particular comparison into direct and indirect evidence (Lu and Ades, 2006). Probability values were summarized and reported as the surface under the cumulative ranking curve (SUCRA), a simple transformation of the mean rank used to provide a hierarchy of the treatments and accounts for both the location and the variance of all relative treatment effects (Lu and Ades, 2004; salanti et al., 2011). Network meta-analyses were performed using the WinBUGS software package (version 1.4.3, MRC Biostatistics Unit) with random effects models for multi-arm trials. The other analyses were performed and presented by the Stata 11.0 and R 2.11.1 software packages.
The first sensitivity analysis was performed on a network excluding trials with low-dose drugs, and the second sensitivity analysis was performed by omitting trials with small sample sizes (each arm of less than ten patients). Moreover, network meta-regression analyses were used to investigate whether potential heterogeneity could be explained by differences in publication year and the placebo response rate. Publication bias was examined using the funnel plot method.
Results
Study Characteristics
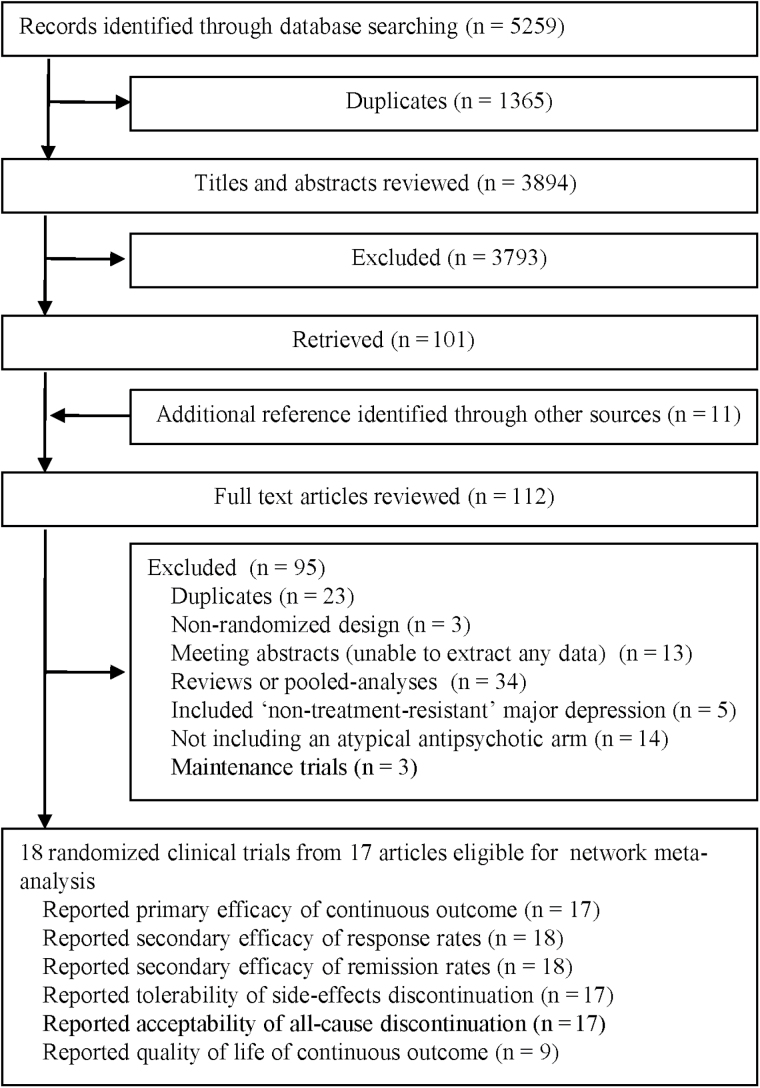
After screening 5 259 citations (Figure 1), 17 articles were included in this review (Shelton et al., 2001, 2005; Corya et al., 2006; Khullar et al., 2006; Mattingly et al., 2006; Berman et al., 2007, 2009; Mahmoud et al., 2007; McIntyre et al., 2007; Thase et al., 2007; Marcus et al., 2008; Reeves et al., 2008; Bauer et al., 2009; Keitner et al., 2009; El-Khalili et al., 2010; Fava et al., 2012; Kamijima et al., 2013) comprised of 18 RCTs with a total of 4 422 patients treated with seven different types (and dosages) of atypical antipsychotic agents: standard-dose aripiprazole (n = 746 patients), low-dose aripiprazole (n = 253 patients), standard-dose olanzapine/fluoxetine (OFC, n = 599 patients), low-dose OFC (n = 59 patients), quetiapine (mean 250–350mg daily, n = 345 patients), quetiapine (mean 150–250mg daily, n = 344 patients), and standard-dose risperidone (n = 217 patients).
Figure 1.
Flowchart of study selection.
Table 1 summarizes the characteristics and outcome measures of all included trials. The studies were published between 2001 and 2013. Sample sizes ranged from 15 to 586 patients, with a median sample size of 240.9 per trial. The mean age of participants was 44.1 years (range: 18–65 years). All studies involved both female and male patients, and the overall female-to-male ratio was approximate 1.8:1. The mean duration of acute treatment was 7.0 weeks (range: 4–12 weeks). Three RCTs had a low-dose treatment arm for comparison with a standard-dose treatment arm or placebo. Most trials (15/18) were augmentation treatment with selective serotonin reuptake inhibitors or selective noradrenalin reuptake inhibitors, and most (11/18) recruited patients that previously failed at least two conventional antidepressant treatment trials.
Table 1.
Baseline Characteristics of Included Studies
| Trial | Treatment comparators | Antidepressants | Patients | Mean Age (years) | Female (%) | Duration (weeks) | Prior Failed Trials | Rating Scale | Primary efficacy (mean) | Side-effects discontinuation | All-cause discontinuation | Quality of Life (mean) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bauer et al., 2009 | Quetiapine (mean 250–400mg daily) vs Quetiapine (mean 150– 250mg daily) vs Placebo | SSRI/SNRI | 487 | 45.4 | 67.6 | 6 | 1 historical | MADRS | -14.94;-15.26;-12.21 | 19/163;11/167;6/161 | 30/163;21/167;16/161 | 12.81;14.7;12.58 |
| Berman et al., 2007 | Standard dose Aripiprazole vs Placebo | SSRI/SNRI | 353 | 45.4 | 62.8 | 6 | 1–3 historical, 1 prospective | MADRS | -8.8;-5.8 | 6/182;4/176 | 22/182;16/176 | -1.09;-0.63 |
| Berman et al., 2009 | Standard dose Aripiprazole vs Placebo | SSRI/SNRI | 343 | 45.3 | 73.1 | 6 | 1–3 historical, 1 prospective | MADRS | -10.1;-6.4 | 11/177;3/172 | 30/177;23/172 | 9.8;5.2 |
| Corya et al., 2006 | Standard dose OFC vs Low dose OFC vs placebo | Fluoxetine | 341 | 45.7 | 72.5 | 12 | 1 or more historical, 1 prospective | MADRS | -14.06;-11.97;-11.7 | 29/243;2/59; 3/60 | 60/243;13/59; 12/60 | - |
| El-Khalili et al., 2010 | Quetiapine (mean 250–400mg daily) vs Quetiapine (mean 150– 250mg daily) vs Placebo | SSRI/SNRI | 432 | 45.5 | 72.5 | 6 | 1 historical | MADRS | -14.7;-13.6;-11.7 | 27/149;16/148;1/148 | 45/149;34/148; 23/148 | 11.82; 10.37; 11.32 |
| Fava et al., 2012 | Low dose aripiprazole vs Placebo | SSRI/SNRI | 221 | 45.1 | 64.4 | 4 | 1–3 historical, 1 prospective | MADRS | -8.54;-8.1 | 0/56;0/169 | 2/56;2/169 | - |
| Kamijima et al., 2013 | Standard dose Aripiprazole vs Low dose aripiprazole vs Placebo | SSRI/SNRI | 586 | 38.7 | 42.0 | 6 | 1–3 historical, 1 prospective | MADRS | -9.6;-10.5; -7.4 | 7/194;8/197; 2/195 | 17/194;17/197; 12/195 | -1.03; -0.96;-0.46 |
| Keitner et al., 2009 | Standard dose Risperidone vs Placebo | Various | 95 | 45.2 | 56.7 | 4 | 1 prospective | MARDS | - | 6/62;1/33 | 8/62;7/33 | 1.2;0.5 |
| Khullar et al., 2006 | Quetiapine (mean 250–400mg daily) vs Placebo | SSRI/SNRI | 15 | - | - | 8 | At least 1 historical | MADRS | -14.88;-5.29 | 0/8;0/7 | 1/8;1/7 | - |
| Mahmoud et al., 2007 | Standard dose Risperidone vs Placebo | Various | 268 | 46.1 | 73.5 | 6 | 1 prospective | HAMD-17 | -10.9;-8.7 | 8/141;3/133 | 26/141;16/133 | 15.2;11 |
| Marcus et al., 2008 | Standard dose Aripiprazole vs Placebo | SSRI/SNRI | 369 | 44.5 | 66.7 | 6 | 1–3 historical, 1 prospective | MADRS | -8.5;-5.7 | 7/191;2/190 | 29/191;28/190 | -1.3;-0.7 |
| Mattingly et al., 2006 | Quetiapine (mean 250–400mg daily) vs Placebo | SSRI/SNRI | 37 | - | - | 8 | 1 historical, 1 prospective | MADRS | -17;-8.7 | 0/24;0/13 | 3/24;2/13 | - |
| McIntyre et al., 2007 | Quetiapine (mean 150–250mg daily) vs Placebo | SSRI/SNRI | 58 | 44.5 | 62.1 | 8 | 1 or more trials | HAMD-17 | -11.37;-5.61 | 8/29;2/29 | 11/29;13/29 | - |
| Reeves et al., 2008 | Standard dose Risperidone vs Placebo | Various | 23 | 43.5 | 69.6 | 8 | 1 prospective | MADRS | -22.09;-14.44 | 0/12;0/11 | 1/12;4/11 | - |
| Shelton et al., 2001 | Standard dose OFC vs placebo | Fluoxetine | 20 | 42.0 | - | 8 | 2 historical, 1 prospective | MARDS | -13.6;–1.2 | 0/10;0/10 | 1/10;3/10 | - |
| Shelton et al., 2005 | Standard dose OFC vs placebo | Fluoxetine | 288 | 42.1 | 69.8 | 8 | 1 or more historical, 1 prospective | MARDS | -8.71;-8.51 | 10/146;4/142 | 30/146;28/142 | - |
| Thase 2007 | Standard dose OFC vs placebo | Fluoxetine | 203 | 44.1 | 61.2 | 8 | 1 or more historical, 1 prospective | MADRS | -10.8;-9.4 | 27/200;5/206* | 52/200;40/206* | -1.6;-1.1* |
| Thase 2007 | Standard dose OFC vs placebo | Fluoxetine | 198 | 44.9 | 68.0 | 8 | 1 or more historical, 1 prospective | MADRS | -14.6;-9 | 27/200;5/206* | 52/200;40/206* | -1.6;-1.1* |
*A pooled result of Thase 2007 HAM-D, Hamilton Depression Rating Scale; MADRS, Montgomery-Åsberg Depression Rating Scale; OFC, olanzapine/fluoxetine; SNRI, selective noradrenalin reuptake inhibitor; SSRI, selective serotonin reuptake inhibitors
The risk of bias of included RCTs was assessed according to Cochrane metrics (Supplementary Figure 1). The overall quality of studies was rated as good, even though many reports did not provide details about randomization and allocation concealment, while none of the RCTs met the criteria for high risk of bias on the basis of question-based entries. Funnel plot asymmetry was seen, showing a potential publication bias (Supplementary Figure 2).
Results of Network Meta-Analysis
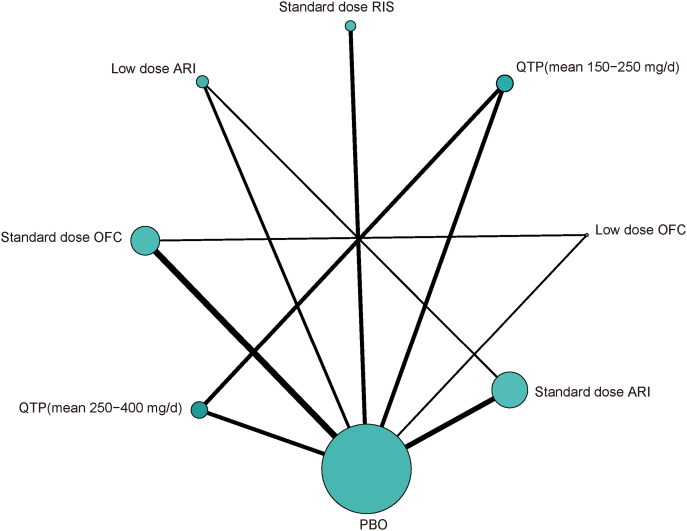
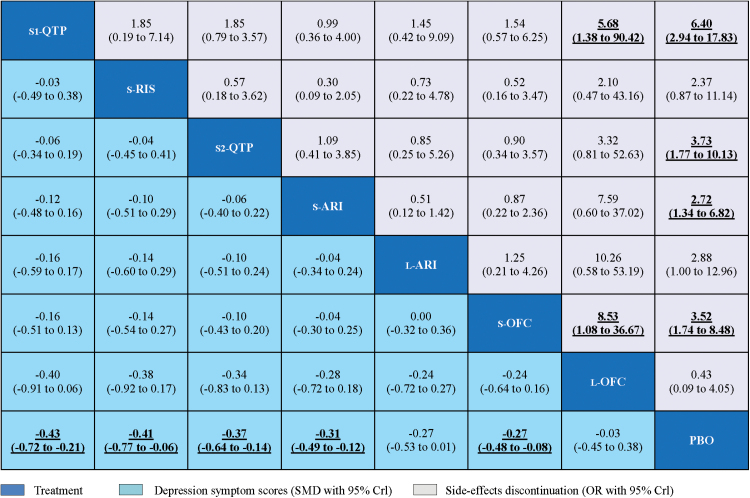
There were eight nodes and ten comparisons of primary outcomes in the network plot of different types (and dosages) of atypical antipsychotic agents (Figure 2). The pooled effect estimates for primary efficacy and tolerability outcomes from the network meta-analysis were provided (Figure 3). Compared with placebo, all standard-dose agents were significantly more effective (SMD ranged from -0.27 to -0.43), while low-dose OFC and low-dose aripiprazole were not. No significant differences in efficacy outcomes were found between these atypical antipsychotics. In terms of tolerability, all standard-dose agents, except for risperidone, had significantly more side-effect discontinuations than placebo (OR ranged from 2.72 to 6.40). Quetiapine (mean dose 250–350mg daily) and standard-dose OFC had significantly more side-effect discontinuations than low-dose OFC (OR = 5.68, 95% credible interval [CrI] 1.38 to 90.42; OR = 8.53, 95% CrI 1.08 to 36.67, respectively).
Figure 2.
Network plot of eligible comparisons for primary outcome. The width of the lines is proportional to the number of trials comparing every pair of treatments, and the size of every node is proportional to the number of randomized participants (sample size). ARI, aripiprazole; OFC, olanzapine/fluoxetine; PBO, placebo; QTP, quetiapine; RIS, risperidone.
Figure 3.
Network meta-analysis of primary efficacy and tolerability outcomes. Drugs are reported in order of efficacy ranking. Comparisons between treatments should be read from left-to-right, and the estimate is in the cell in common between the column-defining treatment and the row-defining treatment. To obtain standardized mean differences (SMDs) for comparisons in the opposite direction, negative values should be converted into positive values, and vice versa. For the primary efficacy, SMDs less than 0 favor the column-defining treatment. For the tolerability, odds ratios (ORs) higher than 1 favor the column-defining treatment. To obtain ORs for comparisons in the opposite direction, reciprocals should be taken. Significant results are in bold and underlined. CrI, credible intervals; L-ARI, low dose aripiprazole; L-OFC, low dose olanzapine/fluoxetine; PBO, placebo; S1-QTP, quetiapine (mean 250–400mg daily); S2-QTP, quetiapine (mean 150–250mg daily); S-ARI, standard dose aripiprazole; S-OFC, standard dose olanzapine/ fluoxetine; S-RIS, standard dose risperidone.
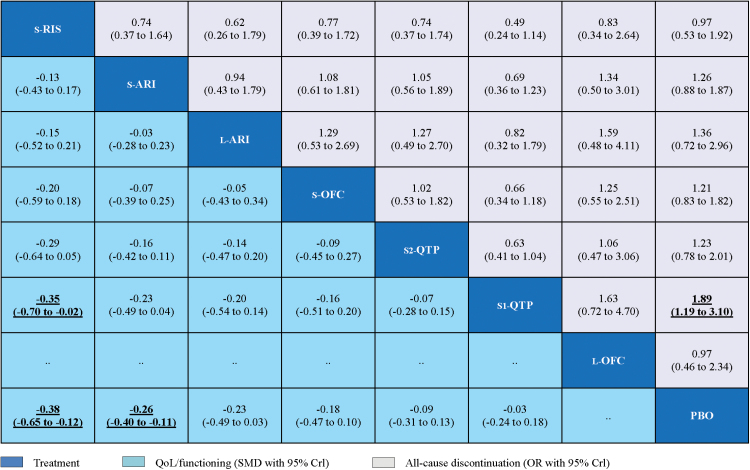
The pooled effect estimates for QoL/functioning and acceptability outcomes from the network meta-analysis were provided (Figure 4). In terms of the QoL/functioning outcome, only standard-dose risperidone and standard-dose aripiprazole were significantly more beneficial than placebo (SMD = -0.38, 95% CrI -0.65 to -0.12, and SMD = -0.26, 95% CrI -0.40 to -0.11, respectively). Moreover, standard-dose risperidone was significantly more beneficial than quetiapine (mean dosage: 250–350mg daily; SMD = 0.35, 95% CrI 0.02 to 0.70). In terms of acceptability outcome, only quetiapine (mean dose 250–350mg daily) had a significantly higher rate of all-cause discontinuation than placebo (OR = 1.89, 95% CrI 1.19 to 3.10).
Figure 4.
Network meta-analysis of quality of life (QoL/functioning) and acceptability outcomes. Drugs are reported in order of efficacy ranking. Comparisons between treatments should be read from left-to-right, and the estimate is in the cell in common between the column-defining treatment and the row-defining treatment. For the QoL/functioning, standardized mean differences (SMDs) lower than 0 favor the column-defining treatment. To obtain SMDs for comparisons in the opposite direction, negative values should be converted into positive values, and vice versa. For the acceptability, odds ratios (ORs) higher than 1 favor the column-defining treatment. To obtain ORs for comparisons in the opposite direction, reciprocals should be taken. Significant results are in bold and underlined. CrI, credible interval; L-ARI, low dose aripiprazole; L-OFC, low dose olanzapine/fluoxetine; PBO, placebo; S1-QTP, quetiapine (mean 250–400mg daily); S2-QTP, quetiapine (mean 150–250mg daily); S-ARI, standard dose aripiprazole; S-OFC, standard dose olanzapine/ fluoxetine; S-RIS, standard dose risperidone.
In regard to the secondary efficacy outcomes for response rates and remission rates (Supplementary Figure 3), the results were consistent with those of the primary outcome for efficacy, except that low-dose aripiprazole was somewhat more effective than placebo.
All loops (i.e. networks of three comparisons that arise when collating studies involving different selections of competing treatments) were consistent, since all their 95% CIs included 0 (i.e. the direct estimate of the summary effect was not significantly differentiated from the indirect estimate; Supplementary Figure 4). Supplementary Table 2 presents the results of the overall SUCRA-based probabilities for all atypical antipsychotic drugs in terms of efficacy, tolerability, acceptability, and QoL/functioning; however, the few significant findings in the network meta-analysis restrict the interpretation of hierarchical evidence based on SUCRA.
Sensitivity Analysis and Meta-Regression
The first sensitivity analysis of the network meta-analysis model was conducted by refitting the model by omitting studies with low-dose atypical antipsychotic agents. No material change in either the groups of pooled estimated effects or rank ordering was found (Supplementary Figure 5). The second sensitivity analysis, performed by omitting studies with small sample sizes, showed that the model was robust, as the statistical significances and rank ordering of efficacy and tolerability outcomes showed little change (Supplementary Figure 6). In the meta-regression analysis to assess potential biases in publication year, there was no statistical significance for this variable (Supplementary Figure 7).
Discussion
This network meta-analysis provides useful and comprehensive evidence regarding the efficacy, acceptability, tolerability, and quality of life of various atypical antipsychotics used for the adjunctive treatment of treatment-resistant depression. All included standard-dose atypical antipsychotic agents were found to be significantly more efficacious than adjunctive placebo. Low-dose atypical antipsychotics were not found to be efficacious. Two standard-dose medications—risperidone and aripiprazole—showed statistically significant benefits in functioning and quality of life. In terms of tolerability, all standard-dose atypical antipsychotics, with the exception of risperidone, had significantly more side-effect discontinuations than placebo. Only quetiapine (mean dosage: 250–350mg daily) had significantly more all-cause discontinuations than placebo. In summary, the reviewed atypical antipsychotic agents demonstrated efficacy in reducing depressive symptoms, but were accompanied by substantial risks of adverse events, including akathisia (aripiprazole), sedation (quetiapine, OFC, aripiprazole), and weight gain (all drugs, especially OFC; Ucok and Gaebel, 2008).
These findings have clinically-relevant implications for comprehensively balancing the risk-benefit profiles of these adjunctive atypical antipsychotics for TRD (Kennedy, et al., 2009). In our network analysis, among these atypical antipsychotics, standard-dose risperidone appeared to be the most beneficial in balancing efficacy, tolerability, and quality of life. However, these findings were restricted to a relatively limited number of trials in which risperidone was compared with other atypical antipsychotics. Moreover, patients in the adjunctive risperidone trials had mild to moderate refractory depression, failing to respond to only one course of conventional antidepressant therapy, while patients in trials with adjunctive other atypical antipsychotics had depressions with inadequate response to at least two courses of antidepressant treatments. Thus, the significant benefits for risperidone require further validation with patients experiencing more refractory depression. Nonetheless, risperidone did show antidepressant efficacy in patients with adult TRD, and should be considered as a recommendation in clinical guidelines although it has not yet received approval for that indication from the FDA.
Low-dose antipsychotic agents, including aripiprazole and OFC, appeared not to be effective in reducing depressive symptoms. There are two possible limitations in the interpretation of these results. First, there were only three trials reported using low-dose atypical antipsychotics, so a significant treatment effect may be obscured by the limited data (Barnes et al., 2006). Second, low-dose aripiprazole did show significant efficacy in comparison to placebo in both response and remission outcomes. Thus, the low-dose atypical antipsychotics may still be promising augmentation agents for TRD and deserve further study. Lower doses of atypical antipsychotic medication show a lower incidence of severe side-effects, thus an increase in tolerability and compliance (Taylor, 2000; Hicks et al., 2010; Bauer et al., 2013).
As outlined in previous studies, acceptability may encompass efficacy and tolerability outcomes (Cipriani et al., 2009, 2011). In our analysis, under the reviewed atypical antipsychotics only quetiapine (mean dosage: 250–350mg daily) had significantly more all-cause discontinuations than placebo. Thus, high doses of atypical antipsychotics may worsen the risk-benefit balance for refractory patients.
This study has several limitations. The first major limitation is that the number of included trials is relatively small, especially with low-dose drugs, which restricts the interpretation of the findings. Second, there are different definitions of treatment resistance, which may have resulted in comparisons of different patient populations. However, the methods used to define treatment resistance in studies reviewed here were similar to those of previous meta-analyses. Third, changes in QoL measures may lag behind reported or observed changes in depressive symptom measures; thus, the interpretation of QoL/functioning is limited by the absence of longer-term data (Bech, 2005). Finally, although there were no significant differences in efficacy among these atypical antipsychotics as a group, individual patients may respond preferentially to one agent or another.
There are additional issues that need to be discussed. First, we do not address the clinically important issue of adjunctive atypical antipsychotic therapy for preventing relapse in the medium and long term (i.e. ≥6 months). One systematic review of long-term, two-armed, parallel randomized controlled antidepressant trials initially identified 2 693 abstracts, but ultimately included only six trials (Deshauer et al., 2008). Even if continuing adjunctive treatment is shown to reduce relapse rates, the increased side-effect burden and cost of continuing two agents raises additional and clinically-important considerations (Papakostas et al., 2004), so that it is not at all clear how long patients should continue to take atypical antipsychotic agents, even if they help in the short term. Second, in this network meta-analysis, placebo responses in these included studies ranged from about 10% to about 40%, which may cause significant problems in interpreting the data, particularly the comparative SMDs. However, a previous meta-analysis reported that the superiority of the drug versus placebo would be a worse performance when examining studies with placebo response rates ≥40% in adjunctive trials (Iovieno and Papakostas, 2012). And the magnitude of the placebo response seems to be related to the study population (e.g. biological validity of illness, baseline severity of illness, chronicity of the index episode of depression, age of participants, medical and psychiatric comorbidity) and study design (probability of receiving placebo, trials durations, study outcome measures) rather than the intervention itself (Brunoni et al., 2009; Papakostas et al., 2015). Third, we found no usable data for analyses of other currently-prescribed atypical antipsychotics, such as ziprasidone, that met our inclusion criteria.
In conclusion, this meta-analysis found evidence that all the reviewed standard-dose atypical antipsychotics were significantly effective in reducing depressive symptoms in patients with treatment-resistant depression. Although no significant differences were found in efficacy between any two atypical antipsychotics, standard-dose risperidone and standard-dose aripiprazole showed more benefits in improving quality of life of patients than a placebo. Clinicians prescribing atypical antipsychotics should be cautious, given the evidence of potential treatment-related side effects.
Supplementary Material
For supplementary material accompanying this paper, visit http://www.ijnp.oxfordjournals.org/
Statement of Interest
The authors have declared that no competing interests exist.
Supplementary Material
Acknowldgments
This study was supported by the National Basic Research Program of China (973 Program; Grant No. 2009CB918300). The funder had no role in the study design; the collection, analysis, and interpretation of data; the writing of the report; or the decision to submit the article for publication.
References
- Alexander GC, Gallagher SA, Mascola A, Moloney RM, Stafford RS. (2011) Increasing off-label use of antipsychotic medications in the United States, 1995–2008. Pharmacoepidemiol Drug Saf 20:177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attari A, Moghaddam Y, Hasanzadeh A, Soltani M, Mahmoodi M. (2006) Comparison of efficacy of fluoxetine with nortriptyline in treatment of major depression in children and adolescents: a double-blind study. J Res Med Sci 11:24–30. [Google Scholar]
- Barnes SA, Lindborg SR, Seaman JW. (2006) Multiple imputation techniques in small sample clinical trials. Stat Med 25:233–45. [DOI] [PubMed] [Google Scholar]
- Bauer M, Pretorius HW, Constant EL, Earley WR, Szamosi J, Brecher M. (2009) Extended-release quetiapine as adjunct to an antidepressant in patients with major depressive disorder: results of a randomized, placebo-controlled, double-blind study. J Clin Psychiatry 70:540–549. [DOI] [PubMed] [Google Scholar]
- Bauer M, Pfennig A, Severus E, Whybrow PC, Angst J, Moller HJ, World Federation of Societies of Biological Psychiatry Task Force on Unipolar Depressive D (2013) World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders, part 1: update 2013 on the acute and continuation treatment of unipolar depressive disorders. World J Biol Psychiatry 14:334–385. [DOI] [PubMed] [Google Scholar]
- Bech P. (2005) Social functioning: should it become an endpoint in trials of antidepressants? CNS Drugs 19:313–324. [DOI] [PubMed] [Google Scholar]
- Berard R, Fong R, Carpenter DJ, Thomason C, Wilkinson C. (2006) An international, multicenter, placebo-controlled trial of paroxetine in adolescents with major depressive disorder. J Child Adolesc Psychopharmacol 16:59–75. [DOI] [PubMed] [Google Scholar]
- Berlim MT, Turecki G. (2007) Definition, assessment, and staging of treatment-resistant refractory major depression: a review of current concepts and methods. Can J Psychiatry 52:46–54. [DOI] [PubMed] [Google Scholar]
- Berman RM, Marcus RN, Swanink R, McQuade RD, Carson WH, Corey-Lisle PK, Khan A. (2007) The efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: a multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychiatry 68:843–853. [DOI] [PubMed] [Google Scholar]
- Berman RM, Fava M, Thase ME, Trivedi MH, Swanink R, McQuade RD, Carson WH, Adson D, Taylor L, Hazel J, Marcus RN. (2009) Aripiprazole augmentation in major depressive disorder: a double-blind, placebo-controlled study in patients with inadequate response to antidepressants. CNS Spectr 14:197–206. [DOI] [PubMed] [Google Scholar]
- Blier P, Szabo ST. (2005) Potential mechanisms of action of atypical antipsychotic medications in treatment-resistant depression and anxiety. J Clin Psychiatry 66(Supp 8):30–40. [PubMed] [Google Scholar]
- Brooks SP, Gelman A. (1998) General Methods for Monitoring Convergence of Iterative Simulations. J Comput Graph Stat 7:434–455. [Google Scholar]
- Brunoni AR, Lopes M, Kaptchuk TJ, Fregni F. (2009) Placebo response of non-pharmacological and pharmacological trials in major depression: a systematic review and meta-analysis. PLOS One 4:e4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipriani A, Furukawa TA, Salanti G, Geddes JR, Higgins JP, Churchill R, Watanabe N, Nakagawa A, Omori IM, McGuire H, Tansella M, Barbui C. (2009) Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet 373:746–758. [DOI] [PubMed] [Google Scholar]
- Cipriani A, Barbui C, Salanti G, Rendell J, Brown R, Stockton S, Purgato M, Spineli LM, Goodwin GM, Geddes JR. (2011) Comparative efficacy and acceptability of antimanic drugs in acute mania: a multiple-treatments meta-analysis. Lancet 378:1306–1315. [DOI] [PubMed] [Google Scholar]
- Corya SA, Williamson D, Sanger TM, Briggs SD, Case M, Tollefson G. (2006) A randomized, double-blind comparison of olanzapine/fluoxetine combination, olanzapine, fluoxetine, and venlafaxine in treatment-resistant depression. Depress Anxiety 23:364–372. [DOI] [PubMed] [Google Scholar]
- Crown WH, Finkelstein S, Berndt ER, Ling D, Poret AW, Rush AJ, Russell JM. (2002) The impact of treatment-resistant depression on health care utilization and costs. J Clin Psychiatry 63:963–971. [DOI] [PubMed] [Google Scholar]
- Deshauer D, Moher D, Fergusson D, Moher E, Sampson M, Grimshaw J. (2008) Selective serotonin reuptake inhibitors for unipolar depression: a systematic review of classic long-term randomized controlled trials. Can Med Assoc J 178:1293–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Khalili N, Joyce M, Atkinson S, Buynak RJ, Datto C, Lindgren P, Eriksson H. (2010) Extended-release quetiapine fumarate (quetiapine XR) as adjunctive therapy in major depressive disorder (MDD) in patients with an inadequate response to ongoing antidepressant treatment: a multicentre, randomized, double-blind, placebo-controlled study. Int J Neuropsychop 13:917–932. [DOI] [PubMed] [Google Scholar]
- Endicott J, Nee J, Harrison W, Blumenthal R. (1993) Quality of Life Enjoyment and Satisfaction Questionnaire: a new measure. Psychopharmacol Bull 29:321–326. [PubMed] [Google Scholar]
- Fava M, Mischoulon D, Iosifescu D, Witte J, Pencina M, Flynn M, Harper L, Levy M, Rickels K, Pollack M. (2012) A double-blind, placebo-controlled study of aripiprazole adjunctive to antidepressant therapy among depressed outpatients with inadequate response to prior antidepressant therapy (ADAPT-A Study). Psychother Psychosom 81:87–97. [DOI] [PubMed] [Google Scholar]
- Finley PR. (2009) Mood disorders: major depressive disorders. In: Applied therapeutics: the clinical use of drugs, 9th ed, (Koda-Kimble MA, Young LY, Alldredge BK, Corelli RL, Guglielmo BJ, Kradjan WA, Williams BR,ds.), pp 1–32. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins. [Google Scholar]
- Frank E, Prien RF, Jarrett RB, Keller MB, Kupfer DJ, Lavori PW, Rush AJ, Weissman MM. (1991) Conceptualization and rationale for consensus definitions of terms in major depressive disorder. Remission, recovery, relapse, and recurrence. Arch Gen Psychiatry 48:851–855. [DOI] [PubMed] [Google Scholar]
- Guo WB, Liu F, Xue ZM, Gao K, Wu RR, Ma CQ, Liu ZN, Xiao CQ, Chen HF, Zhao JP. (2012a) Altered white matter integrity in young adults with first-episode, treatment-naive, and treatment-responsive depression. Neurosci Lett 522:139–144. [DOI] [PubMed] [Google Scholar]
- Guo WB, Liu F, Chen JD, Gao K, Xue ZM, Xu XJ, Wu RR, Tan CL, Sun XL, Liu ZN, Chen HF, Zhao JP. (2012b) Abnormal neural activity of brain regions in treatment-resistant and treatment-sensitive major depressive disorder: a resting-state fMRI study. J Psychiatr Res 46:1366–1373. [DOI] [PubMed] [Google Scholar]
- Hamilton M. (1960) A rating scale for depression. J Neurol Neurosurg Psychiatry 23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy D. (2000) The assessment of outcomes in depression: measures of social functioning. Rev Contemp Pharmacother 11:295–301. [Google Scholar]
- Hicks P, Hicks XP, Meyer H, Shisslak C. (2010) How best to manage treatment-resistant depression? J Fam Pract 59:490–497. [PubMed] [Google Scholar]
- Higgins JPT, Green S. (2011) Cochrane handbook for systematic reviews of interventions version 5.1. 0 [updated March 2011]. Retrieved March 10, 2014. http://handbook.cochrane.org.
- Iovieno N, Papakostas GI. (2012) Correlation between different levels of placebo response rate and clinical trial outcome in major depressive disorder: a meta-analysis. J Clin Psychiatry 73:1300–6. [DOI] [PubMed] [Google Scholar]
- Kamijima K, Higuchi T, Ishigooka J, Ohmori T, Ozaki N, Kanba S, Kinoshita T, Koyama T, Group AS. (2013) Aripiprazole augmentation to antidepressant therapy in Japanese patients with major depressive disorder: a randomized, double-blind, placebo-controlled study (ADMIRE study). J Affect Disord 151:899–905. [DOI] [PubMed] [Google Scholar]
- Keitner GI, Garlow SJ, Ryan CE, Ninan PT, Solomon DA, Nemeroff CB, Keller MB. (2009) A randomized, placebo-controlled trial of risperidone augmentation for patients with difficult-to-treat unipolar, non-psychotic major depression. J Psychiatr Res 43:205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy SH, Lam RW, Parikh SV, Patten SB, Ravindran AV. (2009) Canadian Network for Mood and Anxiety Treatments (CANMAT) clinical guidelines for the management of major depressive disorder in adults. Introduction. J Affect Disord 117(Supp 1):S1-2. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS, National Comorbidity Survey R (2003) The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 289:3095–3105. [DOI] [PubMed] [Google Scholar]
- Khullar A, Chokka P, Fullerton D, McKenna S, Blackamn A. (2006) A double-blind, randomized, placebo-controlled study of quetiapine as augmentation therapy to SSRI/SNRI agents in the treatment of non-psychotic unipolar depression with residual symptoms. Paper presented at: New Research Abstracts of American Psychiatric Association 2006 Annual Meeting; 20 May 2006; Toronto, Canada. [Google Scholar]
- Kirsch I, Moncrieff J. (2007) Clinical trials and the response rate illusion. Contemp Clin Trials 28:348–351. [DOI] [PubMed] [Google Scholar]
- Komossa K, Depping AM, Gaudchau A, Kissling W, Leucht S. (2010) Second-generation antipsychotics for major depressive disorder and dysthymia. Cochrane Database Syst Rev 12:CD008121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leucht S, Cipriani A, Spineli L, Mavridis D, Orey D, Richter F, Samara M, Barbui C, Engel RR, Geddes JR, Kissling W, Stapf MP, Lassig B, Salanti G, Davis JM. (2013) Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet 382:951–962. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhou X, Qin B, Del Giovane C, Zhang Y, Xie P. (2014) Efficacy, quality of life, and acceptability outcomes of atypical antipsychotic augmentation treatment for treatment-resistant depression: protocol for a systematic review and network meta-analysis. Syst Rev 3:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu G, Ades AE. (2004) Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med 23:3105–3124. [DOI] [PubMed] [Google Scholar]
- Lu G, Ades A. (2006) Assessing evidence inconsistency in mixed treatment comparisons. J Am Stat Assoc 101:447–459 [Google Scholar]
- Mahmoud RA, Pandina GJ, Turkoz I, Kosik-Gonzalez C, Canuso CM, Kujawa MJ, Gharabawi-Garibaldi GM. (2007) Risperidone for treatment-refractory major depressive disorder: a randomized trial. Ann Intern Med 147:593–602. [DOI] [PubMed] [Google Scholar]
- Marcus RN, McQuade RD, Carson WH, Hennicken D, Fava M, Simon JS, Trivedi MH, Thase ME, Berman RM. (2008) The efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: a second multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychopharmacol 28:156–165. [DOI] [PubMed] [Google Scholar]
- Mattingly G, Ilivicky H, Canale J, Anderson R. (2006) Quetiapine combination for treatment-resistant depression. Paper presented at: New Research Abstracts of American Psychiatric Association 2006 Annual Meeting; May 20, 2006; Toronto, Canada. [Google Scholar]
- McIntyre A, Gendron A, McIntyre A. (2007) Quetiapine adjunct to selective serotonin reuptake inhibitors or venlafaxine in patients with major depression, comorbid anxiety, and residual depressive symptoms: a randomized, placebo-controlled pilot study. Depress Anxiety 24:487–494. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. The BMJ 339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. (1979) A new depression scale designed to be sensitive to change. Br J Psychiatry 134:382–389. [DOI] [PubMed] [Google Scholar]
- Nelson JC, Papakostas GI. (2009) Atypical antipsychotic augmentation in major depressive disorder: a meta-analysis of placebo-controlled randomized trials. Am J Psych 166:980–991. [DOI] [PubMed] [Google Scholar]
- Papakostas GI, Petersen T, Mahal Y, Mischoulon D, Nierenberg AA, Fava M. (2004) Quality of life assessments in major depressive disorder: a review of the literature. Gen Hosp Psychiatry 26:13–17. [DOI] [PubMed] [Google Scholar]
- Papakostas GI, Shelton RC, Smith J, Fava M. (2007) Augmentation of antidepressants with atypical antipsychotic medications for treatment-resistant major depressive disorder: a meta-analysis. J Clin Psychiatry 68:826–831. [DOI] [PubMed] [Google Scholar]
- Papakostas GI, Østergaard SD, Iovieno N. (2015) The nature of placebo response in clinical studies of major depressive disorder. J Clin Psychiatry 76:456–66. [DOI] [PubMed] [Google Scholar]
- Psychopharmacology Institute (2013) Second generation antipsychotics (atypicals) Retrieved March 27, 2015.http://psychopharmacologyinstitute.com/antipsychotics/.
- Reeves H, Batra S, May RS, Zhang R, Dahl DC, Li X. (2008) Efficacy of risperidone augmentation to antidepressants in the management of suicidality in major depressive disorder: a randomized, double-blind, placebo-controlled pilot study. J Clin Psychiatry 69:1228–1336. [DOI] [PubMed] [Google Scholar]
- Salanti G, Higgins JP, Ades AE, Ioannidis JP. (2008) Evaluation of networks of randomized trials. Stat Methods Med Res 17:279–301. [DOI] [PubMed] [Google Scholar]
- Salanti G, Ades AE, Ioannidis JP. (2011) Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 64:163–171. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Harnett-Sheehan K, Raj BA. (1996) The measurement of disability. Int Clin Psychopharmacol 11(Supp 3):89–95. [DOI] [PubMed] [Google Scholar]
- Shelton RC, Tollefson GD, Tohen M, Stahl S, Gannon KS, Jacobs TG, Buras WR, Bymaster FP, Zhang W, Spencer KA, Feldman PD, Meltzer HY. (2001) A novel augmentation strategy for treating resistant major depression. Am J Psych 158:131–134. [DOI] [PubMed] [Google Scholar]
- Shelton RC, Williamson DJ, Corya SA, Sanger TM, Van Campen LE, Case M, Briggs SD, Tollefson GD. (2005) Olanzapine/fluoxetine combination for treatment-resistant depression: a controlled study of SSRI and nortriptyline resistance. J Clin Psychiatry 66:1289–1297. [DOI] [PubMed] [Google Scholar]
- Shelton RC, Osuntokun O, Heinloth AN, Corya SA. (2010) Therapeutic options for treatment-resistant depression. CNS Drugs 24:131–161. [DOI] [PubMed] [Google Scholar]
- Spielmans GI, Berman MI, Linardatos E, Rosenlicht NZ, Perry A, Tsai AC. (2013) Adjunctive atypical antipsychotic treatment for major depressive disorder: a meta-analysis of depression, quality of life, and safety outcomes. PLOS Med 10:e1001403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. (2000) Low dose typical antipsychotics-a brief evaluation. Psychiatric Bulletin 24:465–468. [Google Scholar]
- Thase ME, Corya SA, Osuntokun O, Case M, Henley DB, Sanger TM, Watson SB, Dube S. (2007) A randomized, double-blind comparison of olanzapine/fluoxetine combination, olanzapine, and fluoxetine in treatment-resistant major depressive disorder. J Clin Psychiatry 68:224–236. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, Norquist G, Howland RH, Lebowitz B, McGrath PJ, Shores-Wilson K, Biggs MM, Balasubramani GK, Fava M, Team SDS. (2006) Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psych 163:28–40. [DOI] [PubMed] [Google Scholar]
- Ucok A, Gaebel W. (2008) Side effects of atypical antipsychotics: a brief overview. World Psychiatry 7:58–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieta E, Colom F. (2011) Therapeutic options in treatment-resistant depression. Ann Med 43:512–530. [DOI] [PubMed] [Google Scholar]
- Ware JE, Jr., Sherbourne CD. (1992) The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 30:473–483. [PubMed] [Google Scholar]
- Zhou X, Ravindran A, Qin B, Del Giovane C, Li Q, Bauer M, Liu Y, Fang Y, da Silva T, Zhang Y, Fang L, Wang X, Xie P. (2014) Comparative efficacy, acceptability and tolerability of augmentation agents in treatment-resistant depression: systematic review and network meta-analysis. J Clin Psychiatry 76:e487–498. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.