Abstract
Background:
Targeting dorsal raphe 5-HT1A receptors, which are coupled to G-protein inwardly rectifying potassium (GIRK) channels, has revealed their contribution not only to behavioral and functional aspects of depression but also to the clinical response to its treatment. Although GIRK channels containing GIRK2 subunits play an important role controlling excitability of several brain areas, their impact on the dorsal raphe activity is still unknown. Thus, the goal of the present study was to investigate the involvement of GIRK2 subunit-containing GIRK channels in depression-related behaviors and physiology of serotonergic neurotransmission.
Methods:
Behavioral, functional, including in vivo extracellular recordings of dorsal raphe neurons, and neurogenesis studies were carried out in wild-type and GIRK2 mutant mice.
Results:
Deletion of the GIRK2 subunit promoted a depression-resistant phenotype and determined the behavioral response to the antidepressant citalopram without altering hippocampal neurogenesis. In dorsal raphe neurons of GIRK2 knockout mice, and also using GIRK channel blocker tertiapin-Q, the basal firing rate was higher than that obtained in wild-type animals, although no differences were observed in other firing parameters. 5-HT1A receptors were desensitized in GIRK2 knockout mice, as demonstrated by a lower sensitivity of dorsal raphe neurons to the inhibitory effect of the 5-HT1A receptor agonist, 8-OH-DPAT, and the antidepressant citalopram.
Conclusions:
Our results indicate that GIRK channels formed by GIRK2 subunits determine depression-related behaviors as well as basal and 5-HT1A receptor-mediated dorsal raphe neuronal activity, becoming alternative therapeutic targets for psychiatric diseases underlying dysfunctional serotonin transmission.
Keywords: Dorsal raphe, GIRK, 5-HT1A, electrophysiology, citalopram
Introduction
Dysfunctional serotonin (5-HT) transmission plays a key role in the etiology and treatment of depression. Increasing 5-HT by selectively blocking its reuptake is the main pharmacological strategy to treat it (Krishnan and Nestler, 2008). The major source of 5-HT in the brain is the dorsal raphe (DR) nucleus (Dahlstroem and Fuxe, 1964), and it is highly regulated by the 5-HT1A autoreceptors, which play a critical role in the development, modulation, and treatment of depression. A functional polymorphism in the promoter region of the human Htr1a gene, which regulates 5-HT1A receptor levels, is linked to predisposition to mental illness as well as anxiety- and depression-related behaviors and response to antidepressants (Strobel et al., 2003; Lemonde et al., 2004; Lesch and Gutknecht, 2004; Le Francois et al., 2008). Diverse genetic manipulations of 5-HT1A receptor levels have confirmed the role of these receptors in anxiety- and depression-like phenotypes in mice (Heisler et al., 1998; Parks et al., 1998; Richardson-Jones et al., 2010; Ferres-Coy et al., 2013). It is widely accepted that the slow onset of the response to antidepressants is related to the progressive desensitization of the inhibitory effects mediated by activation of 5-HT1A receptors onto 5-HT neurotransmission (Blier and de Montigny, 1994; Artigas et al., 1996). Also, the behavioral response to antidepressants has been linked to increased neurogenesis, which is mediated by stimulation of 5-HT1A receptors (Santarelli et al., 2003). The G protein-coupled inwardly rectifying potassium (GIRK) channels are the main inhibitory effectors of 5-HT1A receptors (Williams et al., 1988), and therefore they could be alternative candidates for the study of depression and antidepressant responses involving the 5-HT1A receptor-mediated signaling.
Neuronal GIRK channels are tetramers mainly formed by GIRK1-3 subunits, since the expression of GIRK4 subunits is limited in the brain (Karschin et al., 1996). Specifically, the GIRK2 subunit plays a relevant role in GIRK channel function, given that the predominant form of GIRK channels is a heterotetramer containing GIRK1 and GIRK2 subunits (Liao et al., 1996), and it is responsible for the generation of G-protein coupled receptor-mediated GIRK currents in several brain areas, including the locus coeruleus (LC) and the hippocampus (HPP) (Luscher et al., 1997; Slesinger et al., 1997; Torrecilla et al., 2002; Labouebe et al., 2007; Cruz et al., 2008). Moreover, mutation of GIRK2 subunits causes a GIRK1 protein downregulation (Signorini et al., 1997; Torrecilla et al., 2002), and the constitutive activity of GIRK2 subunit-containing GIRK channels reduces neuronal excitability in vitro (Luscher et al., 1997; Torrecilla et al., 2002). Recently, it has been demonstrated that the maintenance of the tonic noradrenergic activity, which is another important neurotransmission system widely implicated in mood disorders, is under the control of GIRK2 subunit-containing GIRK channels (Torrecilla et al., 2013). Mice lacking GIRK2 subunits exhibit a reduced anxiety-like phenotype (Blednov et al., 2001; Pravetoni and Wickman, 2008), while mice lacking 5-HT1A receptors display increased anxiety-related behavior (Heisler et al., 1998; Parks et al., 1998; Ramboz et al., 1998). Given the involvement of these receptors in the etiology and treatment of depression and their functional relationship with GIRK channels, the aim of our study was to investigate the role of GIRK2 subunit-containing GIRK channels in depression-related behaviors and adult neurogenesis as well as basal and 5-HT1A receptor-mediated electrophysiological activity in DR neurons.
Methods
Animals
We used C57BL6/J wild-type (WT), GIRK2 heterozygous (GIRK2+/-), and GIRK2 homozygous (GIRK2-/-) mice (3 months old) derived from heterozygote crossing (Signorini et al., 1997). In the electrophysiological, behavioral, and locomotor activity experiments, male and female mice were used; data were pooled given the lack of gender differences in any parameter obtained from these studies. Immunohistochemistry and hypothermic studies were performed in male mice. Animals were maintained at 22±2ºC in a 12-h-light/-dark cycle, with food and water provided ad libitum. All procedures were conducted in accordance with the European Community Council Directive The Protection of Animals Used for Experimental and Other Scientific Purposes (86/609/EEC) and the Spanish Law for the care and use of laboratory animals (RD 1201/2005). Experimental protocols were reviewed and approved by the Local Committee for Animal Experimentation at the University of the Basque Country.
Behavioral Tests
All tests were performed between 9:00 am and 1:00 pm. Mice were transferred to a noise-free and temperature-controlled testing room at least 1 hour before the experiments. No more than 1 test was performed in each mouse.
Novelty Suppressed Feeding Test
The novelty suppressed feeding test (NSFT) was performed as previously described (Santarelli et al., 2003). Animals were food deprived 24 hours prior to the test (water ad libitum). The testing apparatus consisted of a plastic box (45×45×20cm) with a wooden bedding-covered floor illuminated by a 70W lamp and a white paper platform with a food pellet in the center. The test was carried out for 10 minutes and the latency to eat was timed. Afterwards, animals were transferred to their home cages, and the amount of food consumed by each mouse in the subsequent 5 minutes was measured.
Tail Suspension Test
In the tail suspension test (TST), mice were suspended 60cm from the surface with adhesive tape placed approximately 2cm from the tip of the tail. Animals were monitored (6 minutes) by video camera for subsequent blind analysis. Mice were considered immobile when they stood completely motionless or hung passively. Intraperitoneal administration of citalopram (10mg/kg) was carried out 30 minutes prior to the experiments.
Locomotor Activity
Locomotor activity was assessed in an open-field (20×30×30cm) bar system. The floor consisted of a stainless-steel grid conformed by 28 bars of 3mm each, associated to a detector of changes in electric resistance. The activity was monitored for 30 minutes and data were collected for horizontal activity.
In Vivo Electrophysiological Procedures
Mice were anesthetized with chloral hydrate (400mg/kg, i.p.), and supplementary doses were administered as needed. Single-barreled glass micropipettes (1–2 µm tip diameter) were lowered into the DR (relative to bregma: AP -4.5mm, ML -1.0mm, DV -2.5 to -4.0mm) with a lateral angle of 20°C to avoid damaging the sagital sinus. DR neurons were identified using established criteria, which included slow (0.5–2.5 Hz) and regular firing rate, and a long duration (0.8–1.5ms) positive action potential. A burst was defined according to Gartside et al. (2000), and burst-firing neurons in mice were identified as previously described (Gobbi et al., 2007), including a first interspike interval of ≤20ms and a termination interval ≥160ms.
Firing pattern analyses were performed using Spike2 software (Cambridge Electronic Design). The following parameters were analyzed offline: firing rate, coefficient of variation, percentage of cells exhibiting burst firing, firing rate of burst firing neurons, number of burst per cell, percentage of spikes in burst, mean spikes per burst, and the response to drug administration. Basal firing rate was recorded for at least 3 minutes before drug administration. One cell per animal was recorded when any drug was administered. All the recorded neurons met the previously mentioned established criteria and were located within the DR.
Intracerebroventricular administrations were performed 10 minutes prior to the recordings using a microsyringe (5 μL, Hamilton, Bonaduz, Switzerland) connected to a 30-gauge needle that was inserted into the right lateral ventricle (relative to bregma: AP -0.5mm, ML -1.0mm, DV -2.0mm). A volume of 1 μL of tertiapin-Q (100 pmol, dissolved in artificial cerebrospinal fluid [ACSF]), or ACSF (used as a control group) was injected directly into the right lateral ventricle.
8-OH-DPAT–Induced Hypothermia
Body temperature was assessed rectally, inserting a lubricated probe approximately 2cm and monitored with a digital thermometer. Ten minutes after baseline measurements, animals received 8-OH-DPAT (0.5mg/kg i.p.) or 0.9% saline (i.p.), and body temperature was measured the subsequent 10, 20, 30, and 60 minutes.
Immunohistochemistry Procedures
Fixation and Tissue Processing
Mice were anaesthetized with sodium pentobarbital (200mg/kg, i.p.). Animals were perfused through the aortic arch with 3.75% acrolein (25mL, TAAB) in a solution of 2% paraformaldehyde and 0.1M phosphate buffer (PB), pH 7.4, followed by 2% paraformaldehyde (75mL). Coronal brain slices were cut into 40-µm thickness using a vibrating microtome. Sections at levels -1.34mm/-2.30mm posterior to bregma and 1.18mm/0.26mm anterior to bregma from HPP and subventricular zone (SVZ), respectively, were selected for immunohistochemistry according to the stereotaxic mouse brain atlas of Franklin and Paxinos (1997).
Antibodies
A polyclonal affinity-purified rabbit antibody raised against phosphorylated Histone H3 (HH3) and a monoclonal mouse antiserum generated against glial fibrillary acidic protein (GFAP) were used for the determination of proliferating cells and glia. We determined the number of proliferating cells by the presence of phosphorylated HH3 that were not colocalized with GFAP to exclude glial phenotype. Omission of primary and/or secondary antibodies resulted in a total absence of target labeling (Rodriguez et al., 2008, 2009).
Immunohistochemistry
This procedure was performed as previously described (Rodriguez et al., 2008, 2009). Sections were first incubated for 30 minutes in 30% methanol in 0.1M PB and 30% H2O2 and then rinsed with 0.1M PB for 5 minutes and placed in 1% sodium borohydride for 30 minutes. After incubating brain sections in 0.5% bovine serum albumin, in 0.1M TS and 0.25% Triton X-100 for 30 minutes, they were incubated for 68 hours at room temperature in 0.1% bovine serum albumin in 0.1M TS and 0.25% Triton X-100 containing rabbit polyclonal antiserum for HH3 (1:1000) and mouse monoclonal antiserum for GFAP (1:60000). For HH3 labeling, sections were placed in 0.1M TS and 0.25% Triton X-100 containing 1:400 dilutions of biotinylated donkey anti-rabbit IgG (Jackson Immunoresearch, Stratech Scientific Ltd., Soham, UK) for 1 hour at room temperature and avidin-bioctin peroxidase complex for 30 minutes at room temperature. The peroxidase reaction product was visualized in a solution prepared from SGZ kits for 2 to 3 minutes. For GFAP labeling, sections were incubated in 0.1M TS and 0.25% Triton X-100 containing 1:400 dilution of biotinylated horse anti-mouse IgG (Vector Laboratories, Peterborough, UK) for 1 hour at room temperature and avidin-biocytin peroxidase complex for 30 minutes at room temperature. The GFAP peroxidase reaction was observed by incubation in a solution containing 0.022% 3,3′diaminobenzidine and 0.003% H2O2 for 1 to 2 minutes. With this procedure, the GFAP labeling was seen in brown, allowing us to differentiate it from the HH3-labeled cells (blue). Sections were mounted onto gelatinized slides and allowed to dry overnight and then dehydrated in increasing concentrations of ethanol (50%, 70%, 80%, 90%, 95%, and 100%) and xylene. Coverslips were applied using Entellan and were left overnight before counting.
Cell Quantification
The number of HH3-immunoreactive neurons and HH3/GFAP colocalized cells was determined by counting the labeled cells in both hemispheres in sections of dentate gyrus (DG) of the HPP and of the SVZ at levels previously mentioned. Cells were counted using light microscopy. The number of HH3-positive cells and the area measurements of the DG and SVZ bounded by the lateral ventricles, corpus callosum, and caudate-putamen nucleus were determined blindly to ensure consistency and reproducibility.
Drugs
Chloral hydrate, 8-OH-DPAT hydrobromide, and WAY100635 maleate were obtained from Sigma-Aldrich. Citalopram hydrobromide and tertiapin-Q were from Tocris Bioscience. Tertiapin-Q was prepared in ACSF. Chloral hydrate, 8-OH-DPAT, WAY100635, and citalopram were prepared in 0.9% saline.
Statistical Analysis
Data obtained from the TST, locomotor activity, and immunohistochemistry studies were compared through genotypes by 1-way ANOVA followed by Newman-Keuls posthoc test. For the 8-OH-DPAT hypothermic response analysis, 2-way ANOVA followed by Bonferroni posthoc test was used. In the NSFT, data were analyzed using Kaplan-Meier survival analysis and Mantel-Cox log-rank test.
Changes in firing rate induced by 8-OH-DPAT and citalopram are expressed as percentages of the baseline firing rate (mean firing rate during 3 minutes prior to drug administration). Data compiled from dose-response curves were analyzed for the best simple nonlinear fit to the 3-parameter logistic equation (Parker and Waud, 1971) using GraphPad Prism Software (v5.01; GraphPad Software Inc). The following equation was used:
where [A] is the concentration of the drug, E is the effect on the firing rate induced by A, Emax is the maximal effect, ED50 is the effective dose for eliciting 50% of the Emax, and n is the slope factor of the dose-response curve. Extra sum-of-squares F test (GraphPad Prism Software) was used for statistical comparison of the response to a drug in dose-response curves and for comparison of ED50 among groups.
Spontaneous firing rate and coefficient of variation were analyzed in selected pair comparisons using unpaired 2-tailed t test. Two-sided χ2 analysis of contingency tables was used to evaluate differences in the percentage of neurons presenting burst firing. Other parameters derived from burst pattern were analyzed by the nonparametric Kruskal-Wallis test followed by Dunn’s posthoc test. The level of significance was considered P<.05.
Results
Behavioral Characterization of GIRK2 Mutant Mice and the Response to Citalopram
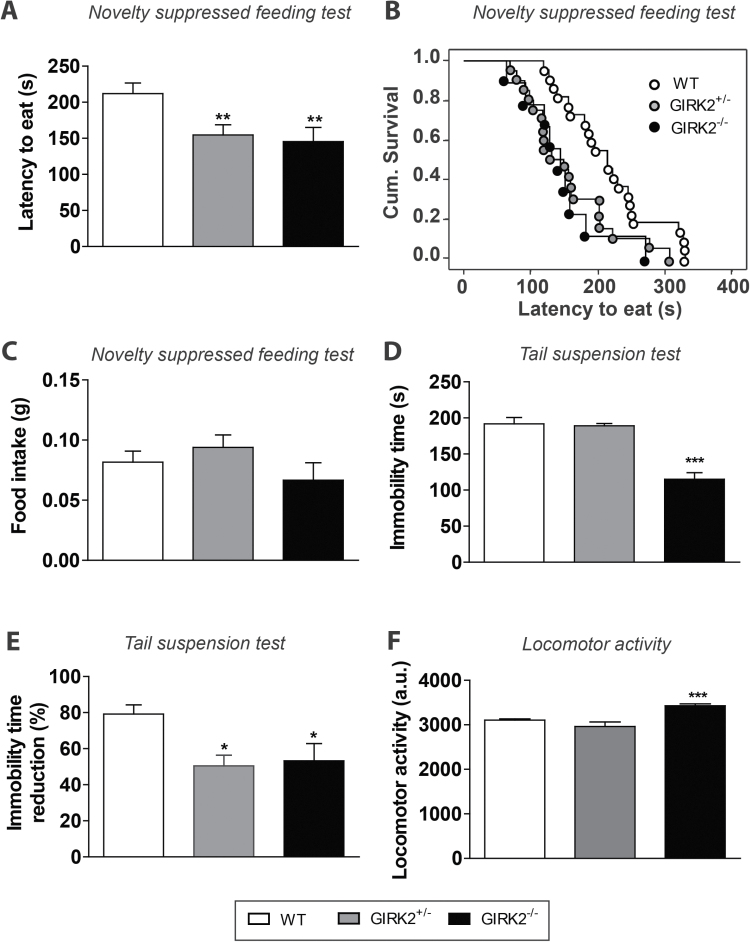
To evaluate whether GIRK2 subunit ablation impacts depressive-related behaviors, GIRK2 mutant mice were evaluated using 2 well-established behavioral models of antidepressant activity, the NSFT and TST (Santarelli et al., 2003; Cryan et al., 2005; Heurteaux et al., 2006). In the NSFT, both GIRK2-/- and GIRK2+/- mice showed a decreased latency to eat compared with WT mice (GIRK2-/-: 145.60±19.63 seconds, n=9; GIRK2+/-: 154.60±14.30 s, n=20; WT: 212.00±14.85 seconds, n=22, P<.01, Kaplan-Meier survival analysis, Mantel-Cox log-rank test) (Figure 1a-b). This decrease in the latency to eat in the mutant groups was not due to an increased appetite (Figure 1c).
Figure 1.
Behavioral characterization of G-protein inwardly rectifying potassium (GIRK)2 mutant mice. (A-B) In the novelty suppressed feeding test (NSFT), both GIRK2+/- and GIRK2-/- mice displayed a lower latency to eat compared with wild-type (WT) mice. Results are expressed as mean latency to eat (seconds) (A) or cumulative survival (B) (n=9–22 mice/group; **P<.01, Kaplan-Meier survival analysis, Mantel-Cox log-rank test). (C) No changes in the amount of food consumed after the NSFT were observed among groups (n=9–22 mice/group). (D) In the tail suspension test (TST), GIRK2-/- mice displayed a lower immobility time compared with GIRK2+/- and WT mice (n=14–16 mice/group; ***P<.001 vs WT, 1-way ANOVA followed by Newman-Keuls test). (E) In the TST, 30 minutes after the citalopram injection (10mg/kg, i.p.), both GIRK2+/- and GIRK2-/- mice showed a lower reduction of the immobility time compared with WT mice (n=7–10 mice/group; *P<.05 vs WT mice, 1-way ANOVA followed by Newman-Keuls test). (F) GIRK2-/- mice showed an increased locomotor activity compared with GIRK2+/- and WT mice (n=12–15 mice/group; ***P<.0001, 1-way ANOVA followed by Newman-Keuls test). Bars represent mean±SEM of n animals.
When evaluating the behavioral characteristics in the TST, GIRK2-/- mice showed a lower immobility time than GIRK2+/- and WT mice (GIRK2-/-: 115.10±9.08 seconds, n=16; GIRK2+/-: 189.10±3.24 seconds, n=16; WT: 191.80±8.87 seconds, n=14, P<.0001, 1-way ANOVA followed by Newman-Keuls test) (Figure 1d). Next, we examined the effect of citalopram on the immobility time in the TST. In all groups, the administration of citalopram (10mg/kg, i.p.) caused a reduction of the immobility time (Figure 1e). However, in GIRK2-/- and GIRK2+/- mice, this reduction was significantly lower than that observed in WT mice (GIRK2-/-: 53.2±9.5%, n=7; GIRK2+/-: 50.5±5.9%, n=7; WT: 79.2±5.1%, n=10, P<.05, 1-way ANOVA followed by Newman-Keuls test). We next tested the locomotor activity of GIRK2 mutant mice. As expected (Blednov et al., 2001; Pravetoni and Wickman., 2008; Arora et al., 2010), GIRK2-/- mice showed an elevated motor activity compared with GIRK2+/- and WT mice (GIRK2-/-: 3429±43.65, n=14; GIRK2+/-: 2962±104.90, n=12; WT: 3105±25.39, n=15, P<.0001, 1-way ANOVA followed by Newman-Keuls test) (Figure 1f).
Electrophysiological Properties of DR Neurons of GIRK2 Mutant Mice
To examine the contribution of GIRK2 subunit-containing GIRK channels to the basal activity of DR neurons, firing rate, coefficient of variation, and burst activity were studied in WT and GIRK2 mutant mice in vivo.
All neurons recorded from WT (n=86), GIRK2+/- (n=79), and GIRK2-/- (n=45) mice fit the standard criteria (see Methods).
Spontaneous firing rate of DR neurons in GIRK2-/- mice was higher than in WT mice (1.99±0.15 Hz, n=45; 1.68±0.08 Hz, n=86, for GIRK2-/- and WT mice, respectively). P<.05, unpaired 2-tailed t test). No changes were seen among groups in either the coefficient of variation or the burst activity. A more extended study of the burst pattern (mean firing rate, number of bursts, percentage of spikes firing in bursts, and mean spikes in burst) showed no differences among groups (Table 1).
Table 1.
In Vivo Electrophysiological Properties of Dorsal Raphe (DR) Neurons Recorded under Basal Conditions in Wild-Type (WT) and G-Protein Inwardly Rectifying Potassium (GIRK2) Mutant Mice
| WT (n=86) | GIRK2 +/- (n=79) | GIRK2 -/- (n=45) | WT TPN-Q (n=15) | |
|---|---|---|---|---|
| Firing rate (Hz) | 1.68±0.08 | 1.88±0.09 | 1.99±0.15* | 2.33±0.27** |
| Coefficient of variation (%) | 37.64±1.21 | 36.57±1.21 | 35.21±1.54 | 40.38±3.25 |
| Burst firing neurons (%) | 7 | 5 | 2 | 13 |
| - Firing rate (Hz) | 2.22±0.36 | 2.01±0.37 | 4.03 | 1.49±0.38 |
| - Number of bursts | 1.33±0.21 | 4.33±1.85 | 1.00 | 2.5±1.50 |
| - Spikes in burst (%) | 1.15±0.46 | 1.01±0.87 | 0.41 | 2.8±1.32 |
| - Mean spikes per burst | 3.00±0.81 | 2.00 | 2.00 | 3.50±0.50 |
Abbreviations: GIRK2+/-, GIRK2 heterozygous mice; GIRK2-/-, GIRK2 homozygous mice; WT, wild type; WT TPN-Q, tertiapin-Q-injected wild-type mice.
*P<.05 and **P<.01 vs WT. Unpaired 2-tailed t-test.
Each cell was recorded for at least 3 minutes (180 seconds were taken for subsequent analysis). All data are presented as the mean±SEM of n experiments.
Next, the effect of the high-affinity GIRK channel blocker, tertiapin-Q (100 pmol, i.c.v.) (Jin and Lu, 1998; Kanjhan et al., 2005), on the electrophysiological properties of DR neurons was assessed. The firing rate of DR neurons in tertiapin-Q–injected WT mice (WT TPN-Q) was statistically higher than in the WT group (WT TPN-Q: 2.32±0.27 Hz, n=15; WT: 1.68±0.08 Hz, n=86, P<.001; unpaired 2-tailed t test). However, no significant changes were seen in either the coefficient of variation or the burst parameters compared with WT mice. No differences were observed in firing rate (1.80±0.27 Hz, n=7), coefficient of variation (35.13±4.42%, n=7), or number of burst firing neurons (14%) in the intracerebroventricular ACSF-injected WT group compared with WT mice (data not shown).
Effect of Girk2 Gene Deletion on the 5-HT1A Receptor-Mediated Inhibition of DR Neuronal Activity
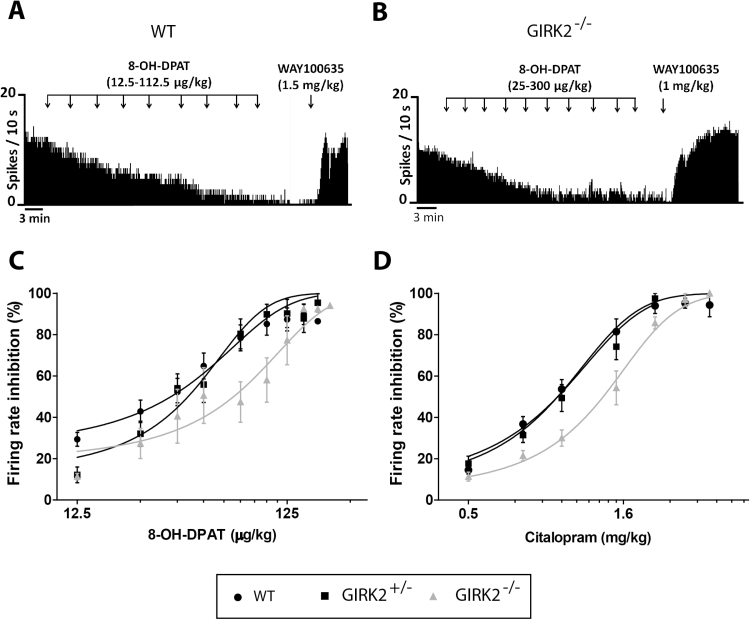
To investigate the role of GIRK2 subunits in the 5-HT1A receptor-mediated transmission, we compared the inhibitory effect of cumulative increasing doses of the 5-HT1A receptor agonist, 8-OH-DPAT (12.5–300 µg/kg, i.p.), on the firing rate of DR neurons of WT and GIRK2 mutant mice. In all groups, 8-OH-DPAT caused a progressive and dose-dependent inhibition of the firing rate (Figure 2a-b). In GIRK2+/-mice, the dose-response curve shifted to the right, so that the ED50 mean value was significantly higher than that obtained in WT mice (ED50: 42.15±2.89 µg/kg, n=8; ED50: 34.93±3.41 µg/kg, n=15, for GIRK2+/- and WT mice, respectively. P<.05, nonlinear fit analysis, extra sum-of-squares F test). In GIRK2-/- mice, the shift in the dose-response curve was even greater, and so the ED50 mean value increased 2-fold compared with WT mice (ED50: 69.50±8.57 µg/kg, n=5, P<.01, nonlinear fit analysis, extra sum-of-squares F test) (Figure 2c). Subsequent administration of the 5-HT1A receptor antagonist, WAY100635 (1–1.5mg/kg, i.p.), completely recovered the firing activity in the 3 groups (WT: 86.22±7.04%, n=13; GIRK2+/-: 92.54±12.98%, n=6; GIRK2-/-: 122.20±23.27%, n=3). No significant differences were found among groups.
Figure 2.
Inhibitory effect of the 5-HT1A receptor agonist 8-OH-DPAT and citalopram on the firing rate of dorsal raphe (DR) neurons in wild-type (WT) and G-protein inwardly rectifying potassium (GIRK2) mutant mice. (A-B) Representative firing rate histograms illustrate the inhibitory effect of 8-OH-DPAT (12.5–300 µg/kg, i.p.) on DR basal activity in WT (A) and GIRK2-/- mice (B). Subsequent adminitration of the 5-HT1A antagonist, WAY100635 (1–1.5mg/kg, i.p.), completely reversed the 8-OH-DPAT-induced inhibitory effect. (C-D) Dose-response curves for 8-OHDPAT (C) and citalopram (0.5–3mg/kg, i.p.) (D) on DR firing rate in WT, GIRK2+/-, and GIRK2-/- mice. Each point represents the mean±SEM of n experiments (n=5–15 mice/group).
Next, the sensitivity of the 5-HT1A receptor to endogenous 5-HT was tested by using the selective serotonin reuptake inhibitor citalopram (0.5–3mg/kg, i.p.), which increases 5-HT levels in the synaptic cleft and indirectly activates these receptors (Pineyro and Blier, 1999). For this purpose, citalopram dose-response curves were performed in WT and GIRK2 mutant mice. In all the groups, cumulative doses of citalopram caused a progressive and dose-dependent inhibition of the firing rate. However, citalopram showed less potency inhibiting DR neurons in GIRK2-/- mice, so that the ED50 mean value for this group was significantly greater than the values obtained for GIRK2+/- and WT mice (ED50: 1.36±0.04mg/kg, n=6; ED50: 1.04±0.04mg/kg, n=7; ED50: 0.97±0.03mg/kg, n=13, for GIRK2-/-, GIRK2+/-, and WT mice, respectively. P<.0001, nonlinear fit analysis, extra sum-of-squares F test) (Figure 2d).
Effect of Pharmacological Blockade of GIRK Channels on the Citalopram-Induced Inhibition of DR Neuronal Activity
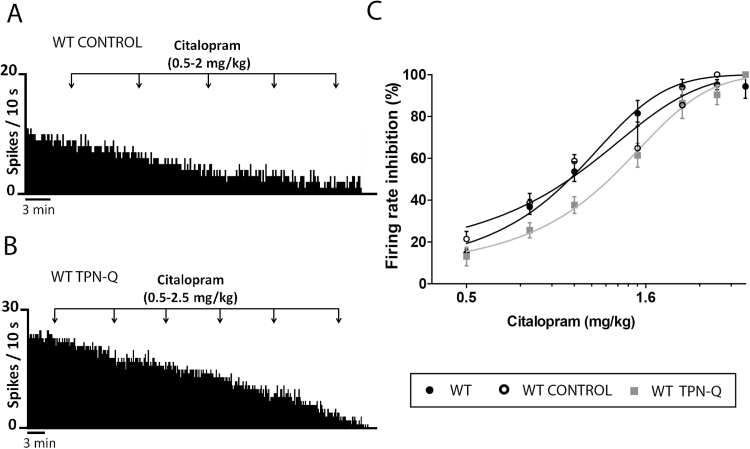
To further investigate the 5-HT1A-GIRK signaling pathway, we performed a pharmacological blocking of GIRK channels with tertiapin-Q, and the sensitivity of the 5-HT1A receptor to the effect of citalopram was evaluated in WT TPN-Q mice and a WT control group (ACSF-injected group, WT control). In both groups, citalopram (0.5–3mg/kg, i.p.) caused a progressive and dose-dependent inhibition of the firing rate (Figure 3a-b). However, it showed less potency inhibiting DR neurons in WT TPN-Q, so that the ED50 mean value for this group was significantly greater than the value obtained for the WT control (ED50: 1.25±0.04mg/kg, n=5; ED50: 0.99±0.06mg/kg, n=4, for WT TPN-Q and the WT control, respectively. P<.0001, nonlinear fit analysis, extra sum-of-squares F test) (Figure 3c). ED50 mean values were similar between GIRK2-/- mice and WT TPN-Q, indicating that citalopram had the same potency in both groups (ED50: 1.25±0.04mg/kg, n=5; ED50: 1.36±0.04mg/kg, n=6, for WT TPN-Q and GIRK2-/- mice, respectively). As expected, the WT control group showed similar ED50 values to the WT mice.
Figure 3.
Inhibitory effect of citalopram on the firing rate of dorsal raphe (DR) neurons in wild-type (WT) and tertiapin-Q injected mice. (A-B) Representative firing rate histograms illustrate the inhibitory effect of citalopram (0.5–2.5mg/kg, i.p.) on DR basal activity in artificial cerebrospinal fluid (ACSF)-injected mice (WT control, i.c.v.) (A) and tertiapin-Q injected mice (WT TPN-Q, 100 pmol, i.c.v.) (B). (C) Dose-response curves for citalopram (0.5–3mg/kg, i.p.) on DR firing rate in WT, WT control, and WT TPN-Q. Each point represents the mean±SEM of n experiments (n=4–5 mice/group).
Characterization of 8-OH-DPAT–Induced Hypothermia in GIRK2 Mutant Mice
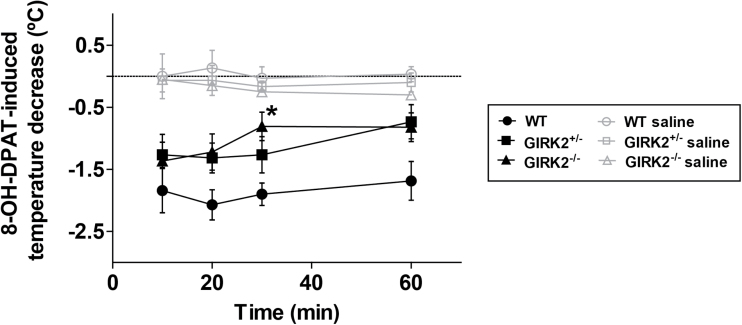
To further study the functional status of 5-HT1A receptors in GIRK2 mutant mice, we evaluated the 8-OH-DPAT–induced hypothermic response. In all groups, 8-OH-DPAT (0.5mg/kg, i.p.) caused a decrease in temperature (n=6–7/group). GIRK2-/- mice showed a reduced 8-OH-DPAT–induced hypothermia in every different time point compared with WT mice. A 2-way ANOVA revealed that in minute 30, the temperature decrease induced by 8-OH-DPAT was significantly smaller in GIRK2-/- mice compared with WT mice ([F(2,68)=10.30, P<.05]) (Figure 4). In addition, 3 other groups of GIRK2+/-, GIRK2-/-, and WT mice were injected with 0.9 % saline (saline groups, i.p.) to check if the manipulation of the animals or the intraperitoneal injection was causing an effect on the response. No significant changes in temperature were observed in these groups.
Figure 4.
Hypothermic response induced by 8-OH-DPAT in wild-type (WT) and G-protein inwardly rectifying potassium (GIRK)2 mutant mice. The temperature decrease induced by 8-OH-DPAT (0.5mg/kg, i.p.) was significantly lower in GIRK2-/- mice relative to WT mice in minute 30. Mice of each genotype injected with 0.9% saline (saline groups) were used as controls. Each point represents the mean±SEM of n animals (n=6–7 mice/group; *P<.05 vs WT, 2-way ANOVA followed by Bonferroni test).
Basal Neurogenesis in GIRK2 Mutant Mice
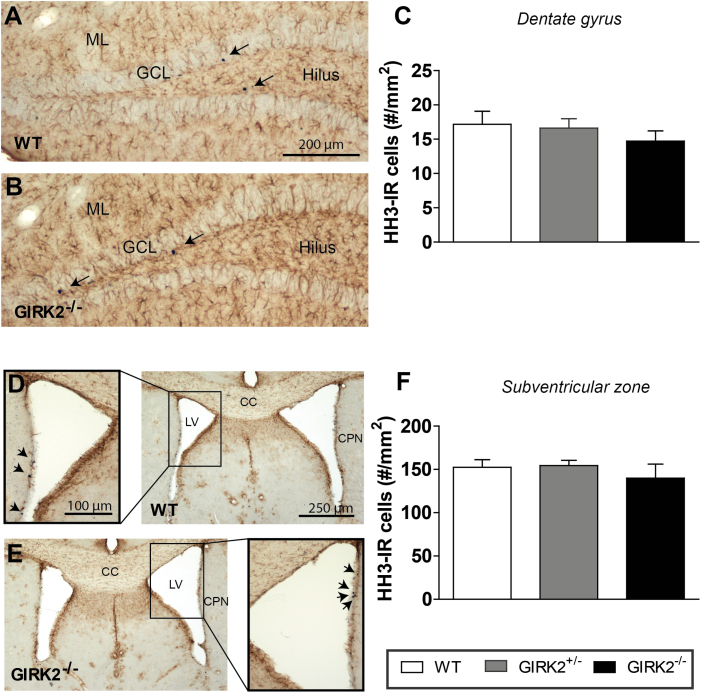
Finally, we investigated whether GIRK2 subunits are involved in basal adult neurogenesis measuring the area density (#cells/mm2, Sv) of new proliferating cells in the DG of the HPP and in the SVZ of WT and GIRK2 mutant mice (n=6–10/group). In both regions of all groups, newly generated cells were observed, as indicated by HH3 immunoreactivity (HH3-IR) (Figure5a-b, d-e).Quantitative analysis of the Sv of HH3-IR cells showed no differences among genotypes in either the DG (WT: 17.18±1.89; GIRK2+/-: 16.69±1.36; GIRK2-/-: 14.71±1.51) (Figure 5c), or in the SVZ (WT: 152.43±8.81; GIRK2+/-: 154.34±6.10; GIRK2-/-: 139.99±16.00) (Figure 5f). Although GFAP immunoreactivity was detected throughout both areas, <2% of HH3-IR cells expressed GFAP in the DG (WT: 1.54%; GIRK2+/-: 1.92%; GIRK2-/-: 1.61%) and the SVZ (WT: 0.48%; GIRK2+/-: 0.36%; GIRK2-/-: 0.50%), showing no statistical differences among genotypes. The low number of HH3-IR cells that colocalize with GFAP found in this study is consistent with other reports and suggests that the proliferating cells are of neural lineage (Rodriguez et al., 2008, 2009, 2011; Fiol-deRoque et al., 2013).
Figure 5.
Brightfield micrographs showing phosphorilated Histone H3 (HH3, a proliferating mitotic marker) labelled cells within the dentate gyrus (DG) and subventricular zone (SVZ) of wild-type (WT) and G-protein inwardly rectifying potassium (GIRK)2 mutant mice. (A-B) Dual labeling of HH3 positive cells (arrows) and glial cells (glial fibrillary acidic protein [GFAP], brown) in the hippocampal DG of WT (A) and GIRK2-/- mice (B). (C) Bar graph showing no differences among groups in the area density of HH3 positive cells within the DG (all layers included). (D-E) Dual labeling of HH3 positive cells (arrows) and glial cells (GFAP, brown) in the SVZ of WT (D) and GIRK2-/- mice (E). (F) Bar graph showing no differences among groups in the area density of HH3 positive cells within the SVZ. Bars represent mean±SEM of n animals (n=6–10 mice/group). CC, corpus callosum; CPN, caudate-putamen nucleus; LV, lateral ventricle; GCL, granular cell layer; ML, molecular layer.
Discussion
The goal of our study was to determine the role that GIRK2 subunit-containing GIRK channels play in depression-related behaviors as well as in the control of 5-HT1A-mediated inhibitory effects. We found that mice lacking GIRK2 subunits of GIRK channels display a depression-resistant phenotype combined with a reduced behavioral response to citalopram, an increase in the firing rate of DR neurons, and a reduction of 5-HT1A receptor-mediated responses. In addition, GIRK2 subunit deletion does not affect basal adult neurogenesis.
Our findings suggest that Girk2 gene deletion promotes a depression-resistant behavior and determines the response to the antidepressant citalopram. Thus, GIRK2-/- mice showed a marked decrease in the immobility time in the TST, and both GIRK2+/- and GIRK2-/- mice presented a lower latency to eat in the NSFT that was not correlated with an increase in appetite. It is important to remark that general locomotor activity status is a confusing factor in these tests (Blednov et al., 2001). Here we observed that GIRK2-/- mice, but not GIRK2+/- mice, have signs of hyperactivity, as reported by other studies (Blednov et al., 2001; Pravetoni and Wickman., 2008; Arora et al., 2010). This has been attributed to D1 receptor activation (Blednov et al., 2002). Given that GIRK2+/- mice do not have hyperactivity but showed depression-resistant phenotype in the NSFT, these results might be attributable to a less depressive-like behavior rather than to a more active state. We suggest that the 5-HT1A-GIRK signaling may be mediating this depression-resistant phenotype: first, studies in 5-HT1A receptor knockout mice show that they display an antidepressant-like phenotype under baseline conditions (Heisler et al., 1998; Mayorga et al., 2001), as we observed in GIRK2 mutant mice. Secondly, citalopram was less potent here in reducing the immobility in the TST. Similarly, it has been reported that the expression of the antidepressant-like behavioral response of selective serotonin reuptake inhibitors in the TST requires the presence of functional 5-HT1A receptors (Mayorga et al., 2001). Additionally, our observations are in line with those showing that chronic administration of fluoxetine exerts a beneficial influence on a rodent model of depression through suppression of GIRK-dependent signaling in the DR (Cornelisse et al., 2007). Interestingly, the depression-resistant phenotype that we observed here is combined with the previously reported reduced anxiety-like behavior of GIRK2 mutant mice (Blednov et al., 2001; Pravetoni and Wickman, 2008). Therefore, this phenotype represents a great advance over the classic depression-resistant phenotype of 5-HT1A knockout mice, since they also display a robust anxiety-like behavior (Heisler et al., 1998; Parks et al., 1998; Ramboz et al., 1998). Given the constitutive and global nature of the Girk2 gene deletion, it is not possible to completely identify the circuit(s) or neurotransmitter system(s) that explain this depression-resistant phenotype. It is conceivable that GABAB receptor-mediated transmission is also affected by the GIRK2 mutation, and this could account for some of the effects seen in this study. However, there are some key points in our work that make this assumption unlikely. First, DR neuronal activity is mainly regulated by 5-HT1A receptors (Sprouse and Aghajanian, 1987), and drugs used in the present work act directly or indirectly on 5-HT1A receptors. Despite the presence of GABAB receptors in DR neurons (Bischoff et al., 1999), there is no evidence of a 5-HT1A-GABAB receptor interaction in DR neurons. Therefore, the responses to 8-OH-DPAT and citalopram observed in this study do not involve the activation of the GABAB receptor-mediated transmission and have to be mediated by the 5-HT1A-GIRK2 signaling. Also, the combined anxiety- and depression-resistant phenotype of GIRK2 mutant mice is contrary to the phenotype of GABAB mutant mice, which show an anxiety-like behavior and behave similarly to control mice in the TST (Mombereau et al., 2004). Overall, although GABAB-GIRK signaling is probably impacted by the mutation, the direct mediation by GABAB receptors of the effects seen in this study is unlikely.
The implication of GIRK2 subunit-containing GIRK channels in the 5-HT1A receptor-mediated neurotransmission is also supported by our in vivo electrophysiological findings. First, we observed that the lack of GIRK2 subunits induces an increase in the firing rate of DR neurons. This increase was also observed by the total blocking of GIRK channels with tertiapin-Q. On one hand, these results suggest that the intrinsic activity of GIRK2 subunit-containing GIRK channels controls the transmission in the DR. Similar findings have been obtained in LC neurons, where it has been observed that GIRK2 subunit-containing GIRK channels regulate the tonic activity of LC neurons in vivo (Torrecilla et al., 2013). In vitro, LC neurons of GIRK2/3-/- mice show an increased firing rate (Cruz et al., 2008). In addition, the contribution of GIRK2 subunits to the resting membrane potential has been reported in different type of neurons, including LC and HPP neurons (Luscher et al., 1997; Torrecilla et al., 2002; Chen and Johnston, 2005; Koyrakh et al., 2005). Nevertheless, in layer 5/6 pyramidal neurons of the prelimbic cortex of GIRK2-/- mice, intrinsic electrophysiological properties remained unaltered compared with WT mice (Hearing et al., 2013). Taken together, the contribution of GIRK2 subunits to neuronal excitability seems to vary across cell types and brain regions. On the other hand, the complete blockade of GIRK channels with tertiapin-Q results in a similar increase in the firing rate of DR neurons to that observed in GIRK2-/- mice. This suggests that GIRK2 subunits, though their expression is low in the DR (Saenz del Burgo et al., 2008), are forming important populations of GIRK channels that control the neuronal tonic activity. In the DR, the most abundant neuronal populations, which are the GABAergic and 5-HT neurons, express GABAB and 5-HT1A receptors coupled to GIRK channels (Bowery, 1987; Innis and Aghajanian, 1987; Waldmeier et al., 1988; Beck et al., 2004; Day et al., 2004). In fact, it is widely accepted that in 5-HT neurons, GABAB and 5-HT1A receptors are coupled to a common pool of G proteins (Innis and Aghajanian, 1987; Williams et al., 1988). However, the specific distribution of GIRK2 subunit-containing GIRK channels in these cells remains unknown. Therefore, further studies are needed to determine the subunit composition of GIRK channels coupled to each particular receptor in specific cell types and their role in the physiology of these cells. Alterations in neurotransmitter levels could also affect the firing pattern of DR neurons in GIRK2 mutant mice, since there are decreased and increased levels of 5-HT and 5-HIAA, respectively (Torrecilla et al., 2013).
Second, our study also reveals that DR 5-HT1A receptors, both endogenously and exogenously activated, are affected by the deletion of GIRK2 subunits, which is shown by reduced receptor functionality. Similarly, µ and α2 receptors of LC neurons in GIRK2 mutant mice were desensitized in vivo (Torrecilla et al., 2013). Our results agree with this study, indicating that in the DR, ablation of the Girk2 gene produces loss of function of the 5-HT1A-GIRK signaling pathway. It is important to remark that the reduced function of 5-HT1A autoreceptors has been proposed as a key consequence of chronic antidepressant treatments, which would explain the delayed onset effect of antidepressants (Artigas et al., 1996; Blier et al., 1998). In fact, new pharmacological or genetic strategies to faster desensitize 5-HT1A and α2 autoreceptors have been of great interest in the study of depression treatment (Sanacora et al., 2004; Richardson-Jones et al., 2010; Portella et al., 2011). The observation that 8-OH-DPAT and citalopram preserved their maximal inhibitory efficacy is consistent with other studies. In LC neurons in GIRK2 mutant mice, the potency of morphine and clonidine was reduced, yet the inhibitory efficacy remained unaltered in vivo (Torrecilla et al., 2013). In hippocampal neurons in GIRK2-/- mice, in vitro postsynaptic GIRK currents induced by stimulation of the 5-HT1A receptor were markedly reduced but not absent (Luscher et al., 1997). Studies conducted to determine the analgesic properties of morphine showed that while the potency of morphine was reduced, its efficacy was preserved in GIRK2-/- and GIRK2/3-/- mice (Mitrovic et al., 2003; Cruz et al., 2008). Also, the pharmacological blockade of GIRK channels caused a reduction in the potency but not in the inhibitory efficacy of citalopram, supporting the primary role of GIRK2 subunit-containing GIRK channels in the inhibitory response of 5-HT1A receptors. The tertiapin-Q dose used was selected according to a previous study, where doses >100 pmol caused dangerous effects (Marker et al., 2004). By testing the 8-OH-DPAT–induced hypothermia, which reflects the sensitivity of 5-HT1A autoreceptors in mice (Goodwin et al., 1985; Richardson-Jones et al., 2011), we confirmed that GIRK2 subunits regulate the functionality of 5-HT1A autoreceptors, as previously reported (Costa et al., 2005).
Our findings show that in basal conditions, Girk2 gene ablation promotes a depression-resistant phenotype without modifying adult neurogenesis in the DG of the HPP or in the SVZ. In line with this, it has also been observed that deletion of the background potassium channel TREK-1 does not alter the adult neurogenesis but results in a depression-resistant phenotype (Heurteaux et al., 2006). Although the role of neurogenesis in the behavioral effects induced by antidepressant drugs remains controversial under certain conditions (see Hanson et al., 2011), compelling work suggests that the increment of hippocampal neurogenesis induced by chronic treatment with fluoxetine reverses some behavioral dysfunctions in animal models of anxiety/depression and control animals (Malberg et al., 2000; Santarelli et al., 2003; Airan et al., 2007; David et al., 2007, 2009; Surget et al., 2008; Wang et al., 2008). Furthermore, specific neurogenic and behavioral effects of fluoxetine require the activation of 5-HT1A receptors (Santarelli et al., 2003). Based on the wide literature on the primary role of GIRK2 subunits in generating GIRK currents by the activation G protein coupled receptors throughout the brain, including the CA1 layer of the HPP (reviewed in Luscher and Slesinger, 2010), and the fact that 5-HT1A receptors in the DR are desensitized, we hypothesize that genetic ablation of GIRK2 subunits could compromise the activity of 5-HT1A receptors also in the DG and therefore the neurogenic effects of fluoxetine. Nevertheless, it could be feasible that the deletion of the GIRK2 subunit strongly determines this behavioral phenotype, and therefore it would limit the importance of neurogenesis mediating the behavioral responses of chronic fluoxetine treatment. In line with this, it has been reported that the complex behavioral phenotype that 5-HT1A knockout mice show is developmentally determined and neurogenesis independent (Santarelli et al., 2003).
In conclusion, our results show the specific role of GIRK2 subunit-containing GIRK channels in the promotion of a depression-resistant phenotype as well as their control of the tonic neuronal activity and mediation of the 5-HT1A receptor inhibitory responses. New strategies targeting the 5-HT1A-GIRK2 pathway could be of great therapeutic interest for the study of pathologies related to an altered 5-HT transmission, such as depression and development of alternative treatments.
Statement of Interest
None.
Acknowledgments
This work was supported by grants from the Government of the Basque Country (S-PE11UN055, IT747-13), the University of the Basque Country (UFI 11/32), and the Spanish Government (FIS PI12/00613) cofinanced by FEDER to N.L., C.B.-C., L.U., and M.T. Plan Nacional de I+D+I 2008–2011 and ISCIII-Subdirección General de Evaluación y Fomento de la Investigación cofinanced by FEDER (PI10/02738) and the Government of the Basque Country grants (AE-2010-1-28, AEGV10/16 and GV- 2011111020) to J.J.R. N.L. has a predoctoral fellowship and C.B.-C. has a postdoctoral fellowship, both from the University of the Basque Country (UPV/EHU). All the authors are entirely responsible for the scientific content of this paper.
References
- Airan RD, Meltzer LA, Roy M, Gong Y, Chen H, Deisseroth K. (2007) High-speed imaging reveals neurophysiological links to behavior in an animal model of depression. Science 317:819–823. [DOI] [PubMed] [Google Scholar]
- Arora D, Haluk DM, Kourrich S, Pravetoni M, Fernandez-Alacid L, Nicolau JC, Lujan R, Wickman K. (2010) Altered neurotransmission in the mesolimbic reward system of girk mice. J Neurochem 114:1487–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artigas F, Bel N, Casanovas JM, Romero L. (1996) Adaptative changes of the serotonergic system after antidepressant treatments. Adv Exp Med Biol 398:51–59. [DOI] [PubMed] [Google Scholar]
- Beck SG, Pan YZ, Akanwa AC, Kirby LG. (2004) Median and dorsal raphe neurons are not electrophysiologically identical. J Neurophysiol 91:994–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff S, Leonhard S, Reymann N, Schuler V, Shigemoto R, Kaupmann K, Bettler B. (1999) Spatial distribution of GABA(B)R1 receptor mRNA and binding sites in the rat brain. J Comp Neurol 412, 1–16. [PubMed] [Google Scholar]
- Blednov YA, Stoffel M, Chang SR, Harris RA. (2001) GIRK2 deficient mice. evidence for hyperactivity and reduced anxiety. Physiol Behav 74:109–117. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Stoffel M, Cooper R, Wallace D, Mane N, Harris RA. (2002) Hyperactivity and dopamine D1 receptor activation in mice lacking girk2 channels. Psychopharmacology 159:370–378. [DOI] [PubMed] [Google Scholar]
- Blier P, de Montigny C. (1994) Current advances and trends in the treatment of depression. Trends Pharmacol Sci 15:220–226. [DOI] [PubMed] [Google Scholar]
- Blier P, Pineyro G, el Mansari M, Bergeron R, de Montigny C. (1998) Role of somatodendritic 5-HT autoreceptors in modulating 5-HT neurotransmission. Ann N Y Acad Sci 861:204–216. [DOI] [PubMed] [Google Scholar]
- Bowery NG, Hudson AL, Price GW. (1987) GABAA and GABAB receptor site distribution in the rat central nervous system. Neuroscience 20:365–383. [DOI] [PubMed] [Google Scholar]
- Chen X, Johnston D. (2005) Constitutively active G-protein-gated inwardly rectifying K+ channels in dendrites of hippocampal CA1 pyramidal neurons. J Neurosci 25:3787–3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelisse LN, Van der Harst JE, Lodder JC, Baarendse PJ, Timmerman AJ, Mansvelder HD, Spruijt BM, Brussaard AB. (2007) Reduced 5-HT1A- and GABAB receptor function in dorsal raphe neurons upon chronic fluoxetine treatment of socially stressed rats. J Neurophysiol 98:196–204. [DOI] [PubMed] [Google Scholar]
- Costa AC, Stasko MR, Stoffel M, Scott-McKean JJ. (2005) G-protein-gated potassium (GIRK) channels containing the GIRK2 subunit are control hubs for pharmacologically induced hypothermic responses. J Neurosci 25:7801–7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz HG, Berton F, Sollini M, Blanchet C, Pravetoni M, Wickman K, Luscher C. (2008) Absence and rescue of morphine withdrawal in GIRK/Kir3 knock-out mice. J Neurosci 28:4069–4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Mombereau C, Vassout A. (2005) The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev 29:571–625. [DOI] [PubMed] [Google Scholar]
- Dahlstroem A, Fuxe K. (1964) Evidence for the existence of monoamine-containing neurons in the central nervous system. I. Demonstration of monoamines in the cell bodies of brain stem neurons. Acta Physiol Scand Suppl 232:1–55. [PubMed] [Google Scholar]
- David DJ, Klemenhagen KC, Holick KA, Saxe MD, Mendez I, Santarelli L, Craig DA, Zhong H, Swanson CJ, Hegde LG, Ping XI, Dong D, Marzabadi MR, Gerald CP, Hen R. (2007) Efficacy of the MCHR1 antagonist N-[3-(1-{[4-(3,4-difluorophenoxy)phenyl]methyl}(4-piperidyl))-4-methylphenyl]-2-m ethylpropanamide (SNAP 94847) in mouse models of anxiety and depression following acute and chronic administration is independent of hippocampal neurogenesis. J Pharmacol Exp Ther 321:237–248. [DOI] [PubMed] [Google Scholar]
- David DJ, Samuels BA, Rainer Q, Wang JW, Marsteller D, Mendez I, Drew M, Craig DA, Guiard BP, Guilloux JP, Artymyshyn RP, Gardier AM, Gerald C, Antonijevic IA, Leonardo ED, Hen R. (2009) Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron 62:479–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day HE, Greenwood BN, Hammack SE, Watkins LR, Fleshner M, Maier SF, Campeau S. (2004) Differential expression of 5HT-1A, alpha 1b adrenergic, CRF-R1, and CRF-R2 receptor mRNA in serotonergic, gamma-aminobutyric acidergic, and catecholaminergic cells of the rat dorsal raphe nucleus. J Comp Neurol 474:364–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferres-Coy A, Santana N, Castane A, Cortes R, Carmona MC, Toth M, Montefeltro A, Artigas F, Bortolozzi A. (2013) Acute 5-HT(1)A autoreceptor knockdown increases antidepressant responses and serotonin release in stressful conditions. Psychopharmacology 225:61–74. [DOI] [PubMed] [Google Scholar]
- Fiol-deRoque MA, Gutierrez-Lanza R, Teres S, Torres M, Barcelo P, Rial RV, Verkhratsky A, Escriba PV, Busquets X, Rodriguez JJ. (2013) Cognitive recovery and restoration of cell proliferation in the dentate gyrus in the 5XFAD transgenic mice model of Alzheimer’s disease following 2-hydroxy-DHA treatment. Biogerontology 14:763–775. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. (1997). The mouse brain in stereotaxic coordinates. San Diego, CA: Academic Press Inc. [Google Scholar]
- Gartside SE, Hajos-Korcsok E, Bagdy E, Harsing LG, Jr, Sharp T, Hajos M. (2000) Neurochemical and electrophysiological studies on the functional significance of burst firing in serotonergic neurons. Neuroscience 98:295–300. [DOI] [PubMed] [Google Scholar]
- Gobbi G, Cassano T, Radja F, Morgese MG, Cuomo V, Santarelli L, Hen R, Blier P. (2007) Neurokinin 1 receptor antagonism requires norepinephrine to increase serotonin function. Eur Neuropsychopharmacology. 17:328–338. [DOI] [PubMed] [Google Scholar]
- Goodwin GM, De Souza RJ, Green AR. (1985) The pharmacology of the hypothermic response in mice to 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT). A model of presynaptic 5-HT1 function. Neuropharmacology. 24:1187–1194. [DOI] [PubMed] [Google Scholar]
- Hanson ND, Owens MJ, Nemeroff CB. (2011) Depression, antidepressants, and neurogenesis: a critical reappraisal. Neuropsychopharmacology 36:2589–2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing M, Kotecki L, Marron Fernandez de Velasco E, Fajardo-Serrano A, Chung HJ, Lujan R, Wickman K. (2013) Repeated cocaine weakens GABA(B)-girk signaling in layer 5/6 pyramidal neurons in the prelimbic cortex. Neuron 80:159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler LK, Chu HM, Brennan TJ, Danao JA, Bajwa P, Parsons LH, Tecott LH. (1998) Elevated anxiety and antidepressant-like responses in serotonin 5-HT1A receptor mutant mice. Proc Natl Acad Sci U S A 95:15049–15054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heurteaux C, Lucas G, Guy N, El Yacoubi M, Thummler S, Peng XD, Noble F, Blondeau N, Widmann C, Borsotto M, Gobbi G, Vaugeois JM, Debonnel G, Lazdunski M. (2006) Deletion of the background potassium channel TREK-1 results in a depression-resistant phenotype. Nat Neurosci 9:1134–1141. [DOI] [PubMed] [Google Scholar]
- Innis RB, Aghajanian GK. (1987) Pertussis toxin blocks 5-HT1A and GABAB receptor-mediated inhibition of serotonergic neurons. Eur J Pharmacol 143:195–204. [DOI] [PubMed] [Google Scholar]
- Jin W, Lu Z. (1998) A novel high-affinity inhibitor for inward-rectifier K+ channels. Biochemistry 37:13291–13299. [DOI] [PubMed] [Google Scholar]
- Kanjhan R, Coulson EJ, Adams DJ, Bellingham MC. (2005) Tertiapin-Q blocks recombinant and native large conductance K+ channels in a use-dependent manner. J Pharmacol Exp Ther 314:1353–1361. [DOI] [PubMed] [Google Scholar]
- Karschin C, Dissmann E, Stuhmer W, Karschin A. (1996) IRK(1–3) and GIRK(1–4) inwardly rectifying K+ channel mRNAs are differentially expressed in the adult rat brain. J Neurosci 16:3559–3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyrakh L, Lujan R, Colon J, Karschin C, Kurachi Y, Karschin A, Wickman K. (2005) Molecular and cellular diversity of neuronal G-protein-gated potassium channels. J Neurosci 25:11468–11478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ. (2008) The molecular neurobiology of depression. Nature 455:894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labouebe G, Lomazzi M, Cruz HG, Creton C, Lujan R, Li M, Yanagawa Y, Obata K, Watanabe M, Wickman K, Boyer SB, Slesinger PA, Luscher C. (2007) RGS2 modulates coupling between GABAB receptors and GIRK channels in dopamine neurons of the ventral tegmental area. Nat Neurosci 10:1559–1568. [DOI] [PubMed] [Google Scholar]
- Le Francois B, Czesak M, Steubl D, Albert PR. (2008) Transcriptional regulation at a HTR1A polymorphism associated with mental illness. Neuropharmacology 55:977–985. [DOI] [PubMed] [Google Scholar]
- Lemonde S, Du L, Bakish D, Hrdina P, Albert PR. (2004) Association of the C(-1019)G 5-HT1A functional promoter polymorphism with antidepressant response. Int J Neuropsychopharmacol 7:501–506. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Gutknecht L. (2004) Focus on the 5-HT1A receptor: emerging role of a gene regulatory variant in psychopathology and pharmacogenetics. Int J Neuropsychopharmacol 7:381–385. [DOI] [PubMed] [Google Scholar]
- Liao YJ, Jan YN, Jan LY. (1996) Heteromultimerization of G-protein-gated inwardly rectifying K+ channel proteins GIRK1 and GIRK2 and their altered expression in weaver brain. J Neurosci 16:7137–7150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Jan LY, Stoffel M, Malenka RC, Nicoll RA. (1997) G protein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons. Neuron 19:687–695. [DOI] [PubMed] [Google Scholar]
- Luscher C, Slesinger PA. (2010) Emerging roles for G protein-gated inwardly rectifying potassium (GIRK) channels in health and disease. Nat Rev Neurosci 11:301–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. (2000) Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci 20:9104–9110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marker CL, Stoffel M, Wickman K. (2004) Spinal G-protein-gated K+ channels formed by GIRK1 and GIRK2 subunits modulate thermal nociception and contribute to morphine analgesia. J Neurosci 24:2806–2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayorga AJ, Dalvi A, Page ME, Zimov-Levinson S, Hen R, Lucki I. (2001) Antidepressant-like behavioral effects in 5-hydroxytryptamine(1A) and 5-hydroxytryptamine(1B) receptor mutant mice. J Pharmacol Exp Ther 298:1101–1107. [PubMed] [Google Scholar]
- Mitrovic I, Margeta-Mitrovic M, Bader S, Stoffel M, Jan LY, Basbaum AI. (2003) Contribution of GIRK2-mediated postsynaptic signaling to opiate and alpha 2-adrenergic analgesia and analgesic sex differences. Proc Natl Acad Sci U S A 100:271–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombereau C, Kaupmann K, van der Putten H, Cryan JF. (2004) Altered response to benzodiazepine anxiolytics in mice lacking GABA B(1) receptors. Eur J Pharmacol 497:119–120. [DOI] [PubMed] [Google Scholar]
- Parker RB, Waud DR. (1971) Pharmacological estimation of drug-receptor dissociation constants. statistical evaluation. I. Agonists. J Pharmacol Exp Ther 177:1–12. [PubMed] [Google Scholar]
- Parks CL, Robinson PS, Sibille E, Shenk T, Toth M. (1998) Increased anxiety of mice lacking the serotonin1A receptor. Proc Natl Acad Sci U S A 95:10734–10739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineyro G, Blier P. (1999) Autoregulation of serotonin neurons: role in antidepressant drug action. Pharmacol Rev 51:533–591. [PubMed] [Google Scholar]
- Portella MJ, de Diego-Adelino J, Ballesteros J, Puigdemont D, Oller S, Santos B, Alvarez E, Artigas F, Perez V. (2011) Can we really accelerate and enhance the selective serotonin reuptake inhibitor antidepressant effect? A randomized clinical trial and a meta-analysis of pindolol in nonresistant depression. J Clin Psychiatry 72:962–969. [DOI] [PubMed] [Google Scholar]
- Pravetoni M, Wickman K. (2008) Behavioral characterization of mice lacking GIRK/Kir3 channel subunits. Genes Brain Behav 7:523–531. [DOI] [PubMed] [Google Scholar]
- Ramboz S, Oosting R, Amara DA, Kung HF, Blier P, Mendelsohn M, Mann JJ, Brunner D, Hen R. (1998) Serotonin receptor 1A knockout: an animal model of anxiety-related disorder. Proc Natl Acad Sci U S A 95:14476–14481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson-Jones JW, Craige CP, Guiard BP, Stephen A, Metzger KL, Kung HF, Gardier AM, Dranovsky A, David DJ, Beck SG, Hen R, Leonardo ED. (2010) 5-HT1A autoreceptor levels determine vulnerability to stress and response to antidepressants. Neuron 65:40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson-Jones JW, Craige CP, Nguyen TH, Kung HF, Gardier AM, Dranovsky A, David DJ, Guiard BP, Beck SG, Hen R, Leonardo ED. (2011) Serotonin-1A autoreceptors are necessary and sufficient for the normal formation of circuits underlying innate anxiety. J Neurosci 31:6008–6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez JJ, Jones VC, Tabuchi M, Allan SM, Knight EM, LaFerla FM, Oddo S, Verkhratsky A. (2008) Impaired adult neurogenesis in the dentate gyrus of a triple transgenic mouse model of alzheimer’s disease. PLoS One 3:e2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez JJ, Jones VC, Verkhratsky A. (2009) Impaired cell proliferation in the subventricular zone in an Alzheimer’s disease model. Neuroreport 20:907–912. [DOI] [PubMed] [Google Scholar]
- Rodriguez JJ, Noristani HN, Olabarria M, Fletcher J, Somerville TD, Yeh CY, Verkhratsky A. (2011) Voluntary running and environmental enrichment restores impaired hippocampal neurogenesis in a triple transgenic mouse model of Alzheimer’s disease. Curr Alzheimer Res 8:707–717. [DOI] [PubMed] [Google Scholar]
- Saenz del Burgo L, Cortes R, Mengod G, Zarate J, Echevarria E, Salles J. (2008) Distribution and neurochemical characterization of neurons expressing GIRK channels in the rat brain. J Comp Neurol 510:581–606. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Berman RM, Cappiello A, Oren DA, Kugaya A, Liu N, Gueorguieva R, Fasula D, Charney DS. (2004) Addition of the alpha2-antagonist yohimbine to fluoxetine: effects on rate of antidepressant response. Neuropsychopharmacology 29:1166–1171. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. (2003) Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301:805–809. [DOI] [PubMed] [Google Scholar]
- Signorini S, Liao YJ, Duncan SA, Jan LY, Stoffel M. (1997) Normal cerebellar development but susceptibility to seizures in mice lacking G protein-coupled, inwardly rectifying K+ channel GIRK2. Proc Natl Acad Sci U S A 94:923–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slesinger PA, Stoffel M, Jan YN, Jan LY. (1997) Defective gamma-aminobutyric acid type B receptor-activated inwardly rectifying K+ currents in cerebellar granule cells isolated from weaver and Girk2 null mutant mice. Proc Natl Acad Sci U S A 94:12210–12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprouse JS, Aghajanian GK. (1987) Electrophysiological responses of serotoninergic dorsal raphe neurons to 5-HT1A and 5-HT1B agonists. Synapse 1, 3–9. [DOI] [PubMed] [Google Scholar]
- Strobel A, Gutknecht L, Rothe C, Reif A, Mossner R, Zeng Y, Brocke B, Lesch KP. (2003) Allelic variation in 5-HT1A receptor expression is associated with anxiety- and depression-related personality traits. J Neural Transm 110:1445–1453. [DOI] [PubMed] [Google Scholar]
- Surget A, Saxe M, Leman S, Ibarguen-Vargas Y, Chalon S, Griebel G, Hen R, Belzung C. (2008) Drug-dependent requirement of hippocampal neurogenesis in a model of depression and of antidepressant reversal. Biol Psychiatry 64:293–301. [DOI] [PubMed] [Google Scholar]
- Torrecilla M, Marker CL, Cintora SC, Stoffel M, Williams JT, Wickman K. (2002) G-protein-gated potassium channels containing Kir3.2 and Kir3.3 subunits mediate the acute inhibitory effects of opioids on locus ceruleus neurons. J Neurosci 22:4328–4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrecilla M, Fernandez-Aedo I, Arrue A, Zumarraga M, Ugedo L. (2013) Role of GIRK channels on the noradrenergic transmission in vivo: an electrophysiological and neurochemical study on GIRK2 mutant mice. Int J Neuropsychopharmacol 16:1093–1104. [DOI] [PubMed] [Google Scholar]
- Waldmeier PC, Wicki P, Feldtrauer JJ, Baumann PA. (1988) Potential involvement of a baclofen-sensitive autoreceptor in the modulation of the release of endogenous GABA from rat brain slices in vitro. Naunyn Schmiedebergs Arch Pharmacol 337:289–295. [DOI] [PubMed] [Google Scholar]
- Wang JW, David DJ, Monckton JE, Battaglia F, Hen R. (2008) Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. J Neurosci 28:1374–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JT, Colmers WF, Pan ZZ. (1988) Voltage- and ligand-activated inwardly rectifying currents in dorsal raphe neurons in vitro. J Neurosci 8:3499–3506. [DOI] [PMC free article] [PubMed] [Google Scholar]