Abstract
Background:
Reduced motivation is an important marker of psychiatric disorders, including depression. We describe the female encounter test, a novel method of evaluating reward-seeking behavior in mice.
Methods:
The test apparatus consists of three open chambers, formed with partitions that allow the animal to move freely from one chamber to another. A test male mouse is habituated in the apparatus, and subsequently a female and male mouse are introduced into a wire-mesh box in the left and right chamber, respectively. The time the test male mouse spends in the female or male area is measured for 10min.
Results:
All six strains of mice tested showed a significant preference for female encounters. The preference was observed in 7–30-week-old mice. The preference was blocked by castration of the resident male test mouse, and was not affected by the phase of the menstrual cycle of the female intruder. The preference was impaired in mouse models of depression, including social isolation-reared, corticosterone-treated, and lipopolysaccharide-treated mice. The impairment was alleviated by fluvoxamine in isolation-reared and lipopolysaccharide-treated mice, and it was improved by the metabotropic glutamate 2/3 receptor antagonist LY341495 in corticosterone-treated mice. Encounter with a female, but not male, mouse increased c-Fos expression in the nucleus accumbens shell of test male mice. Furthermore, both the preference and encounter-induced increases in c-Fos expression were blocked by dopamine D1 and D2 receptor antagonists.
Conclusions:
These findings indicate that motivation in adult male mice can be easily evaluated by quantitating female encounters.
Keywords: Depression model, dopamine, encounter test, nucleus accumbens, sexual motivation
Introduction
Anhedonia, or markedly diminished interest or pleasure, is a characteristic feature of many psychiatric disorders, including major depression (Willner, 1991), and tests that assess this behavior are important for studying the etiology of psychiatric disorders. Previous studies on rodent models using unpredictable chronic mild stress show that the sucrose intake/preference test can measure anhedonia-like behavior (Willner, 1997). However, some reports show no reduction in preference after stress exposure, suggesting that this test has poor reliability (Matthews et al., 1995; Forbes et al., 1996; Willner, 1997; Grønli et al., 2005). The conditioned place preference test has also been used to assess hedonic behavior, but the test is dependent on learning and memory (Mucha and Iversen, 1984; Aguilar et al., 2009). Furthermore, these tests are time-intensive. Mateus-Pinheiro et al. (2014) have recently reported a newly-developed test: the sweet drive test. The test integrates food preference measurement with ultrasonic vocalization recording for measuring anhedonic behavior. However, it takes a long time because of prehabituation to sweet pellets and three test trials. In addition to anhedonia, despair is also a common symptom of depression, and the forced swim and tail suspension tests are widely used to measure this behavior in rodents (Porsolt et al., 1977; Steru et al., 1985). These tests were originally developed to evaluate the activity of antidepressants, and not for assessing the depressive-like state in rodent models of psychiatric disorders. It should be noted that these tests for despair require the prior application of stress. Therefore, the development of simple and noninvasive tests for anhedonia or despair is required for studies on the etiology of psychiatric disorders.
Although the reward system plays a key role in drug dependence, sexual behaviors are also considered to be a reward-seeking behavior or natural motivated behavior. Mendelson and Pfaus (1989) reported that the analysis of anticipatory level-changing behavior can be used to assess sexual motivation. Anticipatory-level-changing behavior is a form of behavior displayed by male rats prior to copulation when tested in a bilevel testing chamber. However, the testing system is complicated, and the analysis is based on physical contact between the male and female rats. Malkesman et al. (2010) demonstrated that the sniffing of estrous female urine can be used to assess reward-seeking behavior in male mice. They showed that sniffing is reduced by the learned helplessness paradigm, and this reduction can be attenuated by the selective serotonin reuptake inhibitor (SSRI) citalopram. Furthermore, Lehmann et al. (2013) recently reported that the urine scent marking activity of a male mouse in response to a female urine spot can be used to measure the strength of hedonic behavior. They showed that urine scent marking activity is diminished by chronic social defeat, a depressive-like condition, and that this effect of chronic social defeat can be abrogated by the SSRI fluoxetine. These observations indicate that sexual motivation is useful for assessing reward-seeking behavior in rodents.
We recently demonstrated that isolation-reared mice, an animal model of psychotic disorders, show encounter-induced abnormal behavior (Ago et al., 2013a; Araki et al., 2014). In this experimental paradigm, encounter stimulation occurs through a mesh partition, and the mice are able to see, hear, and smell their neighbor without physical contact. This social encounter through a mesh partition is a psychological stress and provides information on communication between the two animals. Moy et al. (2004) developed a new test to assess sociability and the preference for social novelty in mice. The procedure uses a rectangular three-chambered box, and the preference for social novelty is quantitated by presenting the test mouse with a choice between the familiar conspecific in one side chamber and an unfamiliar mouse in the other side chamber. Interestingly, we found that a normal male mouse prefers an encounter with a female mouse over an encounter with a male mouse. We hypothesized that female encounter by a male mouse is a sexual behavior, and that it may provide a simple measure of reward-seeking behavior. This paper reports this novel and simple method, which we have termed the female encounter test, for assessing motivation in adult mice.
Methods
Animals
All animal studies were approved by the Animal Care and Use Committee of the Graduate School of Pharmaceutical Sciences, Osaka University. All experimental procedures were conducted in accordance with the guidelines in the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996). Every effort was made to minimize animal suffering and reduce the number of animals used. CD-1 (ICR), ddY outbred, and BALB/c inbred male and female mice were purchased from SHIMIZU Laboratory Supplies Co., Ltd. C57BL/6J, DBA/2J, and C3H/HeJ inbred male and female mice were purchased from CLEA Japan, Inc. All mice, except for social isolation-reared mice, were housed in wire-topped clear polycarbonate cages (28cm × 17cm × 12cm) in groups of five animals under controlled environmental conditions (22±1 °C; 12:12-h light/dark cycle, lights on at 08:00; food and water ad libitum) for at least 1 week before use in experiments. CD-1 mice were used in all experiments except for assessing strain differences (Figure 3). In most experiments, 9-week-old mice were used. When older animals were used, 8-week-old CD-1 male mice were purchased and continuously housed until 40-week-old in our animal facility. For isolation-reared animals, CD-1 male mice were weaned at 3 weeks of age and individually housed for 6 weeks in wire-topped opaque polypropylene cages (28cm × 17cm × 12cm; Ago et al., 2013a). For lipopolysaccharide-treated animals, 9-week-old CD-1 male mice were injected intraperitoneally (i.p.) with lipopolysaccharide (0.5mg/kg), and behaviors were analyzed 24h after the injection (Zhang et al., 2015). For chronic corticosterone-treated animals, 6-week-old CD-1 male mice were subcutaneously (s.c.) injected once daily (between 10:00 and 12:00h) with corticosterone (20mg/kg) for 21 consecutive days (Ago et al., 2008). Mice treated with vehicle for the same period were used as controls. The behavioral experiments were carried out 24h after the last injection of corticosterone.
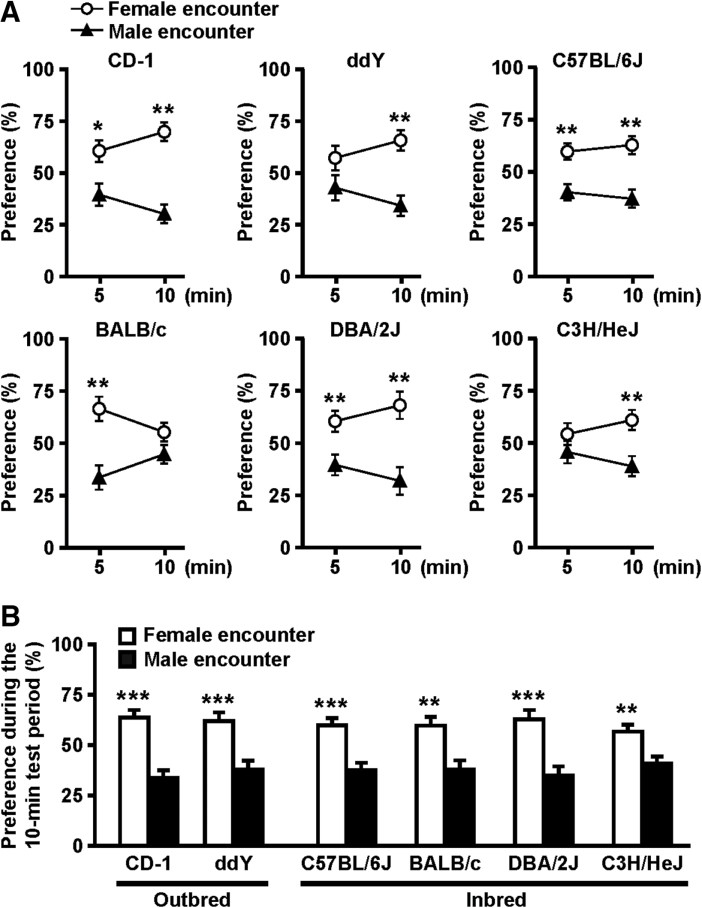
Figure 3.
Effects of mouse strain in the female encounter test. Preference for either male or female encounter during (A) each 5-min period and (B) over the full 10-min test session were analyzed. The data are expressed as the mean ± standard error of the mean of 10 mice/group. * p < 0.05, ** p < 0.01, *** p < 0.001, compared with the male encounter.
Drugs
Fluvoxamine maleate (Abbott Japan Co., Ltd.), LY341495, raclopride, SCH39166 hydrobromide (Tocris Cookson Ltd.), corticosterone, and lipopolysaccharide (Escherichia coli serotype 0127:B8; Sigma) were used. Fluvoxamine and lipopolysaccharide were dissolved in saline (0.9% solution of NaCl). LY341495 was dissolved in 0.067M phosphate buffer (pH 8.0). Raclopride and SCH39166 were dissolved in saline containing less than 0.01% v/v dimethyl sulfoxide. Corticosterone was suspended in 0.5% w/v carboxymethylcellulose. All drugs were injected at a fixed volume of 10ml/kg body weight. The dose of fluvoxamine (10–30mg/kg) was selected according to previous studies where it showed antidepressant-like effects in mice (Redrobe and Bourin, 1998; Renard et al., 2001). LY341495 (0.1–0.3mg/kg) was used at a dose that improved depression-like behavior in the chronic corticosterone-treated mice (Ago et al., 2013b). SCH39166 (0.1–0.2mg/kg) and raclopride (0.03–0.1mg/kg) were used at doses that affected reward-related behaviors in mice (Fish et al., 2014; Price and Middaugh, 2004).
Female Encounter Test
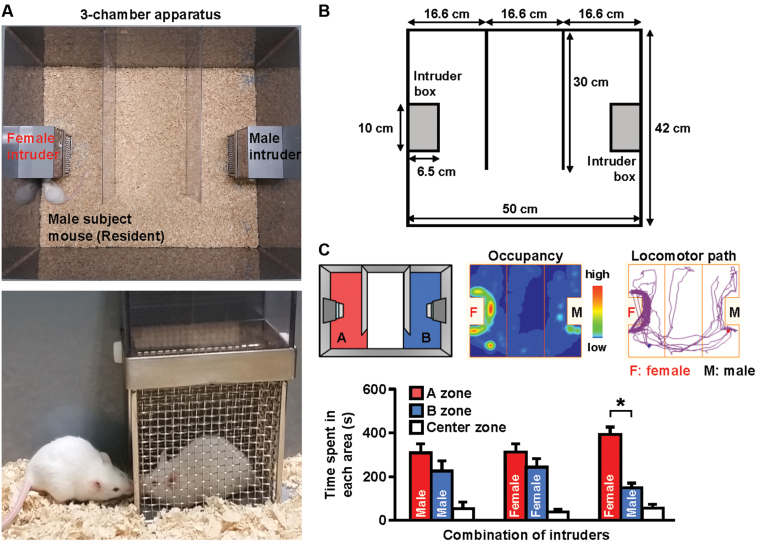
A sexually naïve 9-week-old male CD-1 mouse was placed in the central chamber of an opaque acrylic-modified polyvinyl chloride box (42cm × 50cm × 30cm) divided into three interconnected chambers under an illumination of 400 lx (measuring at center zone; Figure 1A and B). The clear partitions (30cm × 30cm) have openings that allow the animal to move freely from one chamber to another. After a 90-min habituation period, unfamiliar sexually naïve 9-week-old CD-1 male and female mice were introduced into the intruder boxes (10cm × 6.5cm × 20cm). The resident (test) and intruder mice were allowed to interact through the wire-mesh walls for 10min, and then the intruder mice were removed. To eliminate all odors from the previous trial, the intruder boxes were washed with 0.1% chlorhexidine gluconate solution, and the apparatus was wiped with 70% ethanol solution before the start of each experiment. The behaviors of the test mouse were videotaped, and its occupancy in the box and locomotor path was automatically analyzed off-line using the ANY-maze video tracking software (Stoelting Company). The amount of time spent in each of the three chambers was measured to estimate the behavioral reactivity of mice to the intruder. To simplify the indices of motivation, preference to either male or female encounter was also calculated as a percentage score for each intruder: Preference (%) = (time spent in either male or female zone / total time spent in male and female zones during 5- or 10-min period of measurement) × 100. We also used different age groups of CD-1 resident male mice (3–40 weeks old; Figure 2C), and we used different strains of mice (ddY, C57BL/6J, BALB/c, DBA/2J and C3H/HeJ; Figure 3).
Figure 1.
Female encounter test in mice. (A) Images of the three-chambered apparatus and interacting mice. (B) Cage sizes. (C) Representative occupancy in the apparatus and locomotor path of the resident mice during a 10-min encounter analyzed by the ANY-maze video tracking system. A 9-week-old male CD-1 mouse was placed as resident in the central chamber of the apparatus. After a 90-min habituation period, unfamiliar 9-week-old CD-1 male and female mice were introduced into the intruder boxes for 10min. Time spent in each zone by the resident (test) mouse was measured. The data are expressed as the mean ± standard error of the mean of 8 mice/group. * p < 0.01, compared with the male zone.
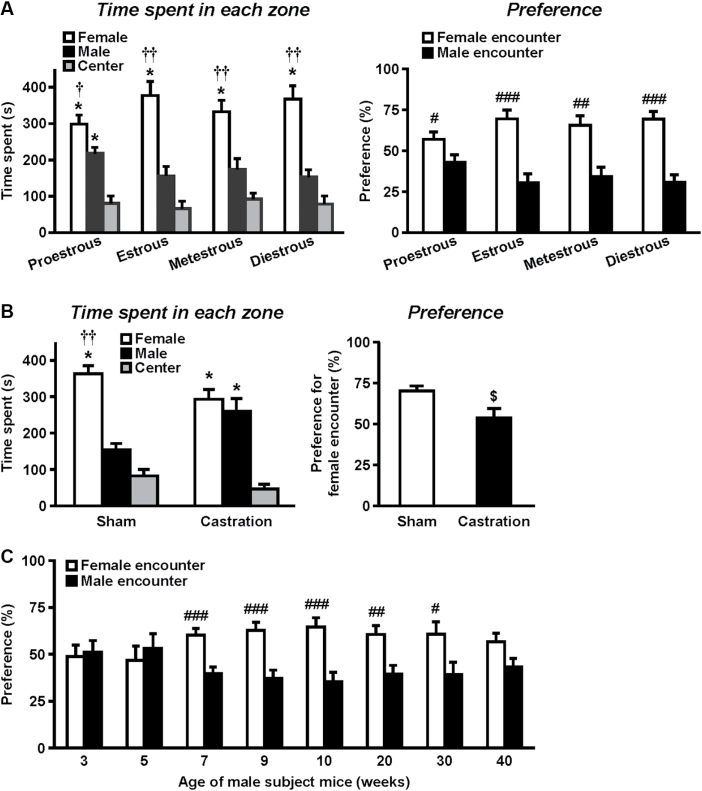
Figure 2.
Effects of gonadal hormones in the female encounter test. (A) Effects of the estrous cycle of the female intruder. Time spent in each zone (left panel) and preference for either male or female encounter (right panel) by the resident mouse were analyzed. The estrous cycle of female intruders was determined immediately after the female encounter test by Giemsa staining. (B) Effects of castration of the male resident test mouse. Time spent in each zone (left panel) and preference for either male or female encounter (right panel) by the resident test mouse were analyzed. (C) Effects of age of the resident mouse in the female encounter test. Preference for either male or female encounter by the resident test mouse is shown. The data are expressed as the mean ± standard error of the mean of (A) 10–11, (B) 8, and (C) 10 mice/group. * p < 0.01, compared with the center zone. † p < 0.05, †† p < 0.01, compared with the male zone. # p < 0.05, ## p < 0.01, ### p < 0.001, compared with the male encounter. $ p < 0.05, compared with the sham-operated mice.
Castration
Each seven-week-old CD-1 male mouse was anesthetized with pentobarbital (40mg/kg, i.p.). A small incision was made in the midline of the scrotum and both testes were externalized. For animals undergoing castration, testes were removed following ligation of the testicular blood supply, while the testes were returned to the scrotum in sham-castrated mice (Hiramatsu et al., 2013). Mice received an injection of buprenorphine (0.1mg/kg, i.p.) and gentamicin (10mg/kg, i.p.), and recovered within a few hours of surgery. Animals were used 2 weeks after surgery.
Estrous Cycle Phase Determination
A vaginal smear was collected immediately after the female encounter test by lavage of the vagina with saline solution using a glass pipette. To identify the phase of the estrous cycle, vaginal smears were fixed on a glass slide using methanol, stained with Giemsa solution, and analyzed by light microscopy. The stage of the estrous cycle was determined based on the presence or absence of leukocytes, cornified epithelial, and nucleated epithelial cells (Meziane et al., 2007; Byers et al., 2012).
c-Fos Immunohistochemistry
Brain expression of the neuronal activity marker c-Fos was determined 2h after a 10-min encounter with either a male or female intruder, as described previously (Koda et al., 2010; Ago et al., 2011, 2013a). Mice that were not exposed to an intruder were used as controls. Each mouse was deeply anesthetized with pentobarbital and then perfused transcardially with saline, followed by a solution of 4% paraformaldehyde in phosphate-buffered saline (PBS). Serial 20 µm thick coronal sections were cut using a cryostat microtome at −20°C. The free-floating sections were preincubated for 30min in 0.3% hydrogen peroxide in PBS and then blocked in 1.5% goat serum in PBS for 20min at room temperature. Thereafter, the sections were incubated with anti-c-Fos rabbit polyclonal primary antibodies (1:1,000; sc-52, Santa Cruz Biotechnology) overnight at room temperature. Subsequently, the sections were washed in PBS and incubated with a secondary antibody solution containing biotinylated anti-rabbit IgG for 30min at room temperature. The sections were then incubated with avidin-biotin-horseradish peroxidase complex for 30min at room temperature. Brown cytosolic products were obtained by reacting with 3,3ʹ-diaminobenzidine. Four independent sections per animal containing the prefrontal cortex, nucleus accumbens, amygdala, ventral tegmental area, or dorsal raphe nucleus were selected. c-Fos-positive cells were counted manually by experienced observers blinded to rearing, treatment, and encounter conditions under bright-field illumination on an Axio Imager.M2 microscope (Carl Zeiss). The number of c-Fos-positive cells in each section was determined in a 500×500 µm area in the left and right hemispheres, and averaged using the ImageJ 1.41 software package (NIH). The average of this average across four sections was then calculated for each subject.
For double immunofluorescence staining of glutamic acid decarboxylase 67 (GAD67) and c-Fos, the brain sections were heated in a microwave oven in a 0.01M sodium citrate buffer (pH 6.0) for 10min. After being rinsed for 10min in PBS containing 0.03% Triton-X100 (PBST), the sections were blocked by 5% donkey serum in PBST for 1h at room temperature. Then, they were incubated with a goat anti-c-Fos polyclonal primary antibody (1:100; sc-52-G, Santa Cruz Biotechnology) and a mouse anti-GAD67 monoclonal primary antibody (1:300; MAB5406, Millipore) at 4°C overnight, followed by an Alexa Fluor 546-labeled anti-goat IgG (1:100, Life Technologies) and an Alexa Fluor 488-labeled anti-mouse IgG (1:300, Life Technologies) for 2h at room temperature. Finally, the sections were rinsed in PBST and mounted in Dako Fluorescence Mounting Medium supplemented with 4’,6-diamidino-2-phenylindole nuclear counterstain (1 μg/ml concentration). All fluorescent images were acquired with an Axio Imager.M2 microscope (Carl Zeiss).
Statistical Analysis
All results are presented as the mean ± standard error of the mean. Data for time spent in each chamber (Figure 2) and time-course of preference to the encounter (Figure 3A) were analyzed using one- or two-way analyses of variance (ANOVAs), with castration or preference as the inter-subject factor and repeated measures with zone or time as the intra-subject factor, followed by the Tukey–Kramer test. For other behavioral measures, the data were analyzed using Student’s t-test (Figures 2A [preference], 2C, and 3B) or one or two-way ANOVA, followed by the Tukey–Kramer test (Figures 2B [preference], 4 and 8A). For the c-Fos expression experiments, the data were analyzed using one or two-way ANOVA, followed by the Tukey–Kramer test. Statistical analyses were performed using a software package, Statview 5.0J, for Apple Macintosh (SAS Institute Inc.). A value of p < 0.05 was considered statistically significant.
Results
Female Encounter Test
We examined the effects of simultaneous encounters with female and male CD-1 intruders on the behavior of male CD-1 resident test mice. A 9-week-old male mouse was placed in the central chamber of a test apparatus divided into three interconnected chambers (Figure 1A and B). After a 90-min habituation period, an unfamiliar female intruder of the same age was introduced into a wire-mesh box located in one side chamber and an unfamiliar male intruder was introduced into an identical box in the opposing side chamber. The amount of time spent by the test male mouse in each of the three chambers was recorded over the 10-min trial. Figure 1C shows a representative occupancy profile and the locomotor path of the test mouse during the 10-min trial. The occupancy and locomotor patterns indicate that male mice spent more time in the female zone than in the male or center zone. Test male mice displayed similar approaches to each side chamber when same-sex intruders were placed in the intruder boxes (Figure 1C).
Figure 2 shows the effects of gonadal hormones in the female encounter test. We examined the effects of the estrous cycle on the preference for the female encounter (Figure 2A). Male CD-1 resident test mice spent more time in the female intruder zone than in the male intruder or center zone at any stage of the estrous cycle. Repeated measures one-way ANOVA revealed a significant effect of zone (F 2,18 = 15.6, p = 0.0001 for proestrous; F 2,18 = 16.2, p < 0.0001 for estrous; F 2,18 = 14.0, p < 0.001 for metestrous; F 2,18 = 20.1, p < 0.0001 for diestrous). Male mice showed a significant preference for the female encounter regardless of the phase of the estrous cycle of the female. In contrast, castrated male resident test mice spent similar amounts of time in the female and male intruder zones (operation: F 1,14 = 10.6, p < 0.01; Figure 2B). The preference for female encounter was not observed in castrated mice. Figure 2C shows the effects of age of the resident test mouse in the female encounter test. Male mice of 7, 9, 10, 20, and 30 weeks, but not 3, 5, or 40 weeks, exhibited a significant preference for female (9 weeks of age) encounters.
Figure 3 shows the effects of mouse strain in the female encounter test. We tested several male mice from four inbred strains (C57BL/6J, BALB/c, DBA/2J, and C3H/HeJ) and one outbred strain (ddY) in addition to CD-1 mice, all at 9 weeks of age. Mice from the same strain and of the same age were used as male and female intruders. Figure 3A shows the time-course of the preference for the encounter during a 10-min test period. CD-1, C57BL/6J, BALB/c, and DBA/2J mice showed a significant preference for the female encounter in the first 5-min period. All strains except BALB/c mice showed a significant preference for the female encounter in the last 5-min period. Collectively, all strains tested showed a significant preference for the female encounter during the 10-min test period (Figure 3B).
Female Preference in Mouse Models of Depression
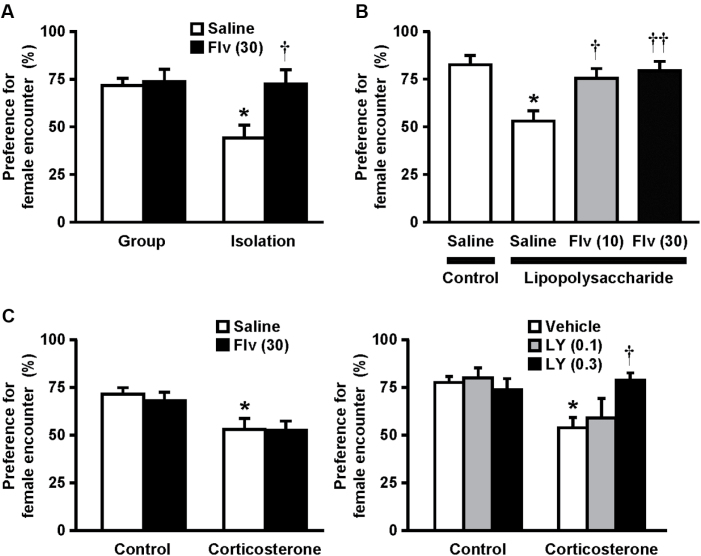
To examine whether the preference for female encounter reflects the depressive-like state, we performed the female encounter test in mouse models of depression, including social isolation-reared, lipopolysaccharide-treated, and corticosterone-treated mice (Figure 4). The preference for female encounter was absent in post-weaning social isolation-reared and chronic corticosterone-treated mice. The preference for female encounter was also absent in lipopolysaccharide-treated mice. Fluvoxamine (10–30mg/kg) significantly improved the impaired preference for female encounter in isolation-reared (interaction: F 1,36 = 3.4, p < 0.05; Figure 4A) and lipopolysaccharide-treated (F 3,28 = 6.8, p < 0.01; Figure 4B) mice. However, fluvoxamine (30mg/kg) did not alleviate the impairment in preference for female encounters in corticosterone-treated mice (F 1,36 = 0.1, p > 0.05; Figure 4C). On the other hand, LY341495 (0.3mg/kg), a metabotropic glutamate 2/3 receptor antagonist, significantly alleviated the impairment in preference for female encounters in corticosterone-treated mice (F 2,54 = 3.4, p < 0.05).
Figure 4.
Female encounter test in mouse models of depression. For (A) isolation-reared and (C) chronic corticosterone-treated resident test mice, fluvoxamine (30mg/kg) and LY341495 (0.1, 0.3mg/kg) were i.p. administered 30min before the encounter with the intruders. For (B) lipopolysaccharide-treated mice, fluvoxamine (10, 30mg/kg) was i.p. injected 30min before the lipopolysaccharide treatment. Preference for the female encounter by resident test mice is shown. The data are expressed as the mean ± standard error of the mean of 8–10 mice/group. * p < 0.01, compared with the (A) saline-treated group-reared, (B) saline, or (C) vehicle-treated control mice. † p < 0.05, †† p < 0.01, compared with the (A) saline-treated isolation-reared, (B) saline/lipopolysaccharide, or (C) vehicle/corticosterone-treated mice.
Involvement of the Reward System in Female Preference
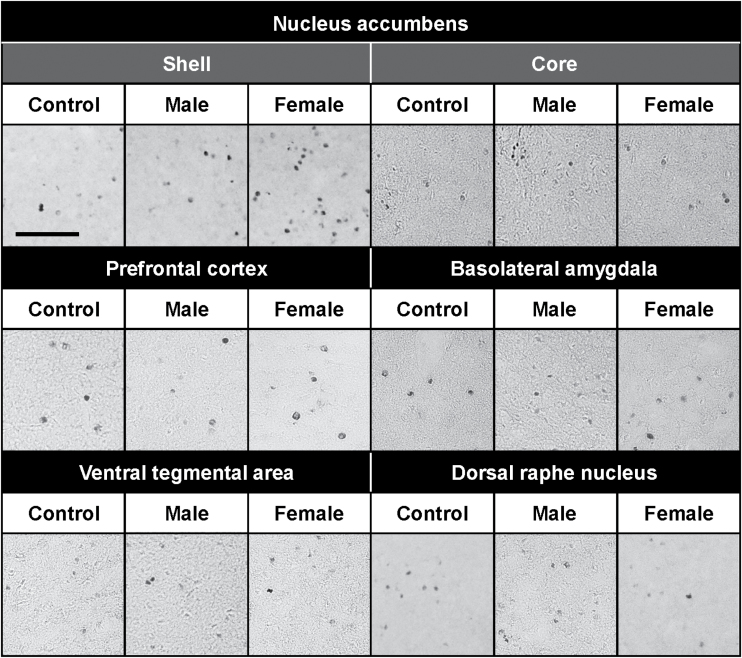
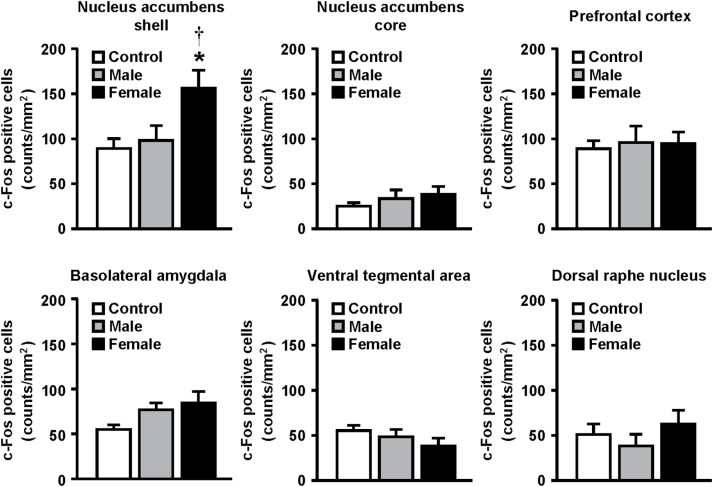
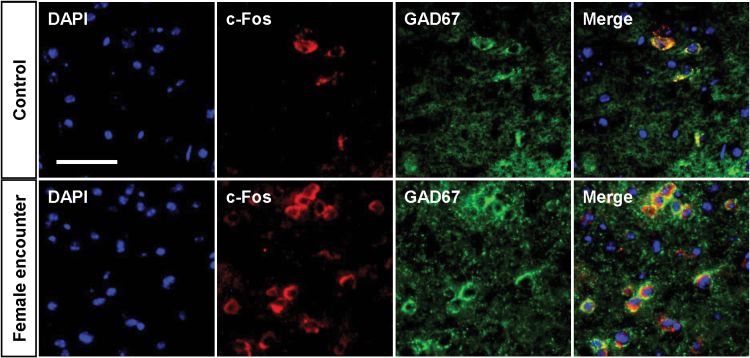
To investigate the neural mechanisms underlying the preference for female encounter, we examined the effects of male and female encounters on c-Fos expression in the brains of male mice (Figures 5 and 6). Representative photomicrographs of c-Fos–positive cells are shown in Figure 5. The female, but not male, encounter caused a significant increase in c-Fos expression in the nucleus accumbens shell (F 2,21 = 5.1, p < 0.05) (Figure 6). Neither the female nor male encounter affected c-Fos expression in the nucleus accumbens core, prefrontal cortex, basolateral amygdala, ventral tegmental area, or dorsal raphe nucleus. By double immunofluorescence staining, we observed that female encounter–induced increases in c-Fos expression were localized mostly to GAD67-expressing cells as markers of GABAergic neurons (Figure 7).
Figure 5.
Representative photomicrographs showing c-Fos immunoreactive cells after encounter with either a male or female intruder in the brain of resident male CD-1 test mice. Mice that were not exposed to an intruder were used as controls (Control). Scale bar, 100 μm.
Figure 6.
Effects of male and female encounter on c-Fos expression in the brain of resident male CD-1 test mice. The data are expressed as the mean ± standard error of the mean of 8 mice/group. * p < 0.05, compared with the control; † p < 0.05, compared with the male encounter.
Figure 7.
Co-localization of c-Fos and glutamic acid decarboxylase 67 (GAD67) immunoreactivities in the nucleus accumbens shell after female encounter. Immunofluorescence staining for c-Fos (red) and GAD67 (green) is shown. 4’,6-diamidino-2-phenylindole was used for nuclear counterstaining (blue). Mice that were not exposed to an intruder were used as controls (Control). Scale bar, 50 μm.
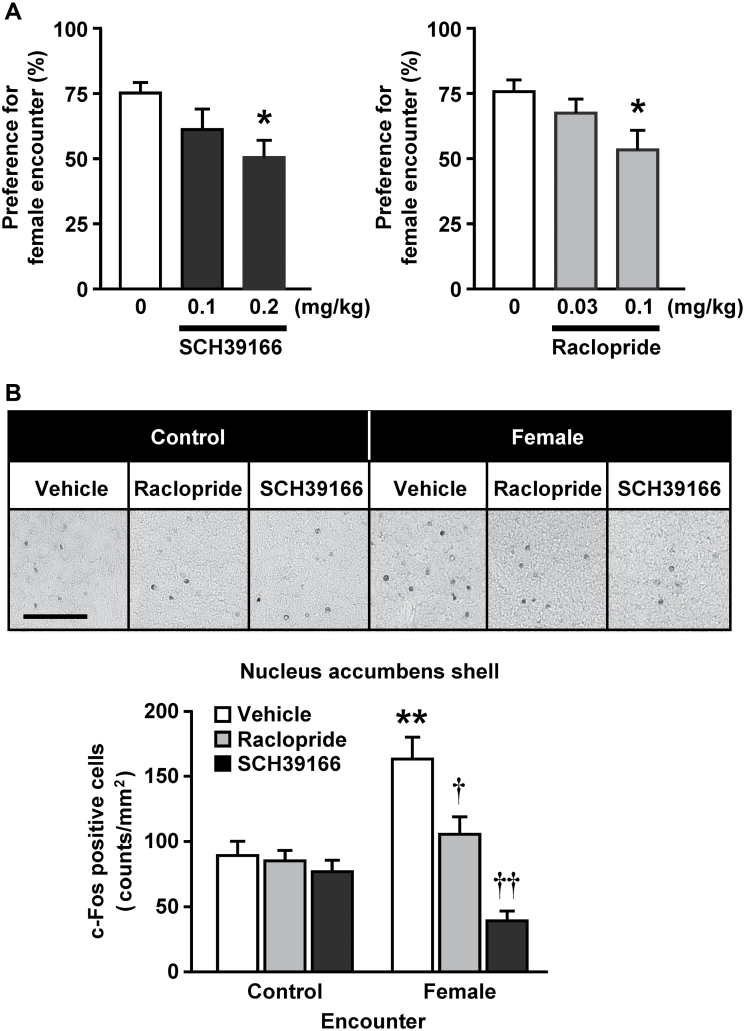
We finally examined the effects of dopamine D1- and D2-like receptor antagonists on the preference for female encounters and female encounter–induced increases in c-Fos expression in the nucleus accumbens shell (Figure 8). SCH39166, a selective dopamine D1/D5 receptor antagonist, and raclopride, a selective dopamine D2/D3 receptor antagonist, significantly attenuated the preference for female encounters in a dose-dependent manner. One-way ANOVA revealed a significant main effect of treatment (F 2,31 = 3.3, p < 0.05 for SCH39166; F 2,27 = 3.6, p < 0.05 for raclopride). Both drugs also inhibited the female encounter–induced increase in c-Fos expression (encounter × treatment: F 2,32 = 3.6, p < 0.05).
Figure 8.
Involvement of the dopaminergic system in the preference for female encounter. Effects of dopamine D1 and D2-like receptor antagonists on (A) the preference for female encounter and (B) the female encounter-induced increase in c-Fos expression in the nucleus accumbens shell. Nine-week-old male CD-1 resident mice were i.p. injected with SCH39166 (0.1, 0.2mg/kg), raclopride (0.03, 0.1), or vehicle 30min before the encounter with 9-week-old CD-1 male and female intruders. The data are expressed as the mean ± standard error of the mean of 10–12 (behavior) and eight (c-Fos) mice/group. * p < 0.05, ** p < 0.01, compared with the vehicle control. † p < 0.05, †† p < 0.01, compared with the vehicle control in the female encounter group.
Discussion
In the present study, we found that a male mouse prefers an encounter with a female mouse over a male mouse. The preference was blocked by castration, suggesting that androgens are involved in the behavior. An important role of androgens is also supported by the finding that the preference is observed from adolescence to adulthood. We further found that the preference for female encounters was absent in three models of depression, and that this impairment in preference could be alleviated by antidepressants. Taken together, our findings indicate that the female encounter test is a simple method for evaluating reward-seeking behavior or motivation and can be used for assessing the depressive-like state in adult male mice. We observed in a preliminary experiment that female mice show a similar preference for male encounters. However, it is not known whether the preference is altered in depression models. Further studies are required for application of the encounter test for investigations in female psychiatric disorders.
In the female encounter test, a three-chambered cage is used for measurement of the preference for female encounter. The test apparatus was originally reported to measure sociability in mice (Nadler et al., 2004) and used for investigations of autism and schizophrenia (Felix-Ortiz and Tye, 2014; Langley et al., 2015; Štefánik et al., 2015). In our pilot experiment, we found that the preference for a female encounter can be measured even using a one-chambered apparatus in ddY, CD-1, and DBA/2J mice, but not in BALB/c, C57BL/6J, or C3H/HeJ mice. In comparison, using the three-chambered apparatus, a similar preference can be observed in all mouse strains. Agmo et al. (2004) and Bai et al. (2009) showed that unconditioned sexual incentive motivation in rats can be observed in the one-chambered apparatus. Furthermore, Hou et al. (2014) showed that chronic unpredictable mild stress attenuates the male preference for an estrous female rat in the one-chambered apparatus. However, the mechanisms underlying the sexual behavior observed in the one-chambered apparatus appear to differ from those underlying the female encounter preference in the present study, because the dopaminergic system does not appear to be involved in the behavior observed in the previous studies (Agmo, 2003; Viitamaa et al., 2006). In the one-chambered paradigm used in these previous studies, the rats were familiarized with the test arena for 3 days, while in our novel three-chambered method, the mice require only 90min for habituation immediately prior to testing. Furthermore, in the habituation phase, spontaneous locomotor activity can be simultaneously measured (data not shown). In our test system, cleaning of the small wire-mesh boxes for the intruders was important for reproducibility. This suggests that substances that interfere with the interaction between the intruder and resident animals may accumulate in the box. The interference may result from urine scent marking by the male resident mouse (Lehmann et al., 2013); however, the mechanisms remain unclear.
Social communication in rodents occurs primarily through olfactory cues (Reynolds, 1971; Davies and Bellamy, 1972; Hurst, 2009). Malkesman et al. (2010) reported that sniffing of estrous female urine is a useful approach for assessing reward-seeking behavior in mice. They showed that female urine activates reward centers in the male rodent brain. Furthermore, Lehmann et al. (2013) showed that male scent marking activity in response to a proestrous female urine spot is a measure of hedonic behavior in mice. These studies suggest that the male interest in female urine is a marker of pleasure. Accordingly, the female urine test (Malkesman et al., 2010) and the urine scent marking test (Lehmann et al., 2013), based on the response to female urine, can be used to evaluate reward-seeking behavior. The female encounter test we present here is also based on sexual behavior, because the preference for female encounter is affected by castration and age. Our test appears to be simpler than previous tests, because there is no requirement for urine collection. Furthermore, unlike the previous tests (Malkesman et al., 2010; Lehmann et al., 2013), our test is not affected by the estrous cycle of the female intruder.
Malkesman et al. (2010) and Lehmann et al. (2013) demonstrated that the female urine test and urine scent marking test could evaluate the depressive state in the learned helplessness and chronic social defeat model, respectively. Indeed, in the present study, we found that the preference for a female encounter was impaired in mouse models of depression, including isolation-reared, chronic corticosterone-treated, and lipopolysaccharide-treated mice. Previous studies found that the isolation-reared (Kawasaki et al., 2011; Ago et al., 2014), chronic corticosterone-treated (Ago et al., 2008, 2013b), and lipopolysaccharide-treated (Haba et al., 2012) mice show increased immobility time in the forced swim test. Depression-like behavior in isolation-reared and lipopolysaccharide-treated mice is improved by an SSRI (Koike et al., 2009; Ohgi et al., 2013), while depressive behavior in chronic corticosterone-treated mice is improved by the metabotropic glutamate 2/3 receptor antagonist LY341495, but not by an SSRI (Ago et al., 2013b). Accordingly, the latter is considered an animal model of treatment-resistant depression. In the present study, we found that the preference for female encounter was impaired in these mouse models of depression. Furthermore, the impaired preference in isolation-reared and lipopolysaccharide-treated mice was improved by the SSRI fluvoxamine, while the impairment in chronic corticosterone-treated mice was improved by LY341495, but not fluvoxamine. These findings indicate that the female encounter test can be used to assess the depressive-like state.
Previous studies show that structural and functional alterations in the brain reward circuitry are associated with anhedonia (Russo and Nestler, 2013; Vialou et al., 2013). To study the neurochemical mechanisms underlying the social preferences, we analyzed c-Fos expression in the brain after a female encounter. An encounter with a female intruder increased c-Fos expression in the nucleus accumbens shell, while an encounter with a male intruder did not affect expression. The effect of a female encounter on c-Fos expression was specific to the nucleus accumbens, because it did not affect c-Fos expression in the other brain regions tested here. This finding is in agreement with previous studies showing that sniffing of female urine increases dopamine release in the nucleus accumbens (Malkesman et al., 2010). The nucleus accumbens is a major brain area that processes incentive–reward responses associated with novel, hedonic, stressful, or aversive stimuli (Kalivas and Duffy, 1995; Reynolds and Berridge, 2002; Jensen et al., 2003; Nicola, 2007). It comprises medium-sized spiny neurons that constitute >90% of all neurons in both the shell and core (Meredith et al., 1993; Zahm, 2000). These neurons are GABAergic and colocalized with various neuropeptides according to their projection patterns (Rogard et al., 1993; Lu et al., 1998; Zahm, 2000). In this line, we found that female encounter–induced increases in c-Fos expression were localized mostly to GAD67-expressing cells, as markers of GABAergic neurons. In addition, GABAergic medium-sized spiny neurons in the nucleus accumbens express either dopamine D1 or D2 receptors or both receptors (Perreault et al., 2012; Gangarossa et al., 2013; Smith et al., 2013). Notably, both the preference for a female encounter and the female encounter–induced increase in c-Fos expression were blocked by dopamine D1 or D2 receptor antagonists. This suggests that the response to a female encounter is mediated by activation of the dopamine reward system, particularly as dopamine D1 and D2 receptors in the nucleus accumbens shell play a key role in reward-seeking behavior (Ikemoto et al., 1997; Schmidt and Pierce, 2006; du Hoffmann and Nicola, 2014).
In conclusion, in the present study, we describe the female encounter test, a novel and simple procedure for assessing motivation. This method is dependent on sexual behavior, similar to the female urine sniffing test (Malkesman et al., 2010) and the urine scent marking test (Lehmann et al., 2013), and the behavior is mediated by activation of the dopamine system in the nucleus accumbens. Our method does not require urine collection and can be performed between the ages of 7 and 30 weeks. Because reduced interest in pleasurable stimuli, such as sex, is a major feature of major depression, our method based on sexual behavior may be more suitable for assessing depressive states and should contribute to advancing our knowledge of mood disorders.
Statement of Interest
None
Acknowledgments
This study was supported in part by KAKENHI (25460099 to Dr Ago; 15K18874 to Mr Onaka; 26293020 to Dr Hashimoto; 26670122 to Dr Hashimoto ; and 15H01288 to Dr Hashimoto), the Neuropsychiatry Drug Discovery Consortium established by Dainippon Sumitomo Pharma Co., Ltd. (Japan) with Osaka University (Drs Matsuda and Hashimoto), Takeda Science Foundation (Japan; Dr Ago), Research Foundation for Pharmaceutical Sciences (Japan; Dr Ago), and the JSPS Program for Advancing Strategic International Networks to Accelerate the Circulation of Talented Researchers (S2603; Drs Hashimoto and Matsuda).
References
- Ago Y, Arikawa S, Yata M, Yano K, Abe M, Takuma K, Matsuda T. (2008) Antidepressant-like effects of the glucocorticoid receptor antagonist RU-43044 are associated with changes in prefrontal dopamine in mouse models of depression. Neuropharmacology 55:1355–1363. [DOI] [PubMed] [Google Scholar]
- Ago Y, Yano K, Hiramatsu N, Takuma K, Matsuda T. (2011) Fluvoxamine enhances prefrontal dopaminergic neurotransmission in adrenalectomized/castrated mice via both 5-HT reuptake inhibition and σ1 receptor activation. Psychopharmacology (Berl) 217:377–386. [DOI] [PubMed] [Google Scholar]
- Ago Y, Araki R, Tanaka T, Sasaga A, Nishiyama S, Takuma K, Matsuda T. (2013a) Role of social encounter-induced activation of prefrontal serotonergic systems in the abnormal behaviors of isolation-reared mice. Neuropsychopharmacology 38:1535–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ago Y, Yano K, Araki R, Hiramatsu N, Kita Y, Kawasaki T, Onoe H, Chaki S, Nakazato A, Hashimoto H, Baba A, Takuma K, Matsuda T. (2013b) Metabotropic glutamate 2/3 receptor antagonists improve behavioral and prefrontal dopaminergic alterations in the chronic corticosterone-induced depression model in mice. Neuropharmacology 65:29–38. [DOI] [PubMed] [Google Scholar]
- Ago Y, Takuma K, Matsuda T. (2014) The potential role of serotonin1A receptors in post-weaning social isolation-induced abnormal behaviors in rodents. J Pharmacol Sci 125:237–241. [DOI] [PubMed] [Google Scholar]
- Agmo A. (2003) Lack of opioid or dopaminergic effects on unconditioned sexual incentive motivation in male rats. Behav Neurosci 117:55–68. [DOI] [PubMed] [Google Scholar]
- Agmo A, Turi AL, Ellingsen E, Kaspersen H. (2004) Preclinical models of sexual desire: conceptual and behavioral analyses. Pharmacol Biochem Behav 78:379–404. [DOI] [PubMed] [Google Scholar]
- Aguilar MA, Rodríguez-Arias M, Miñarro J. (2009) Neurobiological mechanisms of the reinstatement of drug-conditioned place preference. Brain Res Rev 59:253–277. [DOI] [PubMed] [Google Scholar]
- Araki R, Ago Y, Hasebe S, Nishiyama S, Tanaka T, Oka S, Takuma K, Matsuda T. (2014) Involvement of prefrontal AMPA receptors in encounter stimulation-induced hyperactivity in isolation-reared mice. Int J Neuropsychop 17:883–893. [DOI] [PubMed] [Google Scholar]
- Bai YJ, Li YH, Zheng XG, Han J, Yang XY, Sui N. (2009) Orexin A attenuates unconditioned sexual motivation in male rats. Pharmacol Biochem Behav 91:581–589. [DOI] [PubMed] [Google Scholar]
- Byers SL, Wiles MV, Dunn SL, Taft RA. (2012) Mouse estrous cycle identification tool and images. PLOS One 7:e35538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies VJ, Bellamy D. (1972) The olfactory response of mice to urine and effects of gonadectomy. J Endocrinol 55:11–20. [DOI] [PubMed] [Google Scholar]
- du Hoffmann J, Nicola SM. (2014) Dopamine invigorates reward seeking by promoting cue-evoked excitation in the nucleus accumbens. J Neurosci 34:14349–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix-Ortiz A, Tye KM. (2014) Amygdala inputs to the ventral hippocampus bidirectionally modulate social behavior. J Neurosci 34:586–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish EW, DiBerto JF, Krouse MC, Robinson JE, Malanga CJ. (2014) Different contributions of dopamine D1 and D2 receptor activity to alcohol potentiation of brain stimulation reward in C57BL/6J and DBA/2J mice. J Pharm Exp Ther 350:322–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes NF, Stewart CA, Matthews K, Reid IC. (1996) Chronic mild stress and sucrose consumption: validity as a model of depression. Physiol Behav 60:1481–1484. [DOI] [PubMed] [Google Scholar]
- Gangarossa G, Espallergues J, de Kerchove d’Exaerde A, El Mestikawy S, Gerfen CR, Hervé D, Girault JA, Valjent E. (2013) Distribution and compartmental organization of GABAergic medium-sized spiny neurons in the mouse nucleus accumbens. Front Neural Circuits 7;22:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grønli J, Murison R, Fiske E, Bjorvatn B, Sørensen E, Portas CM, Ursin R. (2005) Effects of chronic mild stress on sexual behavior, locomotor activity and consumption of sucrose and saccharine solutions. Physiol Behav 84:571–577. [DOI] [PubMed] [Google Scholar]
- Haba R, Shintani N, Onaka Y, Wang H, Takenaga R, Hayata A, Baba A, Hashimoto H. (2012) Lipopolysaccharide affects exploratory behaviors toward novel objects by impairing cognition and/or motivation in mice: Possible role of activation of the central amygdala. Behav Brain Res 228:423–431. [DOI] [PubMed] [Google Scholar]
- Hiramatsu N, Ago Y, Hasebe S, Nishimura A, Mori K, Takuma K, Matsuda T. (2013) Synergistic effect of 5-HT1A and σ1 receptor activation on prefrontal dopaminergic transmission under circulating steroid deficiency. Neuropharmacology 75:53–61. [DOI] [PubMed] [Google Scholar]
- Hou G, Xiong W, Wang M, Chen X, Yuan TF. (2014) Chronic stress influences sexual motivation and causes damage to testicular cells in male rats. J Sex Med 11:653–663. [DOI] [PubMed] [Google Scholar]
- Hurst JL. (2009) Female recognition and assessment of males through scent. Behav Brain Res 200:295–303. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Glazier BS, Murphy JM, McBride WJ. (1997) Role of dopamine D1 and D2 receptors in the nucleus accumbens in mediating reward. J Neurosci 17:8580–8587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J, McIntosh AR, Crawley AP, Mikulis DJ, Remington G, Kapur S. (2003) Direct activation of the ventral striatum in anticipation of aversive stimuli. Neuron 40:1251–1257. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. (1995) Selective activation of dopamine transmission in the shell of the nucleus accumbens by stress. Brain Res 675:325–328. [DOI] [PubMed] [Google Scholar]
- Kawasaki T, Ago Y, Yano K, Araki R, Washida Y, Onoe H, Chaki S, Nakazato A, Hashimoto H, Baba A, Takuma K, Matsuda T. (2011) Increased binding of cortical and hippocampal group II metabotropic glutamate receptors in isolation-reared mice. Neuropharmacology 60:397–404. [DOI] [PubMed] [Google Scholar]
- Koda K, Ago Y, Cong Y, Kita Y, Takuma K, Matsuda T. (2010) Effects of acute and chronic administration of atomoxetine and methylphenidate on extracellular levels of noradrenaline, dopamine and serotonin in the prefrontal cortex and striatum of mice. J Neurochem 114:259–270. [DOI] [PubMed] [Google Scholar]
- Koike H, Ibi D, Mizoguchi H, Nagai T, Nitta A, Takuma K, Nabeshima T, Yoneda Y, Yamada K. (2009) Behavioral abnormality and pharmacologic response in social isolation-reared mice. Behav Brain Res 202:114–121. [DOI] [PubMed] [Google Scholar]
- Langley EA, Krykbaeva M, Blusztajn JK, Mellott TJ. (2015). High maternal choline consumption during pregnancy and nursing alleviates deficits in social interaction and improves anxiety-like behaviors in the BTBR T+Itpr3tf/J mouse model of autism. Behav Brain Res 278:210–220. [DOI] [PubMed] [Google Scholar]
- Lehmann ML, Geddes CE, Lee JL, Herkenham M. (2013) Urine scent marking (USM): a novel test for depressive-like behavior and a predictor of stress resiliency in mice. PLOS One 8:e69822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu XY, Ghasemzadeh MB, Kalivas PW. (1998) Expression of D1 receptor, D2 receptor, substance P and enkephalin messenger RNAs in the neurons projecting from the nucleus accumbens. Neuroscience 82:767–780. [DOI] [PubMed] [Google Scholar]
- Malkesman O, Scattoni ML, Paredes D, Tragon T, Pearson B, Shaltiel G, Chen G, Crawley JN, Manji HK. (2010) The female urine sniffing test: a novel approach for assessing reward-seeking behavior in rodents. Biol Psychiatry 67:864–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateus-Pinheiro A, Patrício P, Alves ND, Machado-Santos AR, Morais M, Bessa JM, Sousa N, Pinto L. (2014). The Sweet Drive Test: refining phenotypic characterization of anhedonic behavior in rodents. Front Behav Neurosci 8;74:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews K, Forbes N, Reid IC. (1995) Sucrose consumption as anhedonic measure following chronic unpredictable mild stress. Physiol Behav 57:241–248. [DOI] [PubMed] [Google Scholar]
- Mendelson SD, Pfaus JG. (1989) Level searching: A new assay of sexual motivation in the male rat. Physiol Behav 45:337–341. [DOI] [PubMed] [Google Scholar]
- Meredith GE, Pennartz CM, Groenewegen HJ. (1993) The cellular framework for chemical signalling in the nucleus accumbens. Prog Brain Res 99:3–24. [DOI] [PubMed] [Google Scholar]
- Meziane H, Ouagazzal AM, Aubert L, Wietrzych M, Krezel W. (2007) Estrous cycle effects on behavior of C57BL/6J and BALB/cByJ female mice: implications for phenotyping strategies. Genes Brain Behav 6:192–200. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN. (2004) Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav 3:303–314. [DOI] [PubMed] [Google Scholar]
- Mucha RF, Iversen SD. (1984) Reinforcing properties of morphine and naloxone revealed by conditioned place preferences: a procedural examination. Psychopharmacology (Berl) 82:241–247. [DOI] [PubMed] [Google Scholar]
- Nadler JJ, Moy SS, Dold G, Trang D, Simmons N, Perez A, Young NB, Barbaro RP, Piven J, Magnuson TR, Crawley JN. (2004) Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav 3:303–314. [DOI] [PubMed] [Google Scholar]
- National Research Council (1996) Guide for the care and use of laboratory animals. Washington, DC; National Academy Press. [Google Scholar]
- Nicola SM. (2007) The nucleus accumbens as part of a basal ganglia action selection circuit. Psychopharmacology (Berl) 191:521–550. [DOI] [PubMed] [Google Scholar]
- Ohgi Y, Futamura T, Kikuchi T, Hashimoto K. (2013) Effects of antidepressants on alternations in serum cytokines and depressive-like behavior in mice after lipopolysaccharide administration. Pharmacol Biochem Behav 103:853–859. [DOI] [PubMed] [Google Scholar]
- Perreault ML, Fan T, Alijaniaram M, O’Dowd BF, George SR. (2012) Dopamine D1-D2 receptor heteromer in dual phenotype GABA/glutamate-coexpressing striatal medium spiny neurons: regulation of BDNF, GAD67 and VGLUT1/2. PLOS One 7:e33348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M. (1977) Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther 229:327–336. [PubMed] [Google Scholar]
- Price KL, Middaugh LD. (2004) The dopamine D1 antagonist reduces ethanol reward for C57BL/6 mice. Alcohol Clin Exp Res 28:1666–1675. [DOI] [PubMed] [Google Scholar]
- Redrobe JP, Bourin M. (1998) Dose-dependent influence of buspirone on the activities of selective serotonin reuptake inhibitors in the mouse forced swimming test. Psychopharmacology (Berl) 138:198–206. [DOI] [PubMed] [Google Scholar]
- Renard CE, Fiocco AJ, Clenet F, Hascoet M, Bourin M. (2001) Is dopamine implicated in the antidepressant-like effects of selective serotonin reuptake inhibitors in the mouse forced swimming test? Psychopharmacology (Berl) 159:42–50. [DOI] [PubMed] [Google Scholar]
- Reynolds E. (1971) Urination as a social response in mice. Nature 234:481–483. [DOI] [PubMed] [Google Scholar]
- Reynolds SM, Berridge KC. (2002) Positive and negative motivation in nucleus accumbens shell: bivalent rostrocaudal gradients for GABA-elicited eating, taste “liking”/”disliking” reactions, place preference/avoidance, and fear. J Neurosci 22:7308–7320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogard M, Caboche J, Julien JF, Besson MJ. (1993) The rat nucleus accumbens: two levels of complexity in the distribution of glutamic acid decarboxylase (67 kDa) and preproenkephalin messenger RNA. Neurosci Lett 155:81–86. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Nestler EJ. (2013) The brain reward circuitry in mood disorders. Nat Rev Neurosci 14:609–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HD, Pierce RC. (2006) Cooperative activation of D1-like and D2-like dopamine receptors in the nucleus accumbens shell is required for the reinstatement of cocaine-seeking behavior in the rat. Neuroscience 142:451–461. [DOI] [PubMed] [Google Scholar]
- Smith RJ, Lobo MK, Spencer S, Kalivas PW. (2013) Cocaine-induced adaptations in D1 and D2 accumbens projection neurons (a dichotomy not necessarily synonymous with direct and indirect pathways). Curr Opin Neurobiol 23:546–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Štefánik P, Olexová L, Kršková L. (2015) Increased sociability and gene expression of oxytocin and its receptor in the brains of rats affected prenatally by valproic acid. Pharmacol Biochem Behav 131:42–50. [DOI] [PubMed] [Google Scholar]
- Steru L, Chermat R, Thierry B, Simon P. (1985) The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl) 85:367–370. [DOI] [PubMed] [Google Scholar]
- Vialou V, Feng J, Robison AJ, Nestler EJ. (2013) Epigenetic mechanisms of depression and antidepressant action. Annu Rev Pharmacol Toxicol 53:59–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viitamaa T, Haapalinna A, Agmo A. (2006) The adrenergic α2 receptor and sexual incentive motivation in male rats. Pharmacol Biochem Behav 83:360–369. [DOI] [PubMed] [Google Scholar]
- Willner P. (1991) Animal models as simulations of depression. Trends Pharmacol Sci 12:131–136. [DOI] [PubMed] [Google Scholar]
- Willner P. (1997) Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology (Berl) 134:319–329. [DOI] [PubMed] [Google Scholar]
- Zahm DS. (2000) An integrative neuroanatomical perspective on some subcortical substrates of adaptive responding with emphasis on the nucleus accumbens. Neurosci Biobehav Rev 24:85–105. [DOI] [PubMed] [Google Scholar]
- Zhang JC, Wu J, Fujita Y, Yao W, Ren Q, Yang C, Li SX, Shirayama Y, Hashimoto K. (2015) Antidepressant Effects of TrkB Ligands on Depression-Like Behavior and Dendritic Changes in Mice After Inflammation. Int J Neuropsychop, in press. doi:10.1093/ijnp/pyu077 [DOI] [PMC free article] [PubMed] [Google Scholar]