Abstract
Discoveries associated with antibacterial activity of hydrated clays necessitate assessments of in vivo efficacy, practical use and safety. Surface properties of clays can lead to variations in the composition and abundance of bound compounds or ions, thus affecting antibacterial activity. Since exchangeable metal ions released from the clay surface are responsible for in vitro antibacterial activity, we evaluated the in vivo antibacterial efficacy of four natural clays (one illite clay, two montmorillonite clays and one kaolinite clay) and three ion-exchanged, antibacterial clays against superficial, cutaneous meticillin-resistant Staphylococcus aureus (MRSA) infections in mice. Superficial, cutaneous wounds on the back of SKH1-Elite mice were generated and subsequently infected with MRSA. Following twice daily applications of a hydrated clay poultice to infected wounds for 7 days, we observed significant differences in the in vivo antibacterial efficacy between different types of clays. The natural and ion-exchanged illite clays performed best, as measured by bacterial load, inflammatory response and gross wound morphology with significant decreases in bacterial viability and dermatitis. Topical application of kaolinite clay was the least effective, resulting in the lowest decrease in bacterial load and exhibiting severe dermatitis. These data suggest that specific types of clays may offer a complementary and integrative strategy for topically treating MRSA and other cutaneous infections. However, since natural clays exhibit in vitro antibacterial variability and vary vastly in surface chemistries, adsorptive/absorptive characteristics and structural composition, the properties and characteristics of illite clays could aid in the development of standardized and customized aluminosilicates for topical infections.
Introduction
Community-associated meticillin-resistant Staphylococcus aureus (CA-MRSA) infections represent a public health threat, causing serious and sometimes fatal infections in otherwise healthy individuals. While rates of hospital-associated MRSA (HA-MRSA) infections in the US have declined over the past decade, CA-MRSA infections have risen in the past two decades and now represent a significant portion of the disease burden (David & Daum, 2010; Prabaker & Weinstein, 2011). CA-MRSA and HA-MRSA strains cause distinct clinical manifestations and affect different patient populations. HA-MRSA strains, which have circulated in healthcare settings for decades, are associated with pneumonia, bacteraemia and other invasive infections in patients with co-morbid illnesses in healthcare facilities (Mulligan et al., 1993). While CA-MRSA usually causes skin and soft tissue infections in otherwise healthy individuals (Watkins et al., 2012), more severe, invasive diseases may occur and can include necrotizing pneumonia (CDC, 2007; Kwong et al., 2012), pyomyositis and necrotizing fasciitis (Miller et al., 2005; Pannaraj et al., 2006), sepsis (Fortunov et al., 2006), osteomyelitis (Bocchini et al., 2006) or septic arthritis (Arnold et al., 2006).
Vancomycin and daptomycin are the antibiotics of choice for invasive MRSA infections. However, strains of vancomycin-non-susceptible S. aureus are emerging (Liu et al., 2011), and daptomycin is primarily used for the treatment of complicated skin and soft tissue infections and has been associated with eosinophilic pneumonia (Kim et al., 2012). The limited treatment options, in addition to the increase in tissue-destructive S. aureus infections with the emergence of CA-MRSA strains (Chambers & Deleo, 2009; Kennedy et al., 2010), exemplify the need for complementary and integrative strategies to combat these infections. Clays have been used for medicinal applications throughout recorded history to treat such ailments as diarrhoea, dysentery, tapeworm, hookworm, wounds and abscesses (Nunn, 2002). However, use of medicinal clays has fallen out of favour in Western cultures, and despite historical practice, many of the medically related applications have not yet been scientifically validated. Here, we investigate the use of four natural and three ion-exchanged clay minerals for the treatment of cutaneous MRSA infections in mice.
Clays are small phyllosilicate minerals that are commonly grouped into three main classes – smectite, illite and kaolinite – based on their overall crystal layer structure and expandability. Smectite minerals have a large cation exchange capacity (CEC) and exhibit high expansion (swelling) capabilities in the presence of water. Smectites are often referred to as ‘swelling’ or ‘expandable’ clays as these minerals are the most expandable of all clays (Murray, 2006). Montmorillonite clay, a member of the smectite group, is used for numerous industrial applications and commercial products including drilling, foundry sand binders, cat litter, animal feed, cements and ceramics (Murray, 2006). Conversely, illite is a non-expanding clay that has a lower CEC than smectite clays, but a generally greater CEC than that of kaolinite. The ‘swelling’ property of clays is determined by the ability of cations to retain their polar molecule ‘shell’ within the interlayer environment (MacEwan & Wilson, 1980).
Clays have a net negative surface charge, which allows the free exchange of particles from the environment, including bacteria, viruses, proteins, nucleic acids and cations (McLaren, 1963). In hydrated environments, the ionic species adsorbed to clay surfaces can be exchanged into the surrounding medium in a manner that depends on the ionic strength of the aqueous medium and cation selectivity of the clay (Velde, 1995). The in vitro antibacterial activities of the natural clays are associated with the generation of a low pH environment and the chemical desorption of ions from the surface of the clay particles (Cunningham et al., 2010). Moreover, aqueous clay mixture extracts (leachates) exhibit antibacterial activity (Cunningham et al., 2010). Antibacterial clay mixtures supplemented with desferrioxamine, a metal chelator that sequesters Fe, Co, Cu, Ni and Zn ions, rescues Escherichia coli from clay-mediated killing (Cunningham et al., 2010). These five exchangeable ions were subsequently shown to be responsible for in vitro antibacterial activity of natural clays (Otto & Haydel, 2013). Synthetic microbicidal mixtures (SMMs), which are aqueous solutions that contain low concentrations of Fe, Co, Cu and Zn metal chloride salts, exhibit in vitro antibacterial activity (Otto et al., 2014). We used SMMs to ion-exchange natural, non-antibacterial clays and determined that the ion-exchanged clays were imparted with in vitro antibacterial activity. In this study, we evaluated the in vivo antibacterial activity of natural and ion-exchanged clays against MRSA skin infections in mice. To our knowledge, these different clays have not been individually assessed for their respective in vivo antibacterial activity. Moreover, the role of the physical and chemical properties of clays has not been independently evaluated in vivo.
Methods
Bacterial strains and growth conditions
The MRSA strain was obtained from Quest Laboratories (Haydel et al., 2008) and was grown on trypticase soy agar or in trypticase soy broth (TSB). The genome of this bacterial strain is currently being sequenced and is tentatively classified as an MRSA USA300 isolate due to the presence of Panton-Valentine leukocidin (PVL) genes (Medrano and Haydel, unpublished data). Cultures were grown overnight for 14–18 h at 37 °C with gentle rotary mixing. Prior to use, the cells were pelleted via centrifugation for 5 min at 13 000 g, washed with sterile saline (0.85 % NaCl), pelleted via centrifugation for 5 min at 13 000 g and suspended in sterile saline.
Clay mixtures and clay leachate preparation
Clay mixtures were autoclaved for 1 h at 121 °C before experimental use. A 10 % clay suspension refers to 0.1 g of clay mixtures mixed in 1 ml sterile UV-irradiated, ultra-pure H2O (dH2O) (Otto & Haydel, 2013). Leachates (CB-L) were obtained by continuously stirring clay mixtures (1 g per 20 ml) in sterile dH2O for 18–24 h. Subsequently, the hydrated clay mixture suspensions were centrifuged (31 000 g) for 3 h at 4 °C to separate insoluble and soluble fractions. The aqueous supernatant (leachate) was collected and sterilized by passage through a 0.22 μm filter.
Antibacterial susceptibility testing
Exponential-phase MRSA cultures were prepared by diluting overnight cultures into fresh TSB to a concentration of 107 c.f.u. ml− 1 and continuing growth at 37 °C with gentle rotary mixing until the cultures reached mid-exponential phase of growth (OD600 0.4–0.6). Bacterial cells were collected by centrifugation, washed once in sterile saline and suspended in the appropriate clay mixture leachate solution, clay mixture (at 1 % or 10 %, w/v) or sterile dH2O at an initial concentration of 107 c.f.u. ml− 1. Samples were incubated at 37 °C with gentle rotary mixing for 24 h and cell survival was determined by plating duplicate 10-fold serial dilutions (100 μl each) for each sample at 0 and 24 h and enumerating colonies on plates after overnight incubation at 37 °C. Due to sample processing, the indicated 0 h experimental exposure time was equivalent to an initial 2–3 min exposure. All experiments were completed in triplicate. The antibacterial activity of the leachates was previously determined and published elsewhere (Otto & Haydel, 2013; Otto et al., 2014).
Ion-exchanged clay preparation
Clays subjected to ion exchange were purchased from commercial suppliers (kaolinite from Sigma-Aldrich; EC12 illite and DV12 montmorillonite from Argiletz) and were autoclaved for 1 h at 121 °C before experimental use. Ion-exchanged (IE) clays [IE-kaolinite (IE-K); IE-EC12; IE-DV12] were prepared by suspending clay (5 %, w/v) in SMM16 (200 μM FeCl3, 4 μM CoCl2, 80 μM CuCl2 and 40 μM ZnCl prepared in sterile dH2O) (Otto et al., 2014) and mixing at 300 r.p.m. at room temperature for 20–24 h. The clay suspensions were subsequently centrifuged (31 000 g) for 3 h at 4 °C to separate insoluble and soluble fractions. The solid fraction was dried for 24 h at 60 °C, ground to a fine powder with a mortar and pestle and sterilized by autoclaving prior to further use.
ICP-MS of ion-exchanged clays
Measurements of the elemental concentrations in the leachate samples were determined using inductively coupled plasma mass spectrometry (ICP-MS) as previously described (Otto & Haydel, 2013). Table 1 summarizes the settings used for the ICP-MS analyses.
Table 1. ICP-MS settings used to analyse the natural and ion-exchanged clays.
| Parameter | Setting |
|---|---|
| RF power (w) | 1450 |
| Dwell time (ms) | 50 |
| Sweeps per replicate | 40 |
| No. of replicates | 3 |
| Acquisition mode | Peak hopping |
| Argon flow rates (l min− 1): | |
| Nebulizer flow | 0.95 |
| Coolant | 15 |
| Auxiliary | 1.3 |
| Sample uptake (ml min− 1) | 0.400 |
| Presence of oxides as CeO/Ce | < 3 % |
| Presence of doubly charged species (as Ba2+/Ba) | < 3 % |
| Nebulizer type | PFA-ST |
| Spray chamber | Cyclonic quartz |
| Sample and skimmer cones | Pt |
Scalpel-scraping infection model
Six- to eight-week-old female SKH1-Elite mice (Charles River Laboratories, Wilmington, MA, USA) were used for all experiments. The mice were anaesthetized by intraperitoneal injection of 0.05 ml per 25 g of body weight with a mixture containing 21 mg ketamine, 2.4 mg xylazine and 0.3 mg acepromazine. An area of approximately 2 cm2 on the backs of the mice, immediately behind the shoulder blades, was gently scraped with a scalpel blade, taking care not to cut the skin. Following this procedure, the skin became visibly damaged and was characterized by reddening and glistening, but did not bleed. After scraping the skin, a bacterial infection was initiated by inoculating 105 c.f.u. in a 10 μl volume onto the damaged skin and gently spreading the inoculum over the entire surface of the wound. The mice were housed in groups of 3–5 per cage. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. All animal procedures were approved by the Arizona State University Institutional Animal Care and Use Committee (protocols 09-1030R and 12-1240R) and conducted according to relevant national and international guidelines.
Treatment of infected wounds
The first treatment of the wounds occurred at 4 h post-infection. Thereafter, beginning at 16 h after the first treatment, topical treatments were administered twice daily (morning and evening, 8 h interval) for 7 days. For each treatment, 100 to 200 mg (estimated by weighing the pellet of clay on a spatula) of hydrated clay was applied. The topically treated wounds were covered with a 1.5 cm × 1.5 cm square piece of Tegaderm bandage so that the mice were not able to disturb the wound. Twice daily, the wounds were gently swabbed with a sterile, cotton-tipped applicator hydrated with sterile saline, prior to adding the treatment and new bandage. Each day, one of these swabs was added to 1 ml of sterile saline, vortexed for 10–20 s and used to determine c.f.u. ml− 1 of the wound surface. The clays used in this study are as follows: CB10 (Otto & Haydel, 2013), EC12, DV12, kaolinite, (IE)-IC-EC12, IE-DV12 and IE-K. In addition to experimental treatment groups, control groups of mice included (i) a group that was not infected and not treated, (ii) a group that was not infected and treated with each experimental clay sample, (iii) a group that was infected and not treated and finally, (iv) a positive control treatment group that was infected and treated with neosporin triple antibiotic ointment. The number of mice included in each group: no treatment, 13; neosporin triple antibiotic (Abx) ointment, 12; CB10, 13; EC12, 10; IE-EC12, 10; DV12, 10; IE-DV12, 9; kaolinite, 6; IE-K, 10.
Blood and skin sample preparation
Mice were euthanized by CO2 asphyxiation followed by cardiac puncture exsanguination. The collected blood was immediately reserved in 10 μl of 1U μl− 1 heparin, followed by diluting and plating to determine the bacterial load. Immediately after the mice were euthanized, the wounds (approximately 2 cm2) were excised and stored at 4 °C for a maximum of 3 h. Skin samples were divided in half for histology analyses and c.f.u. measurements. Samples for histological analyses were submerged in 10 % neutral buffered formalin. The formalin-fixed specimens were processed and embedded in paraffin, cut into 4 μm-thick sections and subsequently stained with haematoxylin and eosin (H&E). Samples for c.f.u. measurements were weighed (50–200 mg), placed in microcentrifuge tubes containing five 1.6 mm stainless steel beads (Next Advance) and homogenized for 10 min at 4 °C in a Bullet Blender (BBX24, Imgen Technologies). The samples were then diluted and plated onto mannitol salt agar supplemented with 1 μg ampicillin ml− 1 and incubated overnight at 37 °C to determine total c.f.u. g− 1. The calculations were reported as c.f.u. g− 1 to account for the variances in skin weights of each sample.
Histological examination
A board-certified veterinary pathologist, who was blinded to sample source, read and analysed the slides. We developed an easy-to-follow scoring system where dermatitis was assessed as mild, 1; moderate, 2; severe, 3; or marked, 4. The dermatitis severity criteria were as follows: 1) mild – focal or few foci with inflammation confined to the superficial dermis; 2) moderate – multiple foci with inflammation extending to the deep dermis; 3) severe – regionally extensive foci with inflammation occasionally extending to the subcutis; and 4) marked – same as severe, but with inflammation extending to the subcutis through a large portion of the lesion, notable ulceration, down-growth of the rete pegs and hyperkeratosis.
Statistical analyses
We used repeated measures one-way anova and Tukey's multiple comparisons test to assess statistical significance. Each P-value was adjusted to account for multiple comparisons of mean values across all groups. Two skin samples (one Day 0 inoculum control and one Day 7 EC12 sample) and two swab samples (one Day 4 IE-EC12 sample and one Day 6 EC12 sample) were removed from the analysis due to sampling/processing error and inaccurately measured concentrations of 0. Data were analysed using GraphPad Prism 6 with boxplots showing 25th to 75th percentiles and Tukey whiskers.
Results
ICP-MS of ion-exchanged clays
After ion-exchanging the three different clays, we performed ICP-MS to confirm that the clays had successfully been exchanged and to determine the magnitude of this exchange across the samples. ICP-MS analyses determined that the concentration of Fe, Co, Cu and Zn in IE-K increased by 23.8-, 82.0-, 1.74- and 20.5-fold, respectively (Table 2). In the IE-EC12 samples, the concentration of Fe, Co, Cu and Zn increased by 1.1-, 1.8-, 6.7- and 2.0-fold, respectively. Finally, in the IE-DV12 samples, the Fe, Co and Zn concentrations decreased while the Cu concentration increased by 9.3-fold (Table 2).
Table 2. Summary of ion concentrations of EC12, DV12 and kaolinite clays before and after ion exchange.
| Ion | EC12 | DV12 | Kaolinite | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Before [μg g− 1] | After [μg g− 1] | Fold change | Before [μg g− 1] | After [μg g− 1] | Fold change | Before [μg g− 1] | After [μg g− 1] | Fold change | |
| Fe | 10895 | 11797 | 1.1 | 14587 | 79.3.0 | – | 506.0 | 12061 | 23.8 |
| Co | 10.09 | 19.92 | 2.0 | 10.11 | 3.48 | – | 0.2 | 16.25 | 81.3 |
| Cu | 20.62 | 138.69 | 6.7 | 17.09 | 158.39 | 3.5 | 74.14 | 128.75 | 1.7 |
| Zn | 54.87 | 11.34 | – | 45.42 | 34.62 | – | 5.31 | 109.0 | 20.5 |
In vitro antibacterial susceptibility testing
After ion-exchanging the three different clay samples with an antibacterial mixture of metal ion salts (SMM16) (Otto et al., 2014), we assessed the in vitro antibacterial activity of the IE clays and the natural clays (non-IE clays). The SMM16 solution alone was used as a control and exhibited a 2-log10 decrease in viability of MRSA after 24 h (Fig. 1) (Otto et al., 2014). Natural kaolinite did not display antibacterial activity, exhibiting an average 0.4-log10 unit decrease in viability after 24 h, a lesser decrease than the H2O-only control (Fig. 1). IE-K caused an average 5.2-log10 unit decrease in MRSA viability after 24 h exposure, a statistically significant reduction (P = 0.0011) compared with natural kaolinite (Fig. 1). Although ion-exchanged EC12 (IE-EC12) enhanced killing compared with natural EC12, the decrease in viability (0.5-log10 units) was not statistically significant (Fig. 1). Ion-exchanged DV12 (IE-DV12) enhanced MRSA killing with an average 6.5-log10 unit decrease in viability compared with DV12, with an average 4.2-log10 unit decrease (Fig. 1), but the difference was not statistically significant (P = 0.2338).
Fig. 1.
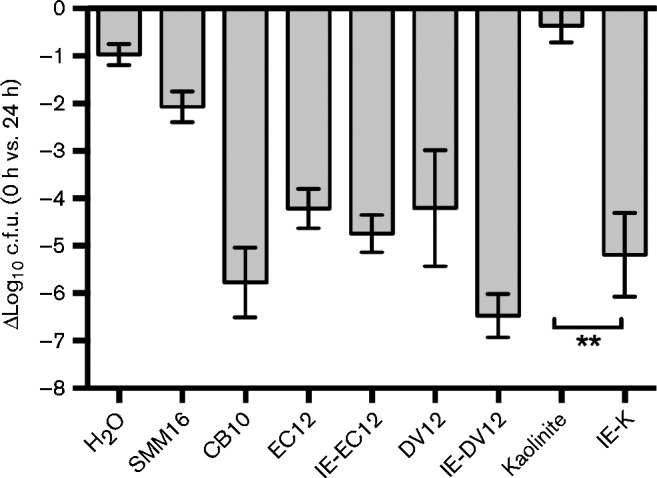
Viability of MRSA following in vitro exposure to SMM16, natural clays and IE clays. Differential c.f.u. counts at 0 h and 24 h of control and experimental exposures are shown. Error bars represent the sem from at least three independent experiments. **, P = 0.0011 in a Tukey's multiple comparison test.
Bacterial load in wound swabs after 7 days of topical, hydrated clay treatment
During the course of treatment, swabs were collected daily to determine the bacterial load on the wound surface. Natural healing, as measured by the no-treatment group, resulted in a 1.4-log10 average decrease in bacterial load after 7 days (Fig. 2a). Mice treated with triple antibiotic ointment (positive treatment control group) and CB10 exhibited average 3.4-log10 and 3.2-log10 decreases, respectively, in bacterial load after 7 days of treatment (Fig. 2a). Treatment with natural kaolinite was the least effective treatment of all the clays, with a 1.4-log10 c.f.u. reduction, while IE-K treatment further reduced the bacterial load and exhibited a 2.4-log10 decrease in bacterial load after 7 days (Fig. 2a). Natural DV12 and IE-DV12 exhibited similar reductions, of 2.5-log10 and 2.4-log10, respectively, after 7 days of treatment (Fig. 2a). Finally, EC12 and IE-EC12 resulted in average 3.1-log10 and 2.5-log10 decreases, respectively, in bacterial load after 7 days (Fig. 2a). Compared with the inoculum control, all treatments reduced the bacterial load after 7 days of treatment (Fig. 2a). However, compared with natural healing with no treatment, only mice treated for 7 days with triple antibiotic ointment (positive treatment control group), CB10 and EC12 exhibited significant average 2.0-log10 (P = 0.0002), 1.9-log10 (P = 0.0005) and 1.7-log10 (P = 0.0058), respectively, c.f.u. reductions (Fig. 2a).
Fig. 2.
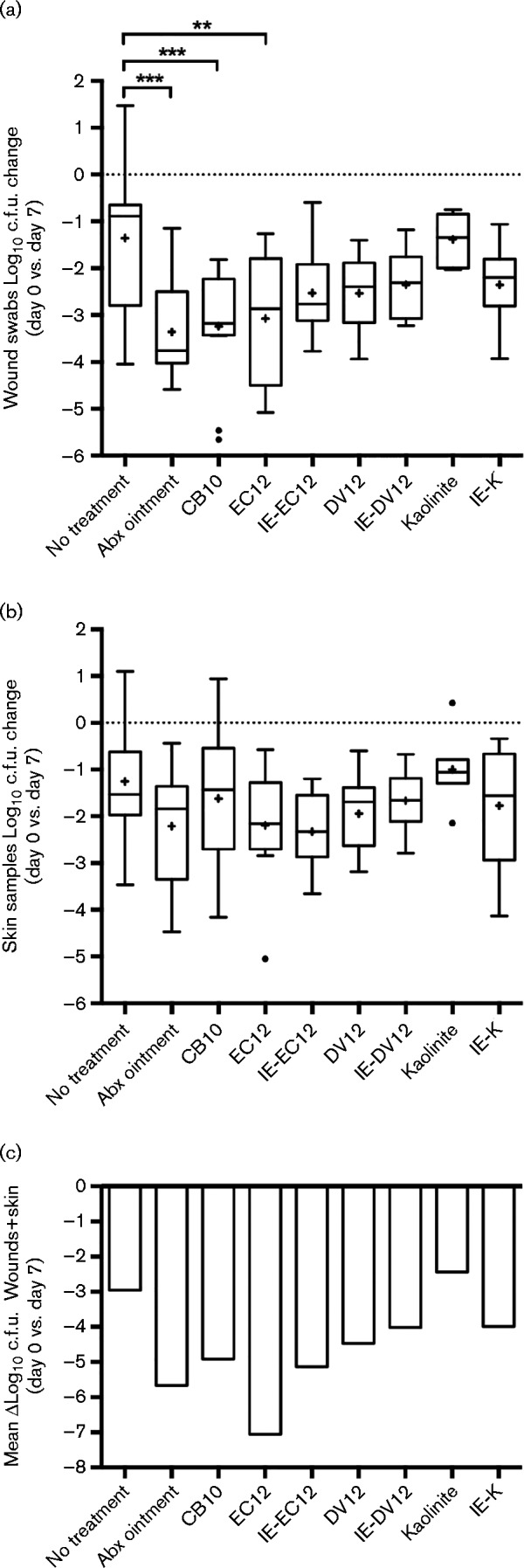
Recovery of viable MRSA cells from (a) wound swabs and (b) skin samples after 7 days of infection (no treatment), triple antibiotic ointment (Abx) application or experimental clay treatment. (a) Log10 c.f.u. fold change of MRSA cells recovered from wound swabs and (b) log10 c.f.u. g− 1 fold change of MRSA cells cultured from skin samples after 7 days of clay treatment. (c) The total log10 reduction in MRSA cells collected from wound swabs (mean value) (a) and skin samples (mean value) (b). Boxplots with median lines extend from the 25th to 75th percentiles and show Tukey whiskers. The dotted lines represent the normalized value of MRSA cells collected 4 h post-infection (inoculum control; day 0). The ‘+’ symbols represent the mean for each group. Outliers are displayed as filled circles. ***, P < 0.001; **, P = 0.0058.
Bacterial load in skin samples after 7 days of topical, hydrated clay treatment
To determine the bacterial load in the skin, we collected skin samples on day 0 from the inoculum control group and from all other mice after the seven-day course of treatment. Similar to the wound swabs, natural healing, as measured by the no-treatment group, resulted in a 1.3-log10 average decrease in skin samples after 7 days (Fig. 2b). Compared with the inoculum control group there was a decrease in bacterial load in all skin samples (Fig. 2b), albeit less than the average c.f.u. decreases from wound swabs (Fig. 2a). Treatment with triple antibiotic ointment, EC12 and IE-EC12 exhibited the greatest decreases in MRSA skin colonization with similar reductions of 2.2-log10, 2.2-log10 and 2.3-log10 c.f.u. g− 1, respectively (Fig. 2b). Conversely, compared with no treatment, kaolinite treatment caused a slight increase in the average bacterial load (Fig. 2b). Despite natural in vitro antibacterial activity and c.f.u. reduction in wound swabs, CB10 treatment only decreased MRSA colonization of skin samples by an average of 0.4-log10 c.f.u. compared with no treatment (Fig. 2b). Compared with natural healing with no treatment, none of the treatments, including triple antibiotic ointment, resulted in statistically significant reductions in MRSA skin colonization (Fig. 2b). To assess the general trends of the experimental treatments, we combined the mean c.f.u. values from the wound swabs and skin samples to determine overall reduction in MRSA wound colonization (Fig. 2c). Although statistical significance could not be determined from mean c.f.u. reductions, EC12 treatment resulted in the largest differential c.f.u. reduction, with an approximately 4.1-log10 decrease compared with no treatment and 1.4-log10 decrease compared with triple antibiotic ointment (Fig. 2c).
Bacterial burden in the blood after 7 days of clay treatment
We recovered less than 10 c.f.u. ml− 1 from blood samples of mice that were not treated or were treated with triple antibiotic ointment or CB10 for 7 days (data not shown). No MRSA cells were recovered from blood samples collected from mice treated with other natural or ion-exchanged clays (data not shown).
Wound observations and histology
We observed macroscopic changes to wounds following treatment with the clays. After 7 days, wounds in the no-treatment group developed a soft scab. All of the clay-treated samples had noticeable acanthosis, or thickening of the skin, with the EC12 and IE-EC12 samples being the least severe and the DV12 and IE-DV12 samples being the most severe (Fig. 3). Table 3 shows a summary of dermatitis scores and rankings from pathological examinations of collected skin samples. The control mice that were neither wounded nor infected, but were treated with clay for 7 days, showed no to mild dermatitis (data not shown). Mice that were wounded and infected, but not treated, had severe dermatitis (Fig. 3d, Table 3). Wound treatment with triple antibiotic ointment resulted in moderate dermatitis (Fig. 3f, Table 3), while CB10 treatment resulted in markedly severe dermatitis (Fig. 3g, h, Table 3). Treatment with IE-EC12 clay resulted in moderate dermatitis (Fig. 3l, Table 3), while EC12 treatment caused mild–moderate dermatitis (Fig. 3j, Table 3), representing a significant reduction in dermatitis compared with no treatment (Table 3). DV12 and IE-DV12 treatment resulted in moderate and severe dermatitis, respectively (Fig. 3n, p, Table 3). Kaolinite clay treatment resulted in markedly severe dermatitis, more severe than the no-treatment group (Fig. 3r, Table 3), while IE-K treatment resulted in moderate dermatitis (Fig. 3t, Table 3).
Fig. 3.
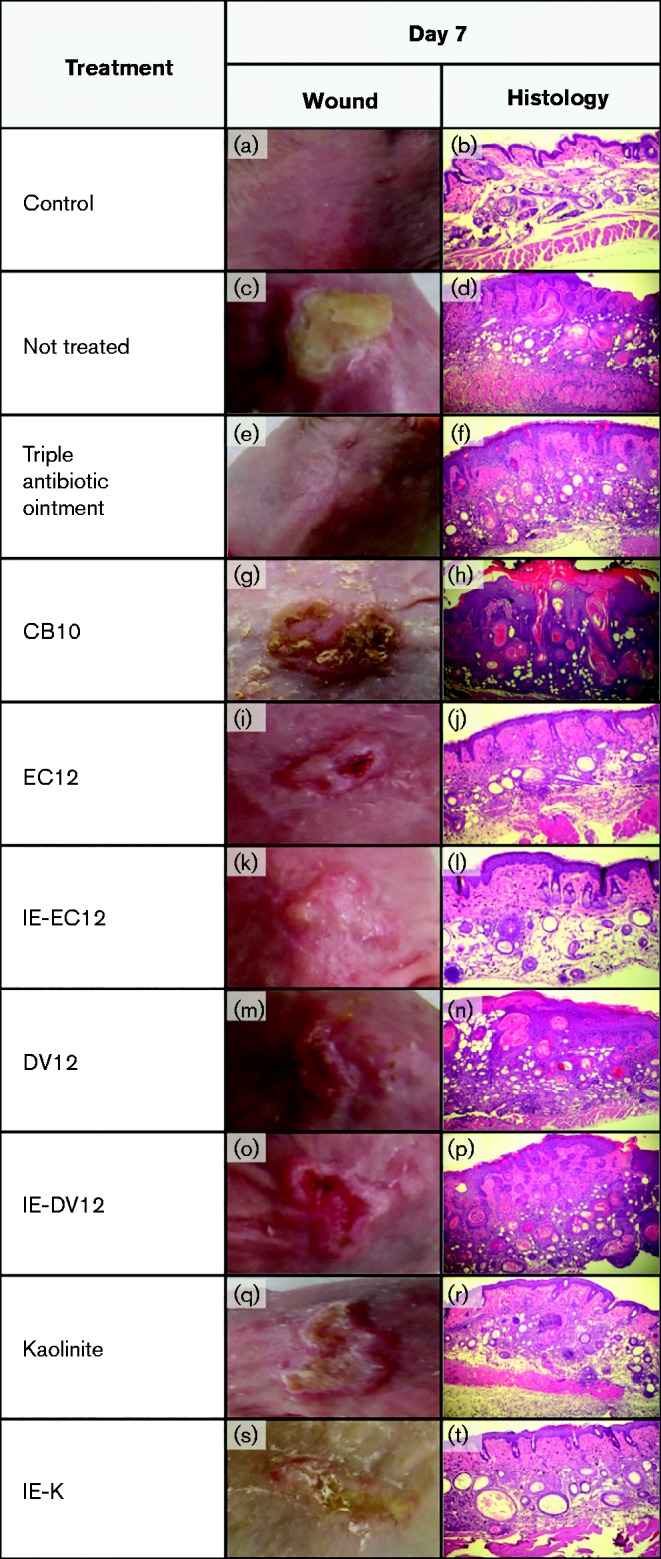
Macroscopic appearance of infected wounds following 7 days of treatment with triple antibiotic ointment (e), CB10 (g), EC12 (i), IE-EC12 (k), DV12 (m), IE-DV12 (o), kaolinite (q) and IE-K (s). Histological appearance (magnification × 400) of wounds following 7 days of treatment with triple antibiotic ointment (f), CB10 (h), EC12 ( j), IE-EC12 (l), DV12 (n), IE-DV12 (p), kaolinite (r) and IE-K (t). Skin specimens were taken immediately after the termination of the experiment, fixed in formalin and embedded in paraffin. The biopsy specimens were H&E stained. All mice, except for the control mice, were wounded and infected with MRSA as described in Methods. Control mouse skin samples were collected on day 0, prior to wounding, infection or initiating any treatments.
Table 3. Summary of dermatitis scores and rankings following 7 days of treatment with natural and IE clays.
| Treatment | Dermatitis score | Dermatitis ranking | Significance |
|---|---|---|---|
| No treatment | 3 ± 0.45 | Severe | NS |
| Triple antibiotic ointment | 2.33 ± 0.88 | Moderate | NS |
| CB10 | 3.67 ± 0.33 | Marked | NS |
| EC12 | 1.7 ± 0.33 | Mild–moderate | * |
| IE-EC12 | 2.1 ± 0.56 | Moderate | NS |
| DV12 | 2.17 ± 0.4 | Moderate | NS |
| IE-DV12 | 2.83 ± 0.48 | Severe | NS |
| Kaolinite | 3.8 ± 0.2 | Marked | NS |
| IE-K | 2.2 ± 0.58 | Moderate | NS |
NS, not significant; *P = 0.037.
Discussion
We have previously demonstrated that the primary in vitro antibacterial mechanism of action of clays is dependent upon soluble metal ions, which are desorbed from the surface of the clays when hydrated (Cunningham et al., 2010; Otto & Haydel, 2013; Otto et al., 2014). While clays have previously been used for the treatment of wounds, little is known about the in vivo mechanism of action and a comprehensive study has not yet been completed to assess the antibacterial characteristics of different types of clays. Here, we aimed to investigate if different clays with in vitro antibacterial activity would yield in vivo efficacy against topical MRSA infections.
Control mice that were neither wounded nor infected, but were treated with daily topical applications of hydrated clay, exhibited mild dermatitis, while mice that were wounded and infected, but were not treated, exhibited severe dermatitis. Thus, any changes or reductions in dermatitis severity were due to the clay treatment and not to the natural state of the skin or the MRSA infection itself. The DV12 montmorillonite clay, IE-DV12 and CB10, composed of approximately 14 % montmorillonite and 37 % illite-smectite clays (Otto & Haydel, 2013), performed poorly. For example, although CB10 resulted in a mean 1.9-log10 unit decrease in cells from the wound surface compared with no-treatment controls (Fig. 2a), there was only an average 0.4-log10 unit decrease observed in the skin samples (Fig. 2b). These data revealed that MRSA colonization of deeper sections of the skin had occurred and clay treatment was unable to kill or remove these cells from infected tissues. Moreover, daily CB10 treatment resulted in the most severe dermatitis (Fig. 3, Table 3). Compared with no-treatment controls, topical treatment with hydrated kaolinite resulted in no or minimal change in bacterial load (Fig. 2) and stimulated the most severe dermatitis (Fig. 3).
MRSA cells recovered from wound swabs were considerably fewer than the number of cells recovered from skin samples collected at the end of the study. This difference in recovery could be an artefact of sampling, since final sterile saline cleansing and swabbing of the wounds was carried out prior to recovering the skin samples. Additionally, during active swabbing, residual clay was often collected. Given the in vitro antibacterial activity of the leachates and SMM16, it is possible that some of the recovered organisms were killed prior to plating. Additionally, S. aureus because is known to invade host tissue and survive intracellularly (Fraunholz & Sinha, 2012), discrepancies in recovery could be due to the inaccessibility of the organisms in the wounds during swab collections. Lastly, it is possible that in vivo efficacy is associated with physical removal of the organisms from the wound. In this scenario, the more accessible organisms would be physically sequestered and removed by the clay, while the organisms in the deeper layers of tissue would be inaccessible. Given that MRSA USA300 strains often cause skin and soft tissue infections (David & Daum, 2010), we note that samples of blood collected from control (no treatment) mice and mice treated with triple antibiotic ointment or CB10 showed an average of < 10 c.f.u. ml− 1. Considering the lack of MRSA present in the blood collected from mice receiving treatment with kaolinite, IE-K, EC12, IE-EC12, DV12 and IE-DV12 and the low number of cells recovered from a limited number of mice in the control (no treatment), triple antibiotic ointment or CB10 treatment groups, we conclude that MRSA did not readily get into the bloodstream after superficial wound infection and topical treatment with hydrated clays.
Notably, the in vitro antibacterial activity of the minerals was superior compared with the in vivo antibacterial activity. Furthermore, in vitro antibacterial activity is dependent on a low pH environment and is attenuated upon the addition of metal ion chelators (Cunningham et al., 2010; Otto & Haydel, 2013; Otto et al., 2014). While ion-exchanging the clays enhanced the in vitro antibacterial activity, there was no significant difference in the in vivo efficacy of the natural and IE clays. The composition of wound exudates is complex, strongly buffered and contains numerous compounds capable of chelation (Trengove et al., 1996; Bal et al., 2013). While nickel, cobalt and chromium permeate damaged full-thickness human skin (epidermis and dermis) in an in vitro diffusion cell system (Filon et al., 2009), it is unclear whether the metal ions present in natural or ion-exchanged clays penetrate into the deeper tissue layers to effect antibacterial activity. Although we cannot completely rule out a chemically derived component associated with in vivo antibacterial efficacy, in vivo benefits appear to be dependent on the physical properties of the clays rather than the ions present on the surface. Taken together with the intracellular lifestyle of S. aureus, numerous factors could contribute to decreased in vivo efficacy compared with in vitro antibacterial activity.
We observed significant differences in the in vivo antibacterial efficacy between the different types of clays. The hydrated EC12 illite clay performed the best as measured by decrease in bacterial load, inflammatory response and gross wound morphology. Illite clays have a moderate ion-exchange capacity and are non-swelling as compared with montmorillonite clays, which are highly absorptive, and kaolinite clays that are minimally absorptive. Notably, the least absorptive clay, kaolinite, was, on average, the least effective of all the clays, resulting in the lowest decrease in bacterial load in the swab and skin samples and exhibiting severe dermatitis.
Wound management largely depends on the characteristics of a particular wound. For example, guidelines established by the Association for the Advancement of Wound Care (AAWC) recommend covering wounds producing large amounts of exudate with absorbent material and hydrating dry wounds with hydrogel preparations (Bolton et al., 2014). A limitation of the work presented here is that we have only considered one wound type. If clays are to be used for the treatment of cutaneous infections, it will be important to address their efficacy for treating various types of skin lesions, such as lacerations, cellulitis or ulcers. In this study, the wounds produced a large amount of exudate for the first 2–3 days after infection. Therefore, according to AAWC guidelines, a layered treatment approach might be necessary whereby absorbent clays are used initially, followed by hydration for the remainder of the treatment.
Natural clays exhibit variability in in vitro antibacterial activity (Fig. 1) (Otto & Haydel, 2013), exhibit chemical variability (Otto & Haydel, 2013), lack structural homogeneity, may contain toxic heavy metals (Cd, As and Pb) (Otto & Haydel, 2013) and display variable in vivo efficacy (Figs 2 and 3). Therefore, investigations of alternative aluminosilicates which offer a) consistent composition, b) defined structure, c) defined chemical composition, d) purity, e) quality assurance, f) consistent and controlled antibacterial activity and g) biocompatibility and safety are necessary for complementary and integrative biomedical applications. Using the comprehensive information gleaned from this study and our previous investigations, we are investigating customized nanostructured and porous aluminosilicates, which offer standardized compositions and controlled antibacterial efficacy, for treatment of topical bacterial infections.
Acknowledgements
This research was supported by Public Health Service grant AT004690 awarded to S. E. H. from the NIH National Center for Complementary and Alternative Medicine. We thank Science Foundation Arizona for graduate research fellowship support for C. C. O, G. Wallstrom for assisting with statistical analyses and J. Pattengill and R. Marler for performing the histological sample preparation and analyses.
Abbreviations:
- CA-MRSA
community-associated MRSA
- CEC
cation exchange capacity
- HA-MRSA
hospital-associated MRSA
- ICP-MS
inductively coupled plasma mass spectrometry
- IE
ion-exchanged
- IE-K
ion-exchanged kaolinite
- MRSA
meticillin-resistant Staphylococcus aureus
- SMM
synthetic microbicidal mixture.
References
- Arnold S. R., Elias D., Buckingham S. C., Thomas E. D., Novais E., Arkader A., Howard C. (2006). Changing patterns of acute hematogenous osteomyelitis and septic arthritis: emergence of community-associated methicillin-resistant Staphylococcus aureus J Pediatr Orthop 26 703–708 10.1097/01.bpo.0000242431.91489.b4 . [DOI] [PubMed] [Google Scholar]
- Bal W., Sokołowska M., Kurowska E., Faller P. (2013). Binding of transition metal ions to albumin: sites, affinities and rates Biochim Biophys Acta 1830 5444–5455 10.1016/j.bbagen.2013.06.018 . [DOI] [PubMed] [Google Scholar]
- Bocchini C. E., Hulten K. G., Mason E. O., Jr, Gonzalez B. E., Hammerman W. A., Kaplan S. L. (2006). Panton-Valentine leukocidin genes are associated with enhanced inflammatory response and local disease in acute hematogenous Staphylococcus aureus osteomyelitis in children Pediatrics 117 433–440 10.1542/peds.2005-0566 . [DOI] [PubMed] [Google Scholar]
- Bolton L. L., Girolami S., Corbett L., van Rijswijk L. (2014). The Association for the Advancement of Wound Care (AAWC) venous and pressure ulcer guidelines Ostomy Wound Manage 60 24–66 . [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2007). Severe methicillin-resistant Staphylococcus aureus community-acquired pneumonia associated with influenza - Louisiana and Georgia, December 2006 - January 2007 MMWR Morb Mortal Wkly Rep 56 325–329. [PubMed] [Google Scholar]
- Chambers H. F., Deleo F. R. (2009). Waves of resistance: Staphylococcus aureus in the antibiotic era Nat Rev Microbiol 7 629–641 10.1038/nrmicro2200 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham T. M., Koehl J. L., Summers J. S., Haydel S. E. (2010). pH-Dependent metal ion toxicity influences the antibacterial activity of two natural mineral mixtures PLoS One 5 e9456 10.1371/journal.pone.0009456 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- David M. Z., Daum R. S. (2010). Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic Clin Microbiol Rev 23 616–687 10.1128/CMR.00081-09 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filon F. L., D'Agostin F., Crosera M., Adami G., Bovenzi M., Maina G. (2009). In vitro absorption of metal powders through intact and damaged human skin Toxicol In Vitro 23 574–579 10.1016/j.tiv.2009.01.015 . [DOI] [PubMed] [Google Scholar]
- Fortunov R. M., Hulten K. G., Hammerman W. A., Mason E. O., Jr, Kaplan S. L. (2006). Community-acquired Staphylococcus aureus infections in term and near-term previously healthy neonates Pediatrics 118 874–881 10.1542/peds.2006-0884 . [DOI] [PubMed] [Google Scholar]
- Fraunholz M., Sinha B. (2012). Intracellular Staphylococcus aureus: live-in and let die Front Cell Infect Microbiol 2 43 10.3389/fcimb.2012.00043 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydel S. E., Remenih C. M., Williams L. B. (2008). Broad-spectrum in vitro antibacterial activities of clay minerals against antibiotic-susceptible and antibiotic-resistant bacterial pathogens J Antimicrob Chemother 61 353–361 10.1093/jac/dkm468 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy L. A., Gill J. A., Schultz M. E., Irmler M., Gordin F. M. (2010). Inside-out: the changing epidemiology of methicillin-resistant Staphylococcus aureus Infect Control Hosp Epidemiol 31 983–985 10.1086/655837 . [DOI] [PubMed] [Google Scholar]
- Kim P. W., Sorbello A. F., Wassel R. T., Pham T. M., Tonning J. M., Nambiar S. (2012). Eosinophilic pneumonia in patients treated with daptomycin: review of the literature and US FDA adverse event reporting system reports Drug Saf 35 447–457 10.2165/11597460-000000000-00000 . [DOI] [PubMed] [Google Scholar]
- Kwong J. C., Chua K., Charles P. G. P. (2012). Managing severe community-acquired pneumonia due to community methicillin-resistant Staphylococcus aureus (MRSA) Curr Infect Dis Rep 14 330–338 10.1007/s11908-012-0254-8 . [DOI] [PubMed] [Google Scholar]
- Liu C., Bayer A., Cosgrove S. E., Daum R. S., Fridkin S. K., Gorwitz R. J., Kaplan S. L., Karchmer A. W., Levine D. P., other authors (2011). Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary Clin Infect Dis 52 285–292 10.1093/cid/cir034 . [DOI] [PubMed] [Google Scholar]
- MacEwan D., Wilson M. (1980). Interlayer and intercalation complexes of clay minerals. In Crystal Structures of Clay Minerals and their X-ray Identification, pp. 197–248. Edited by Brindley G., Brown G. London: Mineralogical Society. [Google Scholar]
- McLaren A. D. (1963). Biochemistry and soil science Science 141 1141–1147 10.1126/science.141.3586.1141 . [DOI] [PubMed] [Google Scholar]
- Miller L. G., Perdreau-Remington F., Rieg G., Mehdi S., Perlroth J., Bayer A. S., Tang A. W., Phung T. O., Spellberg B. (2005). Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles N Engl J Med 352 1445–1453 10.1056/NEJMoa042683 . [DOI] [PubMed] [Google Scholar]
- Mulligan M. E., Murray-Leisure K. A., Ribner B. S., Standiford H. C., John J. F., Korvick J. A., Kauffman C. A., Yu V. L. (1993). Methicillin-resistant Staphylococcus aureus: a consensus review of the microbiology, pathogenesis, and epidemiology with implications for prevention and management Am J Med 94 313–328 10.1016/0002-9343(93)90063-U . [DOI] [PubMed] [Google Scholar]
- Murray H. (2006). Bentonite applications. In Applied Clay Mineralogy: Occurrences, Processing and Applications of Kaolins, Bentonites, Palygorskitesepiolite, and Common Clays, pp. 111–130 Amsterdam: Elsevier Science; 10.1016/S1572-4352(06)02006-X. [DOI] [Google Scholar]
- Nunn J. (2002). Ancient Egyptian Medicine London: Red River Books. [Google Scholar]
- Otto C. C., Haydel S. E. (2013). Exchangeable ions are responsible for the in vitro antibacterial properties of natural clay mixtures PLoS One 8 e64068 10.1371/journal.pone.0064068 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto C. C., Koehl J. L., Solanky D., Haydel S. E. (2014). Metal ions, not metal-catalyzed oxidative stress, cause clay leachate antibacterial activity PLoS One 9 e115172 10.1371/journal.pone.0115172 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannaraj P. S., Hulten K. G., Gonzalez B. E., Mason E. O., Jr, Kaplan S. L. (2006). Infective pyomyositis and myositis in children in the era of community-acquired, methicillin-resistant Staphylococcus aureus infection Clin Infect Dis 43 953–960 10.1086/507637 . [DOI] [PubMed] [Google Scholar]
- Prabaker K., Weinstein R. A. (2011). Trends in antimicrobial resistance in intensive care units in the United States Curr Opin Crit Care 17 472–479 10.1097/MCC.0b013e32834a4b03 . [DOI] [PubMed] [Google Scholar]
- Trengove N. J., Langton S. R., Stacey M. C. (1996). Biochemical analysis of wound fluid from nonhealing and healing chronic leg ulcers Wound Repair Regen 4 234–239 10.1046/j.1524-475X.1996.40211.x . [DOI] [PubMed] [Google Scholar]
- Velde B. (1995). Composition and mineralogy of clay minerals. In Origin and Mineralogy of Clays, pp. 8–42 Berlin: Springer; 10.1007/978-3-662-12648-6_2. [DOI] [Google Scholar]
- Watkins R. R., David M. Z., Salata R. A. (2012). Current concepts on the virulence mechanisms of meticillin-resistant Staphylococcus aureus J Med Microbiol 61 1179–1193 10.1099/jmm.0.043513-0 . [DOI] [PMC free article] [PubMed] [Google Scholar]


