SUMMARY
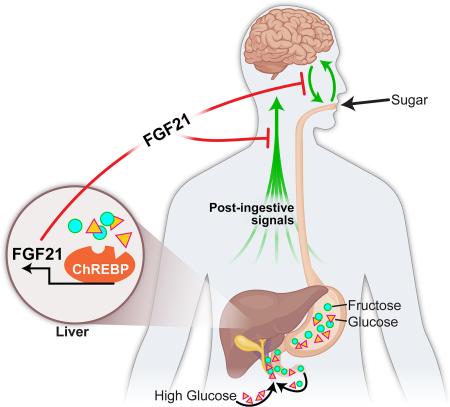
The liver is an important integrator of nutrient metabolism, yet no liver-derived factors regulating nutrient preference or carbohydrate appetite have been identified. Here we show that the liver regulates carbohydrate intake through production of the hepatokine fibroblast growth factor 21 (FGF21), which markedly suppresses consumption of simple sugars, but not complex carbohydrates, proteins, or lipids. Genetic loss of FGF21 in mice increases sucrose consumption, whereas acute administration or overexpression of FGF21 suppresses the intake of both sugar and non-caloric sweeteners. FGF21 does not affect chorda tympani nerve responses to sweet tastants, instead reducing sweet-seeking behavior and meal size via neurons in the hypothalamus. This liver-to-brain hormonal axis likely represents a negative feedback loop as hepatic FGF21 production is elevated by sucrose ingestion. We conclude that the liver functions to regulate macronutrient-specific intake by producing an endocrine satiety signal which acts centrally to suppress the intake of “sweets.”
Graphical Abstract
INTRODUCTION
The recent surge in obesity prevalence has been greatly influenced by food intake and diet composition. Energy from food comes in three macronutrient forms: fat, protein, and carbohydrate. Over the past 50 years, excessive consumption of carbohydrate in the U.S. has been linked with metabolic disease (Imamura et al., 2015; Johnson et al., 2009). Although there is abundant physiological evidence for independent appetites for fats, carbohydrates, proteins and micronutrients in diverse taxa (Simpson et al., 2015), the molecular mechanisms that determine appetite for specific nutrients are largely unknown (Morrison and Laeger, 2015). Carbohydrates represent a major source of food energy for many animal species and are needed to maintain cellular and physiological function. The ingestion of fuels that are readily oxidized or stored as energy would therefore be “rewarding” to an organism. Multiple reward layers, including oral and post-ingestive mechanisms, function to regulate sugar preference and appetite (Drewnowski et al., 2012; Ivan, 2011). While identification of the sweet taste receptors T1R2/T1R3 has provided important insight into the oral signaling elements that mediate the gustatory response to sweets (Damak et al., 2003), the post-ingestive mechanisms regulating carbohydrate intake are not well understood.
It was first proposed in the 1960's that the liver functions to regulate food intake and carbohydrate preference. The so-called “hepatostatic theory” postulated that hepatic signals from the liver provide information about carbohydrate reserves (Russek, 1963, 1970). Since then multiple groups have confirmed and expanded on the role of the liver in carbohydrate preference and food intake (reviewed in (Ivan, 2011)). Although carbohydrate is an important fuel source, excessive carbohydrate consumption can lead to hepatic toxicity and numerous other chronic diseases including obesity and diabetes (Imamura et al., 2015). Therefore, just as there are mechanisms to promote carbohydrate intake, mechanisms likely exist to reduce carbohydrate intake to prevent the negative effects of excessive carbohydrate consumption.
Fibroblast growth factor 21 (FGF21) is an endocrine hormone that regulates energy homeostasis (Markan and Potthoff, 2015). Single-nucleotide polymorphisms in the human FGF21 gene were recently associated with changes in macronutrient intake (i.e., the percentage of diet derived from carbohydrate, fat, or protein) (Chu et al., 2013; Tanaka et al., 2013). In one study, a variant (rs838145) in the region that includes the FGF21 gene was associated with increased carbohydrate intake (Tanaka et al., 2013). A separate human GWAS study identified a synonymous single nucleotide polymorphism (SNP), rs838133, in the first exon of FGF21 as being associated with decreased protein intake and higher carbohydrate intake (Chu et al., 2013). Here we show that FGF21 is induced in the liver in response to high carbohydrate levels and enters circulation where it signals to the brain to suppress carbohydrate intake, thereby completing a negative feedback loop. FGF21 heterozygous and knockout mice exhibit an increased preference for carbohydrates compared to wild-type littermates, whereas genetic or pharmacological elevation of FGF21 levels suppresses the intake of both simple sugars and non-caloric sweeteners, but not lipids or protein. Together, our data demonstrate a post-ingestive mechanism regulating macronutrient-specific intake.
RESULTS AND DISCUSSION
Loss of FGF21 Increases Sugar Preference
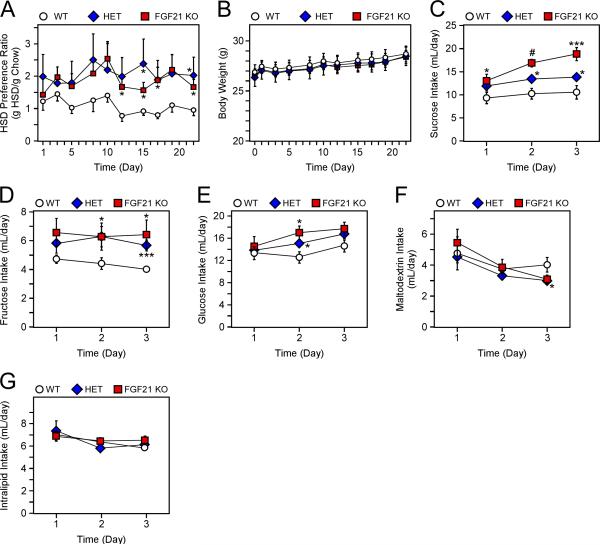
To test the hypothesis that FGF21 regulates macronutrient preference, we first evaluated food preference in mice lacking FGF21. When given free choice between a standard chow diet versus a high-sucrose diet (HSD), both FGF21 heterozygous (HET) and knockout (KO) mice preferred the high-sucrose diet compared to wild-type (WT) littermates (Fig. 1A). Despite preferring the HSD 2:1, FGF21 HET and KO mice consumed the same amount of total energy (Fig. S1A,B), and gained weight at the same rate as WT littermates (Fig. 1B). To investigate the nutrient-specificity of this phenomenon, WT, FGF21 HET, and FGF21 KO littermates, were studied in a series of two-bottle preference tests, where they were offered the choice between water and a range of nutritive and non-nutritive tastants. Consistent with the HSD studies, FGF21 HET and KO mice consumed significantly more sucrose compared to WT littermates in the two-bottle test (Fig. 1C), despite having similar body weights (Fig. S1C). FGF21 HET and KO mice also consumed more glucose or fructose (Fig. 1D,E), and FGF21 KO mice consumed more glucose plus fructose compared to WT littermates (Fig. S1D). Notably, increased preference was not observed for polysaccharide (maltodextrin) or lipid (Fig. 1F,G). FGF21 KO mice also did not specifically prefer a sweet taste, as there was no significant difference in saccharin intake compared to WT mice (Fig. S1E). Together, these data show that loss of FGF21 increases macronutrient-specific intake of mono- and disaccharide sugars.
Figure 1. Loss of FGF21 alters macronutrient-specific intake.
(A) High sucrose diet (HSD) preference ratio (g HSD intake/g Chow intake) in 12-13 week old, male wild-type (WT), FGF21 heterozygous (HET), and FGF21 total knockout (FGF21 KO) littermates (n = 7-8/group). (B) Body weights of mice in (A). (C-G) 16 week old, male WT, HET, and FGF21 KO littermates were offered a two-bottle choice of different nutrients (n = 6-14/group; see methods). Fluid intake per day of (C) 10% sucrose, (D) 10% fructose, (E) 10% glucose, (F) 2% maltodextrin, and (F) 20% intralipid for the indicated mice. Values are mean +/− SEM. (*, P< 0.05; ***, P< 0.005; and #, P<0.001 compared to WT).
Carbohydrate-mediated Activation of ChREBP Increases FGF21 Production From Liver
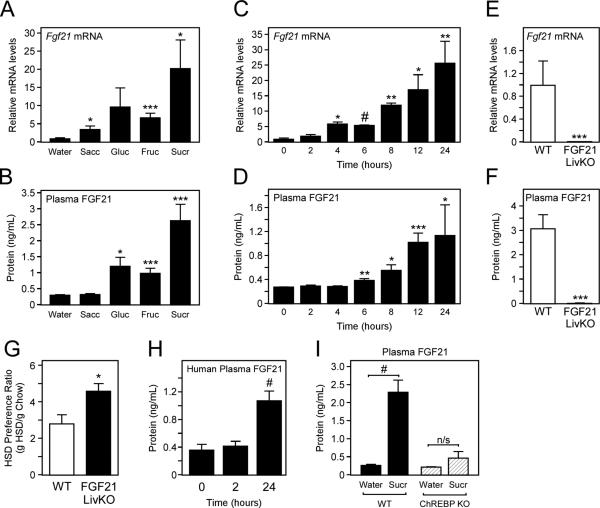
Circulating FGF21 levels are low under ad libitum chow fed conditions. Since loss of FGF21 increased sugar consumption, we hypothesized that FGF21 would be induced in the liver by sugar ingestion to act as a negative-feedback signal, limiting further sugar intake. Hepatic and plasma FGF21 levels were assessed in WT C57Bl/6 mice fed chow ad libitum and provided water, 0.2% saccharin, 10% glucose, 10% fructose, or 10% sucrose ad libitum in a drinking bottle for 24 hours. Hepatic Fgf21 mRNA and plasma FGF21 levels were markedly induced in mice provided glucose, fructose, and sucrose, but not water or saccharin (Fig. 2A,B). A time course for this induction revealed that hepatic and plasma FGF21 levels were significantly increased after 6 hours and continued to increase to reach maximal levels by 12-24 hours (Fig. 2C,D). Plasma FGF21 levels in mice administered sucrose for 1 day did not significantly differ from plasma levels of mice administered FGF21 for 3 days (Fig. S2A). This increase in plasma FGF21 in response to sucrose was derived from the liver as hepatic and plasma FGF21 levels were completely abolished in mice lacking FGF21 specifically from the liver (FGF21 LivKO) (Fig. 2E,F). We therefore assessed whether FGF21 LivKO also demonstrated a preference for HSD. Indeed, FGF21 LivKO preferentially consumed more HSD than WT littermates (Fig. 2G).
Figure 2. Ingestion of carbohydrate stimulates FGF21 production from liver.
(A) Hepatic Fgf21 mRNA and (B) plasma FGF21 protein levels in 11-13 week old, male WT C57Bl/6 mice ad libitum fed chow and provided water, 0.2% saccharin (sacc), 10% glucose (gluc), 10% fructose (fruc), or 10% sucrose (sucr) ad libitum in a drinking bottle for 24 hours (n = 5/group). (C) Hepatic Fgf21 mRNA and (D) plasma FGF21 protein levels in 11-13 week old, male WT C57Bl/6 mice ad libitum fed chow and provided 10% sucrose ad libitum for the indicated time (n = 5/group). (E) Hepatic Fgf21 mRNA and (F) plasma FGF21 protein levels in 10-12 week old, male WT and FGF21 LivKO mice ad libitum fed chow and 10% sucrose ad libitum for 24 hours (n = 5-7/group). (G) HSD preference ratio in 10-12 week old, male WT (FGF21fl/fl) and FGF21 liver-specific knockout (FGF21 LivKO) mice (n = 5/group). (H) Plasma FGF21 levels in human subjects maintained at hyperglycemia via dextrose infusion for 0, 2, and 24 hours (n = 10). (I) Plasma FGF21 levels in WT and ChREBP KO mice ad libitum fed chow and 10% sucrose ad libitum for 24 hours (n = 5-8/group). Values are mean +/− SEM. (*, P< 0.05; **, P< 0.01; ***, P< 0.005; ***, #, P< 0.001 compared to WT).
To determine whether FGF21 is also induced acutely in humans in response to sugars, we measured plasma FGF21 in healthy subjects infused with dextrose to maintain steady state hyperglycemia (90 mg/dL above basal levels) for 0, 2, or 24 hours (Solomon et al., 2012). Consistent with the mouse studies, plasma FGF21 levels were not significantly increased after 2 hours of acute hyperglycemia, but were increased three-fold after 24 hours (from 340 pg/ml to 1012 pg/ml) (Fig. 2H). FGF21 levels were also recently shown to be induced in humans in response to an oral carbohydrate load, although the induction of plasma FGF21 occurred on a different timescale (Dushay et al., 2015). To determine whether FGF21 is induced in hepatocytes directly by high carbohydrate, we treated HepG2 cells with either glucose or fructose and found that both significantly increased FGF21 mRNA levels (Fig. S2B). The transcription factor carbohydrate response element binding protein (ChREBP) has been previously shown to regulate FGF21 levels in vitro in response to high carbohydrate (Iizuka et al., 2009; Uebanso et al., 2011), so we next examined whether the induction of circulating levels of FGF21 in vivo in response to sucrose was mediated by ChREBP. WT and ChREBP KO mice were provided 10% sucrose for 24 hours and plasma FGF21 levels were examined. Compared to WT mice, mice lacking ChREBP (Fig. S2C) failed to significantly induce circulating FGF21 levels in response to sucrose (Fig. 2I). Together these data suggest that sugar ingestion cell-autonomously stimulates FGF21 production in liver through activation of ChREBP, and that loss of this signal increases sugar consumption.
FGF21 Suppresses the Intake of Simple Sugars
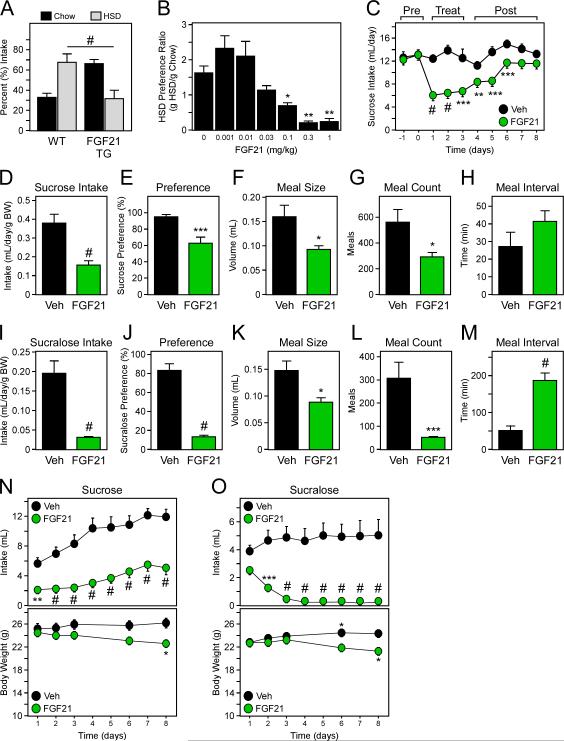
Using gain-of-function models, we next examined the consequence of FGF21 induction in vivo. WT and FGF21 transgenic (TG) mice, which have constitutively high circulating FGF21 levels (Fig. S3A), were offered the choice between chow and HSD as described above. FGF21 transgenic mice preferred chow to HSD, such that the percent preference for chow and HSD was actually opposite of that for WT littermates (Fig. 3A). This occurred in the setting of overall increased energy intake in the FGF21 TG mice (Fig. S3B). Administration of exogenous human FGF21 also dramatically suppressed HSD preference in lean WT mice (Fig. 3B) without affecting total energy intake (Fig. S3C) or body weight (Fig. S3D), consistent with pharmacological injections of FGF21 having only minor effects on body weight in lean mice (Hale et al., 2012). When offered HSD only, FGF21-treated mice consumed the same amount of HSD as vehicle-treated mice (Fig. S3E), suggesting that FGF21 functions as a sugar satiety signal contingent on fullness of stomach or some other signal of repletion. To examine the effect of FGF21 dose on HSD preference, WT mice were administered various amounts of FGF21 for 3 days prior to HSD exposure, which resulted in different levels of circulating FGF21 (Fig. S3F). FGF21 suppressed sucrose consumption at levels as low as 0.3 mg/kg (Fig. 3B), and these data suggest that FGF21's effect on sugar intake is not mediated by conditioned taste aversion because treatment began before the diet was presented. However, to directly test whether FGF21 causes taste aversion, we compared the sweet-appetite reducing properties of FGF21 with lithium chloride (LiCl), which produces illness. While both LiCl- and FGF21-treated animals consumed less sucralose the day following the pairing of FGF21 or LiCl injections with presentation of sucralose solution, only mice administered LiCl exhibited aversion to sucralose following a one week washout period (Fig. S3G). These results show that conditioned taste aversion is not the mechanism by which FGF21 reduces sweet appetite in mice.
Figure 3. FGF21 suppresses the intake of sweet tastants.
(A) Percent intake of chow and high sucrose diet (HSD) in 12-16 week old, male WT and FGF21 transgenic (TG) mice (n = 7-12/group). (B) HSD preference ratio (g HSD intake/g Chow intake) in WT C57BL/6 mice receiving the indicated amount of FGF21 (n = 4/group). (C) Sucrose intake was assessed before (Pre), during (Treat), or after (Post) treatment with vehicle or FGF21 (1 mg/kg) via i.p. injection (Treat) for 3 days (n = 7-8/group). (D-M) WT male C57Bl/6 mice were implanted with identification chips and osmotic minipumps delivering vehicle or human FGF21 protein (n = 16/group). Sucrose and sucralose intake (D,I), preference (E,J), meal size (F,K), meal count (G,L), and meal interval (H,M) (n = 8/group) versus water (n = 8/group). (N) Sucrose and (O) sucralose intake per day of mice in (D) and (I), respectively. Values are mean +/− SEM. (*, P< 0.05; **, P< 0.01; ***, P< 0.005; #, P< 0.001 compared to WT; statistical significance in N,O for body weight are relative to the starting value on day 1 for each group.).
Consistent with the HSD diet experiments, administration of FGF21 via i.p. injection also caused a marked decrease in sucrose intake (Figs. 3C and S3H). Similar results were observed when WT mice were administered vehicle or FGF21 and offered a two-bottle choice of glucose and water (Fig. S3I). Following the treatment period, sucrose intake returned to near pre-treatment levels, demonstrating that the effect of FGF21 is reversible, though persistent for a period of days (Fig. 3C).
To determine what nutrient preferences are modified by FGF21, WT mice were implanted with osmotic minipumps that maintained increased levels of FGF21 or vehicle (Fig. S3J), and then offered ad libitum access to different tastant solutions. In the two-bottle test, mice were given the choice between water and either sucrose, sucralose, lactose, maltose, liposyn, sodium chloride, casein, quinine, or monosodium glutamate (MSG), of which FGF21 treatment only had an impact on total consumption of the disaccharide sucrose and the non-nutritive, artificial sweetener sucralose (decreased consumption by 56% and 49%, respectively) (Fig. S3K). Consistent with these data, FGF21 transgenic mice exhibited an aversion to the artificial sweetener saccharin, but not intralipid or casein (Fig. S3L-N). Collectively, these data demonstrate that genetic and pharmacologic increases in FGF21 selectively suppress sugar and other sweet tastant intake.
To further examine sugar and sweet tastant intake in response to FGF21, we analyzed sucrose and sucralose meal frequency and size in a separate cohort of mice receiving FGF21 via osmotic minipumps. For sucrose, FGF21 administration reduced intake by 59%, preference by 34%, meal size by 42%, and also reduced the number of approaches to the sucrose sipper by 48% while increasing the interval between approaches by 52% (Fig. 3D-H). For sucralose, FGF21 administration reduced intake by 84%, preference by 83%, meal size by 40%, meal count by 83% and increased meal interval by 259% (Fig. 3I-M). Importantly, these effects of FGF21 on intake occur rapidly, prior to a reduction in body weight (Fig. 3N,O). Together, these data show that FGF21 regulates both the appetitive and consummatory components of sweet intake, reducing quantity of sweet-tasting foods consumed in individual meals as well as motivation to seek out sweet foods.
FGF21 Acts on the Hypothalamus to Suppress Sucrose Preference
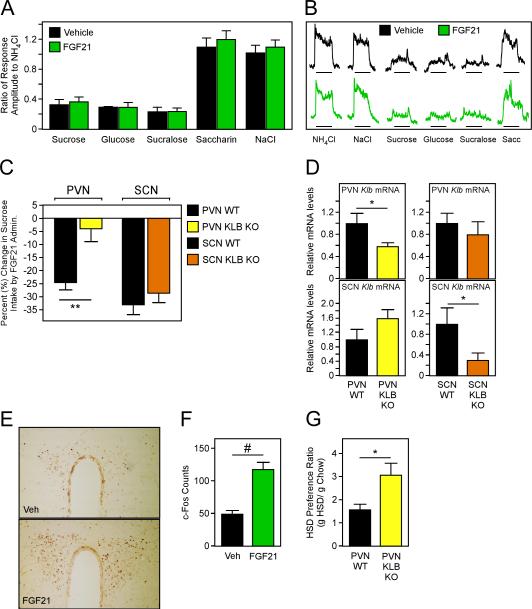
Sweet-taste receptor cells in the taste bud are the starting point of a hard-wired neural circuit that promotes sugar ingestion. FGF21 signals to target tissues through a receptor complex composed of FGFR1c and the co-receptor β-klotho, both of which are required for FGF21 signaling (Adams et al., 2012; Ding et al., 2012). Both Fgfr1c and β-klotho mRNA were undetectable in taste epithelium (Fig. S4A), suggesting that FGF21 does not act directly on the taste bud. Nevertheless, to determine whether FGF21 affects taste, we performed nerve recordings of the chorda tympani nerve in response to various tastants in WT mice that were administered either vehicle or FGF21. Importantly, FGF21 did not affect taste responses for either caloric or non-caloric sweeteners (Fig. 4A,B). We next hypothesized that FGF21 might be suppressing food intake through actions on the central nervous system since FGF21 has been shown to signal to the nervous system (Bookout et al., 2013). To determine whether central FGF21 signaling is sufficient to mediate the suppressive effect of FGF21 on sugar intake, we performed intracerebroventricular (ICV) injections of FGF21 in WT C57Bl/6 mice and assessed sugar preference. Acute ICV injection of FGF21 reduced HSD preference by 62% (HSD/chow ratio: control = 7.08, FGF21-treated = 2.64; P = 0.0038), suggesting that FGF21 may act centrally to reduce sugar intake.
Figure 4. FGF21 signaling to the PVN suppresses sucrose preference.
(A-B) Chorda tympani nerve recordings in male C57Bl/6 mice administered FGF21 (1 mg/kg) or vehicle (n = 5/group). (A) Ratio of nerve recording responses after sucrose (500 mM), glucose (500 mM), sucralose (50 mM), saccharin (50 mM), and NaCl (100 mM) relative to NH4Cl (100 mM). (B) Representative nerve recording tracings from the indicated mice. (C-D) PVN or suprachiasmatic nucleus (SCN) β-klotho (KLB) knockout (KO) mice and control mice were generated by performing bilateral stereotactic injections of AAV-Cre or AAV-GFP into the PVN or SCN of KLBfl/fl mice. Sucrose preference was assessed in each mouse while receiving daily injections of vehicle (3 days) followed by daily injections of FGF21 (3 days). (C) Percent change in sucrose intake in 12 week old male PVN or SCN KLB KO mice and littermate controls by i.p. administration of FGF21 (1 mg/kg) (n = 7-12/group). (D) Klb mRNA expression in the PVN or SCN from brain punches of the indicated mice in (C) as determined by QPCR. (E) Representative photomicrographs depicting the effect of intraperitoneal (i.p.) administration of FGF21 (1 mg/kg) on c-Fos immunoreactivity in the paraventricular nucleus (PVN) in mice. (F) Comparison of the number of immunoreactive c-Fos-positive cells in the PVN between vehicle- and FGF21-treated mice (n = 6/group). (G) High sucrose diet (HSD) preference ratio (g HSD intake/g Chow intake) in a separate cohort of 12 week old male PVN KLB KO mice and WT controls (n = 7-12/group). Values are mean +/− SEM. (*, P< 0.05; **, P< 0.01; #, P< 0.001 compared to WT).
The FGF21 receptor complex is expressed in multiple regions of the brain including the nucleus tractus solitarii (NTS) and the suprachiasmatic nucleus (SCN) (Bookout et al., 2013) and paraventricular nucleus (PVN) (Liang et al., 2014) of the hypothalamus. To determine the brain area underlying FGF21-induced suppression of sucrose intake, we impaired FGF21 signaling specifically in the PVN, SCN, or the hindbrain (NTS). To accomplish this, mice with a floxed β-klotho allele (KLBfl/fl) received bilateral stereotactic injections of either AAV-Cre or AAV-GFP specifically into the PVN (PVN KLB KO or WT) or SCN (SCN KLB KO or WT). To eliminate β-klotho expression from the NTS, we crossed KLBfl/fl mice to Phox2b-cre transgenic mice which express Cre-recombinase in this region (Scott et al., 2011). Notably, reduction of β-klotho expression in the PVN, but not the SCN, impaired FGF21-mediated suppression of sucrose intake (Fig. 4C). Analysis of β-klotho mRNA expression in the PVN and SCN from both sets of mice confirmed region-specific reduction of β-klotho (Fig. 4D). In addition, analysis of Cre mRNA expression in multiple brain regions confirmed site-specific delivery of Cre to the PVN (Fig. S4B). Similar to the SCN, loss of β-klotho in the hindbrain (KLBfl/fl;Phox2b-Cre) did not impair the suppressive effect of FGF21 on sugar consumption (Fig. S4C). c-Fos staining of brain slices from WT mice that were peripherally (i.p.) administered FGF21 also exhibited significantly increased c-Fos staining in the PVN of the hypothalamus (Fig. 4E,F), consistent with a recent report observing increased ERK phosphorylation in the PVN in response to FGF21 administration (Douris et al., 2015). Finally, to examine the physiological significance of FGF21 signaling to the PVN on carbohydrate intake, we assessed HSD preference in a different cohort of PVN KLB KO mice and WT controls. Consistent with FGF21 KO mice exhibiting an increased preference for HSD (Fig. 1A), PVN KLB KO mice demonstrated an approximate 2 fold increase in HSD preference compared to control mice (Fig. 4G). Together, these data suggest that FGF21 suppresses sugar intake by signaling, at least in part, to the PVN which in turn modulates circuits that govern the innate craving and ingestive responses evoked by sweets.
Our work suggests that specific hormonal signals exist to regulate macronutrient-specific intake, and demonstrates that the liver, which is uniquely positioned to sense whole body energy status, also functions as an endocrine regulator of sugar intake. In addition to its role of increasing excess carbohydrate disposal to peripheral tissues, FGF21 may also function to suppress carbohydrate intake as plasma glucose levels start to rise during insulin resistance. These data combined with the human GWAS studies (Chu et al., 2013; Tanaka et al., 2013), suggest that the regulation of macronutrient intake by FGF21 represents a major physiological role for this hormone in the fed state. Interestingly, consumption of low protein diets also increases hepatic and plasma FGF21 levels in humans (Laeger et al., 2014). Therefore, this hepatic hormonal axis regulating sugar satiety may also be utilized to promote foraging for other macronutrients and likely interacts with other nutrient cues to affect feeding. Given the complexity inherent in behavioral studies of diet preference, additional work will be required to determine whether FGF21 also mediates attraction to unidentified types of nutrient or dietary constituents that could contribute to the phenotype reported here. In addition, as the attraction to sweets is mediated by the endogenous opioid and mesolimbic dopaminergic systems, which are involved in the reinforcing and rewarding properties of drugs of abuse (Levine et al., 2003), additional work is necessary to determine whether FGF21 might affect self-administration of other types of rewarding substances. We anticipate that additional hormonal regulators will be identified which regulate both nutrient-specific hunger and satiety. These data raise the interesting possibility that molecular therapies could be developed to treat obesity and type 2 diabetes by qualitatively improving diet.
EXPERIMENTAL PROCEDURES
Mouse Studies
All mice including FGF21 total KO mice (Potthoff et al., 2009) on a mixed C57Bl/6 background, and FGF21 transgenic (TG) mice (Inagaki et al., 2007), FGF21 LivKO mice (Markan et al., 2014), ChREBP KO mice (Iizuka et al., 2004), KLBfl/fl mice (Ding et al., 2012), and Phox2b-Cre mice (Scott et al., 2011) on a pure C57Bl/6 background, have been described. For diet preference studies in KO and TG studies, mice were fed either chow (Teklad 2920X) or high sucrose diet (HSD; Teklad TD.88122) consisting of 23.2% protein, 73.9% carbohydrate (sucrose, 486.6 g/kg) and 2.8% fat by calorie content ad libitum. For two-bottle tastant experiments in WT, FGF21 heterozygous, knockout, and transgenic, and PVN and SCN KLB KO studies, drinking tubes were constructed and test fluids were presented following the Monell Mouse Taste Phenotyping Project specifications (http://www.monell.org/MMTPP/), and mice were offered the indicated amount of each test fluid versus water. Animal experiments were approved by the University of Iowa IACUC and/or the Danish Animal Experiments Inspectorate.
Measurement of FGF21 levels
Mouse FGF21 levels were measured using a commercially available ELISA (Biovendor) in the indicated mice. Human FGF21 levels were measured in described samples (Solomon et al., 2012) using a commercially available ELISA (Biovendor).
Administration of Recombinant FGF21
Recombinant FGF21 was generated and provided by Novo Nordisk. For tastant intake/preference studies of mice receiving exogenous FGF21 via osmotic minipump, wild-type mice were implanted with subcutaneous radio-frequency identification chips and housed in groups of four in an HM2 rodent feeding system (MBROSE, Faaborg, Denmark). The HM2 system records feeding or drinking data from two channels at the level of the individual mouse, based on a chip reading taken when the animal enters the feeding/drinking chamber (which only holds one animal). In addition, the food hopper or water bottle in each channel is mounted on a scale so that any spillage is not logged as food or water intake. Mice were implanted with osmotic minipumps (Alzet) containing recombinant human FGF21 (3 mg/kg/day) or vehicle. Once the infusion began mice were given ad libitum access to water and a test solution for three days (containing maltose (100mM), lactose (100mM), sucrose (100mM), liposyn (20%), casein (8%), monosodium glutamate (100mM), quinine (1.5mM), sodium chloride (0.1%), or sucralose (10mM)). Standard rodent chow was also available ad libitum throughout this time. For feeding behavior in the HM2 system, a meal starts when the scale becomes unstable as the mouse touches the water bottle or food hopper. If the scale is stable for 30 seconds, the system terminates the logging and the intake is defined as a meal. One meal can contain several bouts as long as they are not separated by more than 30 seconds. Following the last day of the treatment period, blood samples were collected by cardiac puncture to determine steady-state differences in circulating FGF21 levels between groups.
Intracerebroventricular injections were performed as described (Davisson et al., 1998), where mice received 5 daily injections of 1 μg/mouse FGF21 or artificial cerebrospinal fluid while being provided chow and HSD.
Stereotactic injections of AAV virus
Stereotactic surgery was performed as previously described (Yang et al., 2006). Using hamilton microsyringe with small hub removable needle, AAV2/5-GFP and –Cre viruses (provided by the University of Iowa Gene Vector Core) were delivered bilaterally to either the SCN or PVN.
c-Fos immunohistochemistry
c-Fos immunostaining was performed as described (Fernandes-Santos et al., 2013).
Nerve Recordings of Tastants
Chorda tympani nerve recordings were performed as described (Vandenbeuch et al., 2015).
Gene expression
Two micrograms RNA from each sample were used to generate cDNA and QPCR was conducted using SYBR green (Invitrogen) as described (Markan et al., 2014). Primer sequences are listed in supplementary information.
Statistical Methods
Data-set statistics were analyzed using the GraphPad Prism software. The Student's t-test and one-way ANOVA were used to compare data sets. For multiple comparison correction, the Benjamini-Hochberg false discovery method was used with Q set to 5%. Data are represented as the mean ± SEM.
Supplementary Material
ACKNOWLEDGMENTS
We thank Drs. David Mangelsdorf and Steven Kliewer (UTSW) for kindly providing FGF21 transgenic, FGF21 total knockout, and KLBfl/fl mice. ChREBP knockout mice were a kind gift from Dr. Kosaku Uyeda (UTSW). We thank Teresa Ruggle for assistance with graphic design, and Omar Shaban, Ananya Munjal, Dr. Kathleen Markan, and Michele Gorecki for technical assistance. We thank Dr. Justin Grobe for his expertise and research support. This work was supported by an American Diabetes Association Junior Faculty Award 7-13-JF-49 (M.J.P.), an Edward Mallinckrodt Jr. Foundation Grant (M.J.P.), the National Institutes of Health (NIH) (R01DK106104) (M.J.P.), T32 GM067795 (L.B.), and P30 DC004657 (S.C.K.), generous research support from the Fraternal Order of Eagles Diabetes Research Center (M.J.P. and K.R.) and the University of Iowa Carver College of Medicine (M.J.P.), the Novo Nordisk Foundation Center for Basic Metabolic Research (M.P.G.), Novo Nordisk scholarship (S.H.R.), the Lundbeck Foundation (M.P.G.), and the Danish Research Council (M.P.G.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
S.H.R. and L.B. designed and performed experiments, interpreted data, and wrote the paper. L.P., M.C.N., M.C.G., A.I.U., A.N.M., T.C.Y., K.E.C., C.R., A.P.T., A.V. and C.B.A. designed and performed experiments and interpreted data. K.K. and T.P.J.S. designed and performed the human studies, and interpreted data. B.H., K.R., M.D.C, A.A.P., and S.C.K. designed experiments, oversaw data collection, interpreted the data, and contributed essential reagents and equipment. M.J.P. and M.P.G. conceived of the project, designed and performed experiments, interpreted data, wrote the paper, and are responsible for the integrity of its content.
REFERENCES
- Adams AC, Cheng CC, Coskun T, Kharitonenkov A. FGF21 requires betaklotho to act in vivo. PloS one. 2012;7:e49977. doi: 10.1371/journal.pone.0049977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookout AL, de Groot MH, Owen BM, Lee S, Gautron L, Lawrence HL, Ding X, Elmquist JK, Takahashi JS, Mangelsdorf DJ, et al. FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nature medicine. 2013;19:1147–1152. doi: 10.1038/nm.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu AY, Workalemahu T, Paynter NP, Rose LM, Giulianini F, Tanaka T, Ngwa JS, Group CNW, Qi Q, Curhan GC, et al. Novel locus including FGF21 is associated with dietary macronutrient intake. Human molecular genetics. 2013;22:1895–1902. doi: 10.1093/hmg/ddt032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Varadarajan V, Zou S, Jiang P, Ninomiya Y, Margolskee RF. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science. 2003;301:850–853. doi: 10.1126/science.1087155. [DOI] [PubMed] [Google Scholar]
- Davisson RL, Yang G, Beltz TG, Cassell MD, Johnson AK, Sigmund CD. The brain renin-angiotensin system contributes to the hypertension in mice containing both the human renin and human angiotensinogen transgenes. Circulation research. 1998;83:1047–1058. doi: 10.1161/01.res.83.10.1047. [DOI] [PubMed] [Google Scholar]
- Ding X, Boney-Montoya J, Owen BM, Bookout AL, Coate KC, Mangelsdorf DJ, Kliewer SA. betaKlotho is required for fibroblast growth factor 21 effects on growth and metabolism. Cell metabolism. 2012;16:387–393. doi: 10.1016/j.cmet.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douris N, Stevanovic DM, Fisher FM, Cisu TI, Chee MJ, Nguyen NL, Zarebidaki E, Adams AC, Kharitonenkov A, Flier JS, et al. Central Fibroblast Growth Factor 21 Browns White Fat via Sympathetic Action in Male Mice. Endocrinology. 2015;156:2470–2481. doi: 10.1210/en.2014-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewnowski A, Mennella JA, Johnson SL, Bellisle F. Sweetness and food preference. The Journal of nutrition. 2012;142:1142S–1148S. doi: 10.3945/jn.111.149575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dushay JR, Toschi E, Mitten EK, Fisher FM, Herman MA, Maratos-Flier E. Fructose ingestion acutely stimulates circulating FGF21 levels in humans. Molecular metabolism. 2015;4:51–57. doi: 10.1016/j.molmet.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes-Santos C, Zhang Z, Morgan DA, Guo DF, Russo AF, Rahmouni K. Amylin acts in the central nervous system to increase sympathetic nerve activity. Endocrinology. 2013;154:2481–2488. doi: 10.1210/en.2012-2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale C, Chen MM, Stanislaus S, Chinookoswong N, Hager T, Wang M, Veniant MM, Xu J. Lack of overt FGF21 resistance in two mouse models of obesity and insulin resistance. Endocrinology. 2012;153:69–80. doi: 10.1210/en.2010-1262. [DOI] [PubMed] [Google Scholar]
- Iizuka K, Bruick RK, Liang G, Horton JD, Uyeda K. Deficiency of carbohydrate response element-binding protein (ChREBP) reduces lipogenesis as well as glycolysis. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:7281–7286. doi: 10.1073/pnas.0401516101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizuka K, Takeda J, Horikawa Y. Glucose induces FGF21 mRNA expression through ChREBP activation in rat hepatocytes. FEBS letters. 2009;583:2882–2886. doi: 10.1016/j.febslet.2009.07.053. [DOI] [PubMed] [Google Scholar]
- Imamura F, O'Connor L, Ye Z, Mursu J, Hayashino Y, Bhupathiraju SN, Forouhi NG. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: systematic review, meta-analysis, and estimation of population attributable fraction. BMJ. 2015;351:h3576. doi: 10.1136/bmj.h3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, Li Y, Goetz R, Mohammadi M, Esser V, et al. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell metabolism. 2007;5:415–425. doi: 10.1016/j.cmet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Ivan E.d.A. Neurobiology of Sensation and Reward. CRC Press; 2011. Multiple Reward Layers in Food Reinforcement. pp. 263–285. [Google Scholar]
- Johnson RK, Appel LJ, Brands M, Howard BV, Lefevre M, Lustig RH, Sacks F, Steffen LM, Wylie-Rosett J, American Heart Association Nutrition Committee of the Council on Nutrition, P.A. et al. Dietary sugars intake and cardiovascular health: a scientific statement from the American Heart Association. Circulation. 2009;120:1011–1020. doi: 10.1161/CIRCULATIONAHA.109.192627. [DOI] [PubMed] [Google Scholar]
- Laeger T, Henagan TM, Albarado DC, Redman LM, Bray GA, Noland RC, Munzberg H, Hutson SM, Gettys TW, Schwartz MW, et al. FGF21 is an endocrine signal of protein restriction. The Journal of clinical investigation. 2014;124:3913–3922. doi: 10.1172/JCI74915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine AS, Kotz CM, Gosnell BA. Sugars: hedonic aspects, neuroregulation, and energy balance. The American journal of clinical nutrition. 2003;78:834S–842S. doi: 10.1093/ajcn/78.4.834S. [DOI] [PubMed] [Google Scholar]
- Liang Q, Zhong L, Zhang J, Wang Y, Bornstein SR, Triggle CR, Ding H, Lam KS, Xu A. FGF21 maintains glucose homeostasis by mediating the cross talk between liver and brain during prolonged fasting. Diabetes. 2014;63:4064–4075. doi: 10.2337/db14-0541. [DOI] [PubMed] [Google Scholar]
- Markan KR, Naber MC, Ameka MK, Anderegg MD, Mangelsdorf DJ, Kliewer SA, Mohammadi M, Potthoff MJ. Circulating FGF21 is liver derived and enhances glucose uptake during refeeding and overfeeding. Diabetes. 2014;63:4057–4063. doi: 10.2337/db14-0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markan KR, Potthoff MJ. Metabolic fibroblast growth factors (FGFs): Mediators of energy homeostasis. Semin Cell Dev Biol. 2015 doi: 10.1016/j.semcdb.2015.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison CD, Laeger T. Protein-dependent regulation of feeding and metabolism. Trends in endocrinology and metabolism: TEM. 2015;26:256–262. doi: 10.1016/j.tem.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potthoff MJ, Inagaki T, Satapati S, Ding X, He T, Goetz R, Mohammadi M, Finck BN, Mangelsdorf DJ, Kliewer SA, et al. FGF21 induces PGC-1alpha and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:10853–10858. doi: 10.1073/pnas.0904187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russek M. Participation of hepatic glucoreceptors in the control of intake of food. Nature. 1963;197:79–80. doi: 10.1038/197079b0. [DOI] [PubMed] [Google Scholar]
- Russek M. Demonstration of the influence of an hepatic glucosensitive mechanism on food-intake. Physiology & behavior. 1970;5:1207–1209. doi: 10.1016/0031-9384(70)90218-0. [DOI] [PubMed] [Google Scholar]
- Scott MM, Williams KW, Rossi J, Lee CE, Elmquist JK. Leptin receptor expression in hindbrain Glp-1 neurons regulates food intake and energy balance in mice. The Journal of clinical investigation. 2011;121:2413–2421. doi: 10.1172/JCI43703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson SJ, Le Couteur DG, Raubenheimer D. Putting the balance back in diet. Cell. 2015;161:18–23. doi: 10.1016/j.cell.2015.02.033. [DOI] [PubMed] [Google Scholar]
- Solomon TP, Knudsen SH, Karstoft K, Winding K, Holst JJ, Pedersen BK. Examining the effects of hyperglycemia on pancreatic endocrine function in humans: evidence for in vivo glucotoxicity. The Journal of clinical endocrinology and metabolism. 2012;97:4682–4691. doi: 10.1210/jc.2012-2097. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Ngwa JS, van Rooij FJ, Zillikens MC, Wojczynski MK, Frazier-Wood AC, Houston DK, Kanoni S, Lemaitre RN, Luan J, et al. Genome-wide meta-analysis of observational studies shows common genetic variants associated with macronutrient intake. The American journal of clinical nutrition. 2013;97:1395–1402. doi: 10.3945/ajcn.112.052183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uebanso T, Taketani Y, Yamamoto H, Amo K, Ominami H, Arai H, Takei Y, Masuda M, Tanimura A, Harada N, et al. Paradoxical regulation of human FGF21 by both fasting and feeding signals: is FGF21 a nutritional adaptation factor? PloS one. 2011;6:e22976. doi: 10.1371/journal.pone.0022976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbeuch A, Larson ED, Anderson CB, Smith SA, Ford AP, Finger TE, Kinnamon SC. Postsynaptic P2X3-containing receptors in gustatory nerve fibres mediate responses to all taste qualities in mice. The Journal of physiology. 2015;593:1113–1125. doi: 10.1113/jphysiol.2014.281014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Gagnon D, Vachon P, Tremblay A, Levy E, Massie B, Michaud JL. Adenoviral-mediated modulation of Sim1 expression in the paraventricular nucleus affects food intake. J Neurosci. 2006;26:7116–7120. doi: 10.1523/JNEUROSCI.0672-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.