Abstract
Introduction
The recent success of early-phase clinical trials for adeno-associated viral (AAV) liver-directed gene therapy for hemophilia B (HB) demonstrates the potential for gene therapy, in the future, to succeed protein-based prophylaxis therapy for HB. Significant obstacles, however, need to be overcome prior to widespread adoption. The largest obstacles include immune responses to the AAV capsid including preexisting neutralizing antibodies (NAbs) and a delayed cellular immune response. Emerging evidence suggests that the latter is vector-dose dependent. Furthermore, the development and eradication of inhibitors remains a significant safety concern. Similarly, biological differences between Factor VIII and Factor IX (FIX) impose challenges to direct adoption of the successes for HB to hemophilia A (HA).
Areas covered
The advantages and limitations of the current strategies addressing these obstacles for gene therapy for HB and HA are discussed, as well as vector manufacturing issues relevant to widespread adoption. Alternative strategies including both ex-vivo and in-vivo lentiviral-based methods are discussed, though we focus on AAV-based approaches because of their recent clinical success and potential.
Expert opinion
Our opinion is that these obstacles can be overcome with current approaches, and AAV-based gene therapy for HB will likely translate into future clinical care. Innovative approaches are, however, likely needed to solve the current problems obstructing HA gene therapy.
Keywords: adeno-associated viral, gene therapy, genetic disease, hemophilia, recombinant vectors
1. Overview
Over the last two decades, a series of preclinical and early-phase clinical studies on gene therapy for hemophilia have demonstrated an impressive amelioration of the disease phenotype, establishing the clinical potential of gene therapy for this disease [1]. Manno et al. described the safety and efficacy of the initial liver gene therapy trial using adeno-associated viral (AAV) (serotype) 2 vectors for hemophilia B (HB) [2] as well as outlining critical limiting features of AAV-based liver-directed gene therapy. These results helped form the basis for the recent success reported by Nathwani et al. of sustained long-term expression of therapeutic levels of FIX in men with severe HB using AAV8 liver-directed gene therapy [3,4]. In this latter trial, five of the six subjects who received the highest vector dose had a greater than 90% reduction in their annual bleeding episodes, and four of the seven subjects who were receiving prophylaxis therapy were able to discontinue prophylaxis factor replacement. These results dramatically highlight the potential of gene therapy to eventually supplant protein factor replacement as the standard therapy for hemophilia prophylaxis. Indeed, in the future, gene therapy may be able to deliver sufficient hemostatic coverage to achieve the aspiration of M.W. Skinner, past President of the World Hemophilia Federation, of “full integration opportunities in all aspects of life” that is “equivalent to someone without a bleeding disorder [5].”
However, significant obstacles exist to achieve this end. Foremost is the ability to extend the technologies to HB patients specifically excluded from these clinical studies including patients with detectable neutralizing antibodies (Nabs) to AAV8, underlying iatrogenic liver disease, and patients at more than a minimal risk of inhibitor development. Although there is a relative high prevalence of anti-AAV NAbs in the general human population, which limits enrollment of current clinical trial subjects, potential successful candidates can now be selected with high certainty. Furthermore, a vector dose-dependent T-cell-mediated immune response against the AAV capsid also limits the vector dose that can be safely administered in human subjects. Although several efficacy and safety concerns were predicted by preclinical studies, models for this cellular immune response remain elusive; thus, a major safety concern cannot be properly researched. Though the experience of a gene therapy for HB may provide a roadmap for how gene therapy for hemophilia A (HA) may navigate similar obstacles, there are important biological differences between FIX and Factor VIII (FVIII) that create their own set of unique barriers for gene therapy for HA. Here we first address how these obstacles for widespread adoption of AAV-based HB gene therapy may be surmounted, and then discuss the biological differences between FIX and FVIII that complicated the direct translation of success in HB to HA. Lastly, we address AAV-vector manufacturing, which will need to be expanded and standardized in order for gene therapy to be widely adopted as a treatment for hemophilia.
2. Overcoming immune responses to AAV
AAV has emerged as the principle vector for in vivo gene therapy [6]. It is derived from nonpathogenic replication-deficient parvovirus and requires co-infection with a helper virus for effective replication [7]. Multiple AAV serotypes are available with distinct tissue tropisms [8]. Its ascendency as the most popular vector for in vivo gene therapy is supported by recent clinical trial successes for HB [3,4] as well as other monogenic diseases such as Leber congenital amaurosis type 2, lipoprotein lipase deficiency and muscular dystrophy [9,10]. Despite having relatively low innate immunity and low transduction efficiency of antigen-presenting cells [11], the responses to AAV capsid proteins by the immune system constitute significant obstacles for extending gene therapy to all patients with hemophilia as well as achieving higher factor levels. Two categories of immune responses limit widespread adoption of AAV-based gene therapy for hemophilia: first, preexisting NAbs against AAV capsid proteins impair transduction [12] and limit AAV-based gene therapy to a single administration; and second, a delayed cellular immune response targets transduced cells, which can diminish sustained factor expression.
2.1 Overcoming preexisting neutralizing antibodies
The presence of preexisting antibodies against AAV blocks AAV-vector transduction by intravascular delivery. The magnitude of the inhibitory effect of these antibodies was first noted in AAV2 delivery through hepatic artery in men with HB in which NAb titers > 1:17 prevented transduction whereas titers < 1:2 did not [2]. Additional studies in nonhuman primates (NHPs), a natural host for AAV8, showed that NAb titers as low as 1:5 prevent liver transduction by AAV8 [13] as well as other serotypes [14,15]. Clinical trials for liver AAV gene therapy are currently excluding candidates with NAb titers ≥ 1:5. However, the prevalence of NAbs titers ≥ 1:5 in boys with hemophilia is 37, 16 and 19% for anti-AAV2, AVV5 and AAV8, respectively [16]. Though higher AAV-vector doses can surmount preexisting NAbs [2,17], this relationship is probably nonlinear and difficult to predict. Simply avoiding NAbs by subject exclusion is a short-term strategy and additional approaches are needed for gene therapy to become a routine treatment for hemophilia. Potential strategies include plasmapheresis and immunosuppression, as well as several strategies to circumvent NAbs by utilizing AAV serotypes with less seroprevalence, employing a therapeutic product with empty ‘decoy’ vectors, or localized perfusion techniques. It is worth noting that these approaches are not mutually exclusive and a combination of these approaches may be eventually required.
Plasmapheresis has demonstrated the ability to diminish anti-AAV NAb titers in human subjects undergoing plasmapheresis for other clinical indications. However, it was most effective in decreasing the titer to < 1:5 when the initial titer was < 1:20 [18]. In contrast, plasmapheresis was able to almost restore transgene expression in NHPs undergoing muscle-directed AAV gene transfer with isolated limb perfusion [19]. Similarly, intensive immunosuppression with rituximab, anti-thymoglobulin, methylprednisolone, mycophenlate mofetil and tacrolimus was only modestly and temporarily able to limit anti-AAV NAb formation in NHPs after an initial dose of liver-directed AAV gene therapy [20]. In contrast, extended immunosuppression with rituximab, rapamycin and IVIG limited anti-AAV antibody formation in a human subject after AAV1-based gene therapy for Pompe disease [21]. These mixed results are, at least partially, due to the distinct conditions of each of the aforementioned reports. Nevertheless, it is unlikely, based on this limited data, that either approach alone will be sufficient to overcome the current obstacle that anti-AAV NAbs present. It remains to be seen whether the inclusion of either plasmapheresis or immunosuppression with other approaches will provide additional benefit.
Engineered structurally distinct hybrid AAV serotypes demonstrate significantly lower seroprevalences than naturally occurring serotypes, but even at the 1:20 titer threshold their seroprevalence is not zero [22]. Utilizing vectors with the lowest NAb seroprevalence will expand the number of patients eligible for AAV-based gene therapy, but by itself, it will probably not allow for either universal adoption or repeated administration.
The ability of empty viral capsids to act as decoys against preexisting NAbs is also being investigated. Empty virions are produced during the manufacturing and packaging processes of viral vectors and can either be removed or reintroduced at specified ratios to the final therapeutic product. In the clinical trial reported by Manno et al. using AAV2-FIX, these empty capsids were stringently removed. Manno et al. observed no FIX expression in their medium dose (4 × 1011 vg/kg) cohort including in subjects without detectable anti-AAV antibodies [2]. In contrast, in the clinical trial reported by Nathwani et al. using AAV8-FIX in which empty capsids were not removed from the vector preparation and constituted approximately 80% of the final product [23], FIX levels ranging from 2 to 4% in both the low- and medium-dose cohorts (2 × 1011 and 6 × 1011 vg/kg, respectively) were achieved [3,4]. Direct comparison between these two trials is complicated by the use of different vector serotypes; however, in the high-dose cohort (2 × 1012 vg/kg), the peak FIX expression was comparable. These data tabulated in (Table 1) suggest that total capsid (cp) doses of at least 1 × 1012 cp/kg may be necessary to surmount even low level of preexisting NAbs. Indeed, Mingozzi et al. showed in a mouse model of anti-AAV NAbs that empty capsids can enhance FIX transgene expression in a dose-dependent manner that is also a function of NAb titer; however, excessive empty capsids also lead to decreased transgene expression, likely due to competition with vector at cellular binding sites [24] since the addition of empty capsids significantly decreases transduction in mice without NAbs [25]. Together, the human and mouse data suggest that reintroduction of empty viral capsids is a promising strategy for avoiding NAbs, especially at low titer levels. Of note, high total capsid dose is a potential concern for enhanced cellular immune response, though as discussed in Section 2.2, this has not been observed in clinical trials.
Table 1.
Comparison of AAV-based liver-directed gene therapy for hemophilia B.
| AAV2 liver-directed FIX trial [2] |
AAV8 liver-directed FIX trial [3] |
|||||||
|---|---|---|---|---|---|---|---|---|
| Dose cohort |
Vector dose (vg/kg) *Empties removed |
Total capsid dose (cp/kg) |
Initial FIX level (%) |
Prevalence of transaminitis (onset time) |
Vector dose (vg/kg) *Contains fourfold empties |
Total capsid dose (cp/kg) |
Initial FIX level (%) |
Prevalence of transaminitis (onset time) |
| Low | 8 × 1010 | 8 × 1010 | 0 | 0/2 | 2 × 1011 | 1 × 1012 | 2 | 0/2 |
| Medium | 4 × 1011 | 4 × 1011 | 0 | 1/2 (4 weeks) | 6 × 1011 | 3 × 1012 | 2 – 3 | 0/2 |
| High | 2 × 1012 | 2 × 1012 | 10 – 12 | 1/2 (4 weeks) | 2 × 1012 | 1 × 1013 | 4 – 10 | 4/6 (7 – 9 weeks) |
All subjects who developed transaminitis had anti-AAV NAbs < 1:5 in both trials.
AAV: Adeno-associated viral; FIX: Factor.
Localized perfusion techniques of the target tissue may also help circumvent NAbs. These procedures aim to replicate the ability of direct introduction of AAV to target tissues such as the eye [9] to avoid preexisting NAbs. Manno et al. utilized direct hepatic artery infusion for AAV2-FIX gene transfer and were able to achieve transient FIX levels of 3% in a subject with a 1:17 NAb titer [2]. In NHPs, both direct portal infusion after a blood-removing saline flush and fluoroscopically guided infusion with a microballoon catheter (which temporarily isolates the infusate) allowed for comparable levels of FIX transgene expression in animals with NAb titers between 1:14 and 1:56 as in animals without detectable NAbs [26]. Though certainly more invasive than systemic infusion, these techniques [27] and other localized perfusion techniques such as percutaneous hepatic perfusion [28] are likely translatable to patients. However, isolated muscle perfusion was unable to overcome high-titer NAbs to AAV2 (1:30, 1:300) due to previous vector administration in HB dogs, though the combined use of an AAV6 serotype vector and localized perfusion achieved FIX levels between 5 and 2% despite very high preexisting anti-AAV2 NAb titers of 1:100 and 1:1000, respectively [29]. This last result underscores the potential of combining approaches in order to overcome NAbs.
Various approaches including plasmapheresis, immunosuppression, selection or manipulation of AAV serotypes, empty decoy capsids, or localized perfusion techniques have demonstrated modest success in improving AAV transduction in the presence of low titer NAbs; however, consistently overcoming high-titer NAbs remains a significant obstacle for extending AAV-based gene therapy for at least 20 - 40% patients with hemophilia who have AAV NAb titers ≥1:5. It has yet to be determined if a combination of these approaches would provide additional benefit.
2.2 Overcoming the cellular immune response
Both Manno et al. and Nathwani et al. observed that the majority of human HB subjects (combined 5 of 8) who received the high dose (2 × 1012 vg/kg) of AAV2 or AAV8 vector, respectively, for liver-directed FIX gene therapy, develop a CD8+ T-cell immune response directed against vector-capsid antigens presented by transduced hepatocytes; if untreated, this cellular immune response diminishes FIX expression [2-4,30]. Importantly, this cellular immune response is not directed against FIX. The damage to the transduced hepatocytes is clinically asymptomatic, but readily evident as a transient rise in liver enzymes, which is a convenient biomarker of this phenomenon. In addition to the high-dose cohort, elevated liver enzymes were also seen in one of the combined four subjects who received the intermediate doses (4 – 6 × 1011 vg/kg), but not observed in the four subjects who received the low doses (8 – 20 × 1010 vg/kg). It is also intriguing that peripheral blood mononuclear cells from subjects in the latter AAV8 trial who received the intermediate and high dose, but not the low dose, demonstrated a capsid-specific T-cell response even though the intermediate-dose subjects did not display elevated liver enzymes. This disconnect may be secondary to differences between liver and peripheral T cells. Of note, this anti-AAV capsid T-cell response was also not seen in preclinical murine, canine or NHP models, including chimpanzee at any dose [31].
Manno et al. initially reported that liver transaminitis developed at 4 weeks after vector administration and was associated with a loss of FIX expression from 11% to < 1%. Interestingly, Nathwani et al. reported liver transaminitis developed between weeks 7 and 9 after vector administration. Moreover, Nathwani et al. were able to effectively prevent the complete loss of FIX expression observed in the earlier trial with an 8 – 12-week tapered course of glucocorticoid therapy. Based on this experience, there appears to be a direct relationship between the magnitude of the decrease in FIX levels and the length of the delay between the elevation of the liver enzymes and the initiation of glucocorticoids therapy. Stringent monitoring of liver enzymes and rapid initiation of immunosuppression appear to be essential to maintain transgene expression at high vector dose. However, this capsid-directed cellular immune response limits the possibility of additional vector dose escalations to achieve higher FIX levels.
The difference in the timing of the transaminitis between the two clinical trials is also not well understood. Preclinical studies in mice had suggested that the AAV8 vector genome uncoats faster than the AAV2 vector [32]; an earlier onset of the capsid-mediated immune response was therefore anticipated in the AAV8 trial, which was the opposite of the clinical experience. This discrepancy emphasizes the limitations of preclinical models. It also remains unclear if preexisting NAbs, at titers low enough to allow for efficient transduction, are a risk factor for developing the cellular immune response.
The lack of a reliable preclinical model demonstrating destruction of transduced hepatocytes due to the cellular immune response has significantly hampered progress in understanding this phenomenon, though the recent development of a mouse model utilizing adoptive transfer of anti-capsid T cells a day after vector administration may help [33]. There is an emerging picture from mice data that AAV vectors activate the innate immune response through the TLR9 pathway including MyD88, which in turn promotes the adaptive anti-AAV capsid CD8+ T cells’ immune response [33-36]. Intriguingly, the composition and structure of the AAV-vector genome may influence the initial innate and subsequent adaptive immune responses. Data from mice suggest that TLR9 activation depends on the structure of the vector genome: immune activation is stronger for the self-complementary compared to single-stranded vectors. Conversely, the time period of upregulation of capsid-specific CD8+ T cells is shorter for the self-complementary vectors than the single-stranded vector [37]. Similarly, depletion of CpG sequences in the vector genome, which is a ligand for TLR9, led to enhanced transgene expression and decreased anti-capsid cellular immune response following AAV muscle gene therapy in mice [38]. However, Nathwani et al. still observed the anti-capsid cellular immune [3] despite the depletion of CpG sequences in their vector genome [37].
Overall, these studies suggest that the optimization of the vector genome to minimize TLR9 signaling and thus avoid initiation of the innate and adaptive immune responses is not currently sufficient to prevent T-cell-mediated destruction of transduced hepatocytes in human subjects. Collectively, these findings in mouse models showed that both self-complementary and single-stranded vectors have risk factors that could explain, at least in part, a clinically significant anti-capsid cellular immunity. Furthermore, the cellular immune response occurs in multiple AAV serotypes. As summarized in (Table 1), however, the emerging concept is that capsid-mediated immune response in human subjects is related to the vector genome dose, but not total capsid dose.
An alternative strategy, therefore, is to enhance the efficiency of the gene therapy product such that lower vector doses, which have not been associated with the cellular immune response, can still provide clinically significant levels of FIX. Protein variants of FIX with increased specific activity may provide a route to lower the vector dose while still achieving clinically relevant FIX activity levels. Probably the most promising such variant is FIX Padua, which has an 8 – 12-fold increased specific activity compared to wild type [39]. This FIX variant has a single missense mutation where a leucine is substituted for an arginine at position 338. In severe HB dogs, AAV muscle and liver gene therapy utilizing FIX Padua has significantly reduced spontaneous bleeds by achieving sustained FIX activity levels up to 240% (antigen level of 29%) [40,41]. Importantly, there has been no thrombotic complications in these dogs; similarly, markers of thrombosis (TAT, D-dimer) were comparable between wild-type mice expressing wild-type FIX and FIX Padua [41-43]. Most excitingly, in severe HB dogs with increased risk for inhibitor formation to canine FIX wild type, liver gene therapy utilizing FIX Padua not only prevented inhibitor formation but also induced immune tolerance induction (ITI) in an animal with a preexisting inhibitor to canine FIX wild type despite subsequent challenges with FIX wild-type protein concentrate [41]. FIX Padua is currently being utilized in at least one of the open gene therapy clinical trials for HB (NCT01687608); an update on this trial has recently been reported with subjects achieving 3 – 25% sustained FIX activity in a dose-dependent manner [44]. This level of FIX activity is likely sufficient to prevent all joint bleeds in most men and would probably allow for at least near “full integration opportunities in all aspects of life [5].” However, the anti-capsid cellular immune response was still observed in at least two subjects who received vector doses equal to or greater than 1 × 1012 vg/kg, which is consistent with the observations from the previous clinical trials (Table 1).
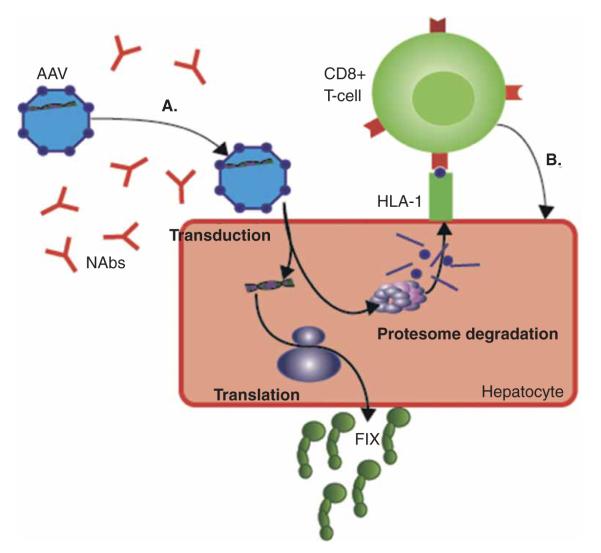
A complementary approach to increase the efficiency of AAV gene therapy to achieve clinically significant levels of FIX at lower vectors doses is the use of novel promoters based on the genome-wide transcriptional modules that result in enhanced FIX gene expression in mouse and NHP models [45]. Development of novel AAV serotypes based on direct evolution [46], and, more recently, using mouse models chimeric with human hepatocytes are also promising approaches to identify more efficient AAV vectors [47]. Notably, as depicted in (Figure 1), the combination of these distinct strategies offers the opportunity to potentially synergistically lower the therapeutic doses of AAV vector to prevent the capsid-mediated immune responses.
Figure 1. Current obstacles posed by the immune system for liver-directed gene therapy for hemophilia.
(A) NAb titers greater than 1:5 prevent meaningful transduction of AAV vectors. Strategies to circumvent NAbs are discussed in Section 2.1. At higher vector doses, there is a T-cell response toward the AAV capsid proteins (purple spheres) (B), which, if untreated, attenuates factor expression due to destruction of transduced hepatocytes. As discussed in Section 2.2, one strategy to overcome this cellular immune response is to improve the efficiency of transduction, translation, and the activity of FIX. Figure components are individually scaled for clarity.
AAV: Adeno-associated viral; FIX: Factor; Nabs: Neutralizing antibodies.
3. Translation for pediatric patients
To date, only adult subjects have been eligible for HB gene therapy trials, and ongoing long-term follow-up for 15 years was advised by the FDA to determine the safety of a gene-based strategy. In addition to ethical considerations, including the higher threshold for efficacy and safety necessary to enroll minor subjects, there are important biological differences between adult and pediatric patients that need to be considered with regard to liver-directed gene therapy. Foremost is the ongoing concern for decreasing levels of FIX over time due to normal growth of both the intravascular space and liver. The approximate fourfold difference in the blood volume between a five-year-old and twenty-year-old requires an equivalent increase in the amount of FIX secreted to maintain the plasma concentration of FIX. In unaffected individuals, this is accomplished by normal liver growth and development. In contrast, in a pediatric recipient of AAV liver gene therapy, liver growth would lead to dilution of the episomal transgene due to hepatocyte division. There is also an ongoing concern about the potential for episomal loss due to hepatocellular proliferation. Both of these mechanisms have been suggested to account for the observed decline of FIX plasma levels over time after AAV gene therapy in utero or perinatally in both sheep and NHPs [48,49]. Integration of the vector genome into the recipient’s DNA-utilizing vectors such as lentivirus, which allows for transgene replication during cell division, may provide for sustained transgene expression during normal growth and development. There are, however, heightened safety concerns involved with integrative approaches: most significantly, genotoxicity due to insertional mutagenesis and germline transmission.
3.1 Safety concerns: genotoxicity and germline transmission
Genotoxicity is always a safety concern for any gene therapy product. Though AAV has very low rates of integration [50], AAV vectors have been shown in mouse models to predispose to hepatocellular carcinoma (HCC). Over a decade, a series of preclinical studies in mouse models have generated conflicting data on the relationship between AAV and HCC. Only recently in a large and carefully designed study by Chandler et al. has this complicated relationship been conclusively demonstrated to be a function of vector dose, vector design including serotype, enhancer and/or promoter as well as age of administration. Notably, the use of hAAT promoter, which is present in most of the ongoing clinical trials, is among the safest vector system evaluated [51]. Furthermore, long-term safety data is maturing from both preclinical dogs and the human subjects that have received liver- and muscle-directed AAV therapy, with greater than 10 years of follow-up in some cases: none of the dogs or human subjects have developed cancers attributable to AAV-based gene therapy [52-54].
The current bioethical consensus is that gene therapy should be limited to somatic corrections and inheritable germline modifications should be avoided [55]. Collective data have demonstrated that rabbits are an excellent preclinical model that well predicts relevant parameters for this safety concern in human subjects. In male rabbits, vector dissemination depends on the route of vector administration, vector dose and tissue tropism. The kinetics of vector clearance from semen was dose- and time-dependent but serotype-independent for AAV serotypes 2, 6 and 8 [56-58], while AAV5 displayed the shortest duration of vector shedding [58]. In all these studies, no late recurrence of AAV sequences was found in semen over several consecutive cycles of spermatogenesis, suggesting that transduction of an early spermatogenesis precursor exposed to AAV did not occur during hematogenous dissemination to the gonads. AAV sequences were also detected in the semen of vasectomized animals, indicating that vector sequences can be found in the semen even in the absence of accessible germ cells [57]. These findings are in agreement with data from early-phase clinical trials on AAV-mediated gene transfer showing only transient shedding of vector sequences mainly following intravascular delivery of the vector [2,3] compared to intramuscular injection [59,60]. Close monitoring is prudent for inadvertent transmission of vector sequences and is required in the advent of novel AAV serotypes and other vector systems such as integrating vectors and gene repair strategies. Clinically, sperm banking and the use of barrier contraception until definitive vector clearance is required to prevent germline and sexual transmission of vectors.
3.2 Integrating vector approaches
Retrovirus is such a class of integrating vector. Pioneering studies by Katherine Ponder on direct in vivo delivery of gamma-retrovirus for liver-specific expression of FIX or FVIII in neonatal dogs with severe hemophilia resulted in long-term expression of therapeutic levels of the missing factor, 12 – 36% for FIX and 116% for FVIII [61,62]. However, there are two major limitations of this strategy: i) this strategy requires cell division of the targeted hepatocyte, which restricts this approach to neonatal animals or adult animals with induced hepatocyte division; and ii) there is ongoing concern for oncogenesis with this vector, as discussed below. Recently, FIX lentiviral liver-directed gene therapy was shown to significantly decrease the bleeding frequency of adult HB dogs with sustained FIX activity of between 1 and 2% for 1.5 – 2.5 years for two animals that received a codon-optimized FIX wild type or FIX Padua; moreover, in provocative mice models for oncogenesis, no genotoxicity was observed with this lentiviral approach [63]. Also, notable is the recent use by Mark Kay and coworkers of a promotorless FIX construct that specifically integrates just upstream from the mouse albumin stop codon and then utilizes highly efficient ribosome skipping to translate both functional albumin and FIX from the same mRNA. AAV8-based gene therapy using this construct resulted in 7 – 20% FIX levels in HB mice [64]. Kay and coworkers also demonstrated that all FIX expressed is due to on-target integration, though the rate of nontranscribed off-target integration was not reported.
However, the history of unanticipated oncogenic risk years after integrating gene therapy (using gamma-retroviral vector) for X-linked SCIDS is a cautionary tale [65]. As highly efficacious protein therapy currently exists for hemophilia, almost no insertional mutagenesis risk is tolerable. Similarly, germ-line transmission of vector-coded sequences is a significant safety concern for in vivo gene therapy using viral vectors, which has profound bioethical implications [55]; this risk will need to be meticulously addressed for integrative approaches. Mature data from long-term follow-up of large animal models receiving integrating FIX and FVIII gene therapy, as well as mature data from integrating gene therapy clinical trials for other benign diseases, is likely necessary before integrating FIX gene therapy clinical trials are contemplated.
4. Inhibitors
In addition to antibody and cellular immune responses to AAV capsids, immune response to expressed FIX protein is also a concern for gene therapy for hemophilia. NABs to protein therapy, termed inhibitors, are already a significant complication of factor replacement. Inhibitors occur in about 20 – 30% of patients with severe HA, in about 10% in patients with non-severe HA, and in around 1 – 5% of patients with HB [66,67]. The risk of inhibitor development is related to the underlying hemophilia causing mutation, though other factors such as the clinical scenario of factor exposure and immunological genetic background also contribute [68]. Patients with mutations resulting in the transcription of cross-reactive material are less likely to develop inhibitors. Eradication of inhibitors can be accomplished using ITI, which usually requires daily factor infusion, and often necessitates placement of a central venous catheter in pediatric patients as well as substantial health-care expenditures. In HB, more than half of patients who develop inhibitors will also manifest an allergic reaction toward FIX protein, which almost always requires medical intervention [69]. In these patients ITI is often complicated by nephrotic syndrome [70]. These complications in combinations with the low incidence and lack of clinical data make treating patients with HB and inhibitors very challenging.
To date, no human subjects receiving AAV FIX gene therapy have developed inhibitors. However, the eligibility criteria for these clinical trials was prudently constructed to minimize the risk of inhibitor formation by including only patients with prolonged exposure history to FIX protein and a reassuring HB mutation [2-4,59]. Nonetheless, significant preclinical data have accumulated, suggesting that liver direction FIX gene therapy is unlikely to provoke inhibitor formation and, moreover, may be a strategy to induce immune tolerance.
FIX is normally secreted from the liver. Early studies in inhibitor-prone mice with large FIX gene deletions demonstrated that AAV liver gene therapy could induce immune tolerance, though the efficacy was strain dependent [71]. Similarly, AAV liver gene therapy in two inhibitor-prone HB dogs successfully induced immune tolerance such that these animals tolerated subsequent FIX protein infusions without inhibitor formation. In these dogs, a single FIX concentrate protein infusion is generally sufficient to provoke an inhibitory antibody. Furthermore, three similar animals receiving AAV muscle gene therapy all developed inhibitors [72,73]. These results highlight that liver gene therapy has a low risk of inhibitor development, especially compared to muscle gene therapy or protein infusion. Most intriguingly, Crudele et al. recently described inhibitor eradication and tolerization with AAV8 liver gene therapy using canine FIX Padua transgene in an inhibitor prone HB dog that had previously developed cross-reactive inhibitor to canine FIX after exposure to human FIX. Importantly, no evidence of allergic or nephrotic syndrome symptoms was observed. This dog maintained tolerance despite a subsequent challenge with wild-type canine FIX concentrate protein [41]. Together these preclinical animal studies suggest that liver-directed gene therapy may be a mechanism to promote tolerance even after inhibitor formation due to previous protein exposure. Indeed, FIX expression via transgene translation provides the continuous uninterrupted exposure to factor that is necessary to achieve ITI. Additionally, a series of studies in HA dogs and NHPs showed a central role of T-regulatory cell in AAV liver gene therapy [74,75]. Based on these canine studies, we speculate that expression of FIX (and possibly other proteins) from human hepatocytes may provide additional protolerogenic benefits.
5. Ectopic-directed gene therapy for HB
The liver is the endogenous source of FIX. However, it may not always be the most advantageous target for hemophilia gene therapy. Subjects with evidence of liver dysfunction or active HBV, HCV and HIV have been excluded from the clinical trials described by Manno et al. and Nathwani et al. Though the prevalence of these viral infections in hemophilia patients has dramatically declined due to a combination of recombinant factors and significantly improved safety of plasma-derived factors, the prevalence of markers for HBV and HCV infection in men born with HB between 1976 and 1980 is still 35 and 80%, respectively, and increases to 50 and 90%, respectively, in men born between 1961 and 1970 [76]. The recent FDA approval of the anti-HCV combination therapy, Viekira Pak, will likely result in a decrease in the number of men with hemophilia with active HCV, but ongoing concerns about liver dysfunction or cirrhosis will still likely limit liver-directed gene therapy in this population.
An alternative approach is muscle-directed gene therapy. The first clinical trial utilizing AAV for gene therapy for HB achieved a slight increase in FIX levels and modest decrease in FIX infusions in some subjects after intramuscular injection of vector without inhibitor development [59]. Furthermore, sustained FIX expression has been demonstrated by muscle biopsy up to 3.5 and 10 years, without evidence of malignant transformation after intramuscular injection [53,54]. Moreover, FIX levels of up 5% have been achieved after AAV muscle-directed gene therapy with peripheral transvenular delivery and isolated limb perfusion in HB dogs [29] and, more recently, FIX activity levels of up to 8% have been achieved with the similar vectors and delivery combined with the hyperactive FIX Padua variant [40]; only 1 of the 11 dogs combined from these 2 studies developed an inhibitor, which was transient and peaked at 1.5 BU. Together, the modest success of this early human trial [59] and the ongoing demonstration of the efficacy and safety of AAV muscle-directed gene therapy in preclinical canine models of HB establishes this approach as a worthwhile alternative to liver-directed gene therapy, particularly for patients with liver dysfunction, which is especially applicable to older men with hemophilia and a history of iatrogenic liver disease.
Hematopoietic stem cells (HSC) are also an attractive target for ex vivo lentiviral-mediated ectopic expression of clotting factors [77]. Promoters and enhancers can be chosen to be either lineage-specific, usually platelets [78-80], or non-lineage specific. The rationale is that platelet-specific FIX or FVIII would be stored in the platelet α-granules, which may minimize the deleterious effect on clotting factor-dependent hemostasis from circulating factor inhibitors, as has been demonstrated in mice models by several groups [78-80]. However, additional studies in large animal hemophilia models, such as dogs, are needed to determine the safety and efficacy of this approach (the first such study of HA dogs was recently reported, as discussed below in Section 6 [81]). Specifically, studies in large animal models with preexisting inhibitors are needed to test the hypothesis that platelet-stored factor can circumvent circulating inhibitors to provide therapeutic hemostasis. This experiment is critical for understanding the translational potential of this approach as patients with refractory inhibitors are the most likely benefit from such a strategy. Furthermore, such an experiment would also help define the risks of insertional mutagenesis and bone marrow conditioning in the setting of inhibitors, as well as to determine if these risks are outweighed by a long-term hemostatic benefit despite circulating inhibitors. This result would be of significant clinical interest as there are very limited treatment options for patients with refractory inhibitors.
6. Gene therapy for HA
Though many of the approaches that have allowed for successful gene therapy for HB can be appropriated for gene therapy for HA, biological differences between FVIII and FIX produce specific obstacles in the development of the latter. FVIII is a significantly larger protein than FIX, 280 kDa compared to 55 kDa, respectively [82], which presents AAV-vector packaging problems. However, this obstacle can be overcome by utilizing B-domain-deleted (BDD)-FVIII, since the large B domain of FVIII, which accounts for 40% for the protein, can be truncated to 14-amino acids without loss of activity [83]. In addition, since FVIII is normally secreted as a heterodimer of a heavy and light chain coupled through noncovalent interactions, two separate vectors can be utilized, each encoding one chain [84]. More problematic, however, is the observation that FVIII is intrinsically poorly expressed and inefficiently secreted even when compared to similar-size genes (as reviewed in [85]). Conversely, the normal plasma concentration of FVIII is significantly lower than for FIX: 1 and 90 nM, respectively [82]. These differences disallow direct adoption of successful therapeutic vector doses for HB gene therapy to HA gene therapy. Rather, preclinical evaluation is required to determine the likely therapeutic vector dose. Lastly, recent work has demonstrated that FVIII is likely exclusively expressed in endothelial cells, including mostly in the liver sinusoid endothelial cells, rather than hepatocytes [86,87]. Liver-directed gene therapy for HA, therefore, relies on ectopic expression of FVIII, which to date has been satisfactory.
Several innovations have enhanced the efficiency of AAV gene therapy for HA. A novel bioengineered BDD-FVIII variant with a histidine substituted for an arginine in the residual B-domain at position 1645 (R1645H) resulted in a twofold enhanced expression after AAV8 gene therapy in HA mice; most excitingly, this variant also demonstrated superior hemostasis even at comparable antigen levels of BDD-FVIII [88]. Codon optimization of BDD-FVIII has also resulted in an unprecedented 30 – 40-fold increase in expression of BDDFVIII [89]. Most recently, codon optimization was incorporated into an innovative gene therapy construct that also included both a novel 17-amino acid peptide (termed V3), which re-introduced all six glycosylation triplets of the undeleted B-domain into the residual B-domain as well as an improved liver-specific promoter [90]. In combination, this construct in an AAV8 vector was able to achieve levels of 12 – 25% and 40 – 75% with vector doses of 2 - 7 × 1012 and 2 × 1013 vg/kg, respectively, in NHPs; however, both vector doses were associated with the development of FVIII inhibitors for more than 20 weeks, which resolve only after immunosuppression with rituximab and cyclophosphamide. Based on clinical experience in patients receiving ITI, rituximab does prevent an amnestic response to FVIII protein in patients with inhibitors. However, the strength of the tolerance in the NHPs who received FVIII-V3 gene therapy is unknown since no challenges with FVIII protein were reported. The use of non-species-specific FVIII in these animals also hampers the assessment of immunogenicity as the expression of human factors in animal models is associated with a high rate of inhibitors. The use of this construct in canine HA models will better inform on the risk of inhibitor development. Together, these preclinical studies demonstrate the potential of AAV-based gene therapy for HA. However, significantly higher vector doses have been required to achieve clinically relevant FVIII levels than were required to achieve similar FIX levels in comparable preclinical models. Caution is therefore warranted as, based on the FIX experience detailed in Section 2, these vector doses are likely to elicit a strong cellular immune response in human subjects. Additional innovations that further enhance the efficiency of gene therapy for HA will likely be necessary before AAV-based gene therapy can begin clinical trials.
An alternative approach to overcome the current obstacle of relative high vector doses required for in vivo FVIII gene therapy is ex vivo FVIII gene therapy, targeting HSC as already described for HB in Section 5. Non-myeloablative conditioning bone marrow transplant of transduced HSC utilizing porcine-human chimeric BDD-FVIII, which demonstrates an order of magnitude greater secretion than human BDD-FVIII, has achieved therapeutic levels of between 2 and 4% even with mixed chimerism in mice [91,92]. Similarly, bone marrow transplant of HSC utilizing a platelet-specific human FVIII in severe HA dogs resulted in an improvement of the disease phenotype without evidence of toxicity including FVIII inhibitors [81]. However, recent evidence also suggests that FVIII expression in platelets may potentially perturb megakaryopoiesis [93]. A recent study using lentiviral vector for ex vivo of blood outgrowth endothelial cells also showed early evidence of efficacy [94]. Additional studies in large animal models are needed to determine the safety and efficacy of these approaches, especially the risk associated with conditioning chemotherapy and associated thrombocytopenia in hemophilia.
Inhibitor formation remains a major concern in the development of HA therapeutics, especially gene therapy. However, as discussed in Section 4, liver expression of coagulation factors may be tolerogenic. Canine FVIII AAV liver-directed gene therapy was able to completely tolerize three out of four HA dogs with preexisting FVIII inhibitors such that subsequent protein administrations did not induce an anamnestic response; these dogs had sustained FVIII levels of 2 – 8% and a greater than 90% reduction in bleeding episodes. The fourth dog experienced a very strong anamnestic response to 216 BU, which subsequently decreased to 0.8 BU [74]. The 75% complete success of liver-directed gene therapy tolerizing these animals is at least comparable to current ITI regimens [95]. This experience in HA dogs suggests that liver-directed gene therapy may provide extra tolerogenic benefits in addition to sustained FVIII expression.
7. AAV manufacturing Issues
The long-term success of several clinical trials using AAV vectors for local or systemic delivery as well as the recent approval of alipogene tiparvovec (Glybera) by the European Medicine Agency [96] has energized the field and subsequently increased the interest of many pharmaceutical companies in gene therapy and vector manufacturing. Over the last two decades, the development of recombinant AAV vector has moved from limited technologies and modest production yields to what is now necessary for the ongoing and planned gene therapy trials. Continuing improvement in the manufacturing process using adherent mammalian cells with transient transfection, suspension culture and invertebrate cells, aimed at large-scale production of recombinant AAV vectors, will be critical for the success of vector commercialization [97-99]. Similarly, better characterization of vector preparations, such as the recent multicenter studies using reference standard material [100], will provide critical information to allow for direct comparison between vector parameters, and preclinical and clinical study outcomes. Notably, most of these clinical successes are primarily focused on orphan diseases, but the progression to more common diseases, including vaccines, is anticipated. Accordingly, the manufacturing processes will need to be further optimized.
8. Expert opinion
Protein factor replacement therapy, where it is available, has dramatically improved the quality of life and life expectancy of patients with hemophilia [101,102]. However, many unmet needs remain, including the necessity of repeated intravenous access, the morbidity of inhibitor development and eradication, the continued limitations on social integration imposed by the bleeding phenotype, and the treatment gap between the developing and developed world. Recent clinical trial results of AAV-based liver-directed gene therapy for HB demonstrate the possibility of AAV-based gene therapy for meeting many of these needs [2,3] and in the future, potentially succeeding factor replacement as the standard prophylactic therapy for HB. Over two decades of preclinical and clinical research preceded these results, but cautious optimism is warranted about this future. Alipogene tiparvovec recently become the first gene therapy product approved in the western world, which provides an example of successful commercialization of such therapies [96].
Expanding treatment to the 80% of patients with hemophilia who live in the developing world is an ethical imperative [103]. The potential of a single infusion of a gene therapy product to provide the equivalent hemostasis of a lifetime of multiple times-per-week intravenous protein replacement, in other words a ‘one and done’ therapy, is analogous to the ability of vaccines to provide lifetime protection against infectious diseases.
It is also worthwhile to compare FIX gene therapy with the recently approved recombinant FIX Fc fusion protein and the other similar products employing various half-life extending technologies in late clinical trials (as reviewed in [104,105]). These novel protein therapeutics decrease the frequency of venipuncture needed to provide effective prophylaxis and will likely enhance the quality of life of patients, especially pediatric patients. The overall immunogenicity of these long-acting factors will have to be determined in a previously untreated patient study. However, the comparative advantages of gene therapy include ‘one and done’ definitive therapy as well as the potential pro-tolerogenic benefits of liver expressed FIX. For example, at least 304 infusions of long-acting FIX (assuming a conservative 2-week frequency) would have been necessary to provide comparable prophylaxis to the 4 subjects now off prophylaxis after AAV liver gene therapy for 152 total months reported recently [3].
Despite these advantages, obstacles remain as summarized in (Table 2). As discussed, the ongoing and planned incorporation of the FIX Padua variant with 8 – 12-fold increased specific activity with other strategies to enhance AAV efficiency may allow for therapeutic FIX levels with a vector dose insufficient to trigger the anti-capsid cellular immune response. This would be a major achievement as the cellular immune response was not predicted by large animal models and, to date, it is not possible to identify the high-risk group for this complication. Notably, FIX Padua may also provide FIX levels that are sufficiently high, such that the contemplation of FIX AAV liver-directed gene therapy in boys with hemophilia can begin, if even a fourfold dilution of the FIX level due to normal growth would continue to provide therapeutic benefit. A larger obstacle is the translation of the success of AAV-based FIX gene therapy to FVIII gene therapy, which has been less effective to date. Both in vivo and ex vivo approaches are being investigated, with the development of inhibitors continuing to be a significant safety concern for all approaches. However, the emerging data from both HA and HB dogs that FVIII and FIX AAV-directed liver gene therapy, respectively, may induce sustained immune tolerance to the transgene could favor this approach. An incremental design will probably be necessary to translate these results into human patients where subjects that are slightly more prone to inhibitors or with slightly higher titers are included with each subsequent iteration.
Table 2.
Comparison of obstacles and solutions to widespread adoption of AAV-based gene therapy for hemophilia B.
| Obstacles | Solutions | Advantages | Limitations |
|---|---|---|---|
| Preexisting NAbs (Section 2.1) |
Plasmapheresis | Modest success in human subjects Can be combined with other solutions |
Most effective lowering titers < 1:20 |
| Immunosuppression regimen |
Can be combined with other solutions | Low efficacy Infectious risk |
|
| Serotype variation | Low toxicity Can be combined with other solutions |
Unlikely to achieve 0% seroprevalence in many subjects Safety will need to be demonstrated for every serotype |
|
| Empty capsids | Demonstrated relative efficacy Low toxicity Can be combined with other solutions |
Theoretical concern of strengthening cellular immune response |
|
| Localized perfusion | Demonstrated improved transgene expression in humans and large animal models Can be combined with other solutions |
Invasive procedure | |
| Cellular immune response (Section 2.2) |
Immunosuppression | Proven efficacy | Narrow therapeutic window to maintain FIX expression Duration of therapy is not clear May not be effective in all patients |
| Genome optimization | Can be combined with other solutions | Unlikely to be sufficient in isolation | |
| Lower vector dose | Substantial efficacy | Requires system-specific strategies to enhance transduction, translation and/or activity |
|
| Inclusion of pediatric patients (Section 3) |
High initial factor activity |
May be attainable with current strategies Safety could be determined in adult subjects |
Thrombotic risk |
| Inclusion of patients with liver disease (Section 5) |
AAV-based muscle- directed gene therapy |
Proven long-term efficacy in large animal models |
Requires transient immunosuppression to limit inhibitor formation |
AAV: Adeno-associated viral; FIX: Factor; Nabs: Neutralizing antibodies.
This is an optimistic time for gene therapy for hemophilia. Ongoing and planned clinical trials for HB are likely to clarify some of the unresolved safety concerns. For example, AAV8 single-stranded DNA genome with distinct amounts of empty capsid will be compared with the long-term successful strategy using self-complementary genome and mostly empty capsid. Moreover, the utilization of FIX Padua with AAV8 self-complementary genome and about 10% empty capsids is ongoing and will be highly informative in terms of immune responses to the vector capsid and this hyperactive FIX variant. Additionally, the determination if the vector genome (single-stranded versus self-complementary) has a significant role in the outcome of immune responses will be critical since the self-complementary strategy is not applicable for AAV-based gene therapy for HA and many other disorders with relatively larger genes. Innovative approaches are necessary to overcome the current obstacles hindering HA gene therapy. Despite continued obstacles, our opinion is that current approaches for AAV-based gene therapy for HB will translate into future clinical care.
Article highlights.
Proof-of-concept studies of adeno-associated viral (AAV) liver gene therapy over the last two decades have paved the way for current successful clinical trials demonstrating evidence of AAV-transduced human hepatocytes resulting in long-term expression of therapeutic factor levels in men with severe hemophilia B (factor IX deficiency) without formation of antibodies to the transgene.
The main safety concern following AAV delivery is the cellular immune response to the vector capsid that can limit the efficacy of gene transfer in a vector-dose-dependent manner. This complication can usually be controlled with transient immunosuppression.
The presence of neutralizing antibodies to AAV vectors is the main limiting factor preventing inclusion of many patients. Novel developments in vector capsid, vector delivery and potentially other viral vectors are needed to extend promising studies to all patients.
Translational studies for hemophilia A (Factor VIII deficiency) for liver expression by AAV vectors are encouraging, and the use of transgene with advantageous biological activity is likely to enhance efficacy, but careful and extensive assessment of immunogenicity is critical to define the safety profile.
Ectopic expression of transgene may be required for those with underlying liver disease, and continued development in these areas is needed to demonstrate translational potential.
Liver gene therapy for young patients may provide a simplified strategy for early onset of uninterrupted prophylactic therapy while facilitating immune tolerance to the transgene. Cumulative data from clinical studies in adults will guide the risk and benefit assessment of a given strategy for boys with hemophilia.
This box summarizes key points contained in the article.
Acknowledgments
This work was supported by NIH/NIHLBI grant P01 HL64190: Gene Therapy for Hemophilia (Poncz, PI: Arruda, Leader Project 1). BSJ is the recipient of an HTRS/Novo Nordisk 2015 Mentored Research Award in Hemophilia or Rare Bleeding Disorders from the Hemostasis and Thrombosis Research Society, Inc., which was supported by Novo Nordisk Inc. He also received a Bayer Fellowship Project Award 2015.
Footnotes
Declaration of interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.High KA. The gene therapy journey for hemophilia: are we there yet? Hematology Am Soc Hematol Educ Program. 2012;2012:375–81. doi: 10.1182/asheducation-2012.1.375. [DOI] [PubMed] [Google Scholar]
- 2.Manno CS, Pierce GF, Arruda VR, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12(3):342–7. doi: 10.1038/nm1358. •• First AAV-based liver gene therapy for hemophilia B.
- 3.Nathwani AC, Reiss UM, Tuddenham EG, et al. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N Engl J Med. 2014;371(21):1994–2004. doi: 10.1056/NEJMoa1407309. •• Most recent report of long-term clinical trial using AAV-based liver gene therapy for hemophilia B.
- 4.Nathwani AC, Tuddenham EG, Rangarajan S, et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med. 2011;365(25):2357–65. doi: 10.1056/NEJMoa1108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skinner MW. WFH: closing the global gap–achieving optimal care. Haemophilia. 2012;18(Suppl 4):1–12. doi: 10.1111/j.1365-2516.2012.02822.x. [DOI] [PubMed] [Google Scholar]
- 6.Mingozzi F, High KA. Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nat Rev Genet. 2011;12(5):341–55. doi: 10.1038/nrg2988. [DOI] [PubMed] [Google Scholar]
- 7.Zinn E, Vandenberghe LH. Adeno-associated virus: fit to serve. Curr Opin Virol. 2014;8:90–7. doi: 10.1016/j.coviro.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao GP, Alvira MR, Wang L, et al. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci U S A. 2002;99(18):11854–9. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maguire AM, Simonelli F, Pierce EA, et al. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N Engl J Med. 2008;358(21):2240–8. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaudet D, Methot J, Dery S, et al. Efficacy and long-term safety of alipogene tiparvovec (AAV1-LPLS447X) gene therapy for lipoprotein lipase deficiency: an open-label trial. Gene Ther. 2013;20(4):361–9. doi: 10.1038/gt.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nayak S, Herzog RW. Progress and prospects: immune responses to viral vectors. Gene Ther. 2010;17(3):295–304. doi: 10.1038/gt.2009.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masat E, Pavani G, Mingozzi F. Humoral immunity to AAV vectors in gene therapy: challenges and potential solutions. Discov Med. 2013;15(85):379–89. [PubMed] [Google Scholar]
- 13.Jiang H, Couto LB, Patarroyo-White S, et al. Effects of transient immunosuppression on adenoassociated, virus-mediated, liver-directed gene transfer in rhesus macaques and implications for human gene therapy. Blood. 2006;108(10):3321–8. doi: 10.1182/blood-2006-04-017913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calcedo R, Wilson JM. Humoral immune response to AAV. Front Immunol. 2013;4:341. doi: 10.3389/fimmu.2013.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basner-Tschakarjan E, Mingozzi F. Cell-mediated immunity to AAV Vectors, evolving concepts and potential solutions. Front Immunol. 2014;5:350. doi: 10.3389/fimmu.2014.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li C, Narkbunnam N, Samulski RJ, et al. Neutralizing antibodies against adeno-associated virus examined prospectively in pediatric patients with hemophilia. Gene Ther. 2012;19(3):288–94. doi: 10.1038/gt.2011.90. [DOI] [PubMed] [Google Scholar]
- 17.Wang L, Calcedo R, Bell P, et al. Impact of pre-existing immunity on gene transfer to nonhuman primate liver with adeno-associated virus 8 vectors. Hum Gene Ther. 2011;22(11):1389–401. doi: 10.1089/hum.2011.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monteilhet V, Saheb S, Boutin S, et al. A 10 patient case report on the impact of plasmapheresis upon neutralizing factors against adeno-associated virus (AAV) types 1, 2, 6, and 8. Mol Ther. 2011;19(11):2084–91. doi: 10.1038/mt.2011.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chicoine LG, Montgomery CL, Bremer WG, et al. Plasmapheresis eliminates the negative impact of AAV antibodies on microdystrophin gene expression following vascular delivery. Mol Ther. 2014;22(2):338–47. doi: 10.1038/mt.2013.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Unzu C, Hervas-Stubbs S, Sampedro A, et al. Transient and intensive pharmacological immunosuppression fails to improve AAV-based liver gene transfer in non-human primates. J Transl Med. 2012;10:122. doi: 10.1186/1479-5876-10-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corti M, Elder M, Falk D, et al. B-cell depletion is protective against Anti-AAV capsid immune response: A human subject case study. Mol Ther Methods Clin Dev. 2014 doi: 10.1038/mtm.2014.33. doi:10.1038/mtm.2014.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calcedo R, Vandenberghe LH, Gao G, et al. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J Infect Dis. 2009;199(3):381–90. doi: 10.1086/595830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allay JA, Sleep S, Long S, et al. Good manufacturing practice production of self-complementary serotype 8 adeno-associated viral vector for a hemophilia B clinical trial. Hum Gene Ther. 2011;22(5):595–604. doi: 10.1089/hum.2010.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mingozzi F, Anguela XM, Pavani G, et al. Overcoming preexisting humoral immunity to AAV using capsid decoys. Sci Transl Med. 2013;5(194):194ra92. doi: 10.1126/scitranslmed.3005795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao K, Li M, Zhong L, et al. Empty virions In AAV8 vector preparations reduce transduction efficiency and may cause total viral particle dose-limiting side-effects. Mol Ther Methods Clin Dev. 2014;1(9):20139. doi: 10.1038/mtm.2013.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mimuro J, Mizukami H, Hishikawa S, et al. Minimizing the inhibitory effect of neutralizing antibody for efficient gene expression in the liver with adeno-associated virus 8 vectors. Mol Ther. 2013;21(2):318–23. doi: 10.1038/mt.2012.258. • Demonstration that isolated hepatic perfusion can circumvent anti-AAV capsid preexisting neutralizing antibodies.
- 27.Raper SE, Wilson JM, Nunes FA. Flushing out antibodies to make AAV gene therapy available to more patients. Mol Ther. 2013;21(2):269–71. doi: 10.1038/mt.2013.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reddy SK, Kesmodel SB, Alexander HR., Jr Isolated hepatic perfusion for patients with liver metastases. Ther Adv Med Oncol. 2014;6(4):180–94. doi: 10.1177/1758834014529175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arruda VR, Stedman HH, Haurigot V, et al. Peripheral transvenular delivery of adeno-associated viral vectors to skeletal muscle as a novel therapy for hemophilia B. Blood. 2010;115(23):4678–88. doi: 10.1182/blood-2009-12-261156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mingozzi F, Maus MV, Hui DJ, et al. CD8(+) T-cell responses to adeno-associated virus capsid in humans. Nat Med. 2007;13(4):419–22. doi: 10.1038/nm1549. • Molecular mechanism of the anti-AAV capsid T-cell response delineated.
- 31.Flotte TR, Goetzmann J, Caridi J, et al. Apparently nonspecific enzyme elevations after portal vein delivery of recombinant adeno-associated virus serotype 2 vector in hepatitis C virus-infected chimpanzees. Hum Gene Ther. 2008;19(7):681–9. doi: 10.1089/hum.2007.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomas CE, Storm TA, Huang Z, Kay MA. Rapid uncoating of vector genomes is the key to efficient liver transduction with pseudotyped adeno-associated virus vectors. J Virol. 2004;78(6):3110–22. doi: 10.1128/JVI.78.6.3110-3122.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martino AT, Basner-Tschakarjan E, Markusic DM, et al. Engineered AAV vector minimizes in vivo targeting of transduced hepatocytes by capsid-specific CD8+ T cells. Blood. 2013;121(12):2224–33. doi: 10.1182/blood-2012-10-460733. • Mouse model for anti-AAV capsid cellular immune response.
- 34.Martino AT, Suzuki M, Markusic DM, et al. The genome of self-complementary adeno-associated viral vectors increases toll-like receptor 9-dependent innate immune responses in the liver. Blood. 2011;117(24):6459–68. doi: 10.1182/blood-2010-10-314518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rogers GL, Suzuki M, Zolotukhin I, et al. Unique roles of TLR9- and MyD88-dependent and -independent pathways in adaptive immune responses to AAV-mediated gene transfer. J Innate Immun. 2015;7(3):302–14. doi: 10.1159/000369273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu J, Huang X, Yang Y. The TLR9-MyD88 pathway is critical for adaptive immune responses to adeno-associated virus gene therapy vectors in mice. J Clin Invest. 2009;119(8):2388–98. doi: 10.1172/JCI37607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu TL, Li H, Faust SM, et al. CD8+ T cell recognition of epitopes within the capsid of adeno-associated virus 8-based gene transfer vectors depends on vectors’ genome. Mol Ther. 2014;22(1):42–51. doi: 10.1038/mt.2013.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Faust SM, Bell P, Cutler BJ, et al. CpG-depleted adeno-associated virus vectors evade immune detection. J Clin Invest. 2013;123(7):2994–3001. doi: 10.1172/JCI68205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simioni P, Tormene D, Tognin G, et al. X-linked thrombophilia with a mutant factor IX (factor IX Padua) N Engl J Med. 2009;361(17):1671–5. doi: 10.1056/NEJMoa0904377. [DOI] [PubMed] [Google Scholar]
- 40.Finn JD, Nichols TC, Svoronos N, et al. The efficacy and the risk of immunogenicity of FIX Padua (R338L) in hemophilia B dogs treated by AAV muscle gene therapy. Blood. 2012;120(23):4521–3. doi: 10.1182/blood-2012-06-440123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crudele JM, Finn JD, Siner JI, et al. AAV liver expression of FIX-Padua prevents and eradicates FIX inhibitor without increasing thrombogenicity in hemophilia B dogs and mice. Blood. 2015;125(10):1553–61. doi: 10.1182/blood-2014-07-588194. • Demonstration of efficacy of hyperactive FIX in AAV-based liver gene therapy in dogs without increased thromobogenicity as well as ability of gene therapy to eradicate preexisting FIX inhibitors.
- 42.Monahan PE, Sun J, Gui T, et al. Employing a gain-of-function factor IX variant R338L to advance the efficacy and safety of hemophilia B human gene therapy: preclinical evaluation supporting an ongoing adeno-associated virus clinical trial. Hum Gene Ther. 2015;26(2):69–81. doi: 10.1089/hum.2014.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cantore A, Nair N, Della Valle P, et al. Hyperfunctional coagulation factor IX improves the efficacy of gene therapy in hemophilic mice. Blood. 2012;120(23):4517–20. doi: 10.1182/blood-2012-05-432591. [DOI] [PubMed] [Google Scholar]
- 44.Monahan PE, Walsh CE, Powell JS, et al. Update on phase 1/2 open-label trial of BAX335, an adeno-associated virus 8 (AAV8) vector-based gene therapy for program for phemophilia B. J Thromb Haemost. 2015;13(Suppl 2):87. [Google Scholar]
- 45.Chuah MK, Petrus I, De Bleser P, et al. Liver-specific transcriptional modules identified by genome-wide in silico analysis enable efficient gene therapy in mice and non-human primates. Mol Ther. 2014;22(9):1605–13. doi: 10.1038/mt.2014.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kotterman MA, Schaffer DV. Engineering adeno-associated viruses for clinical gene therapy. Nat Rev Genet. 2014;15(7):445–51. doi: 10.1038/nrg3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lisowski L, Dane AP, Chu K, et al. Selection and evaluation of clinically relevant AAV variants in a xenograft liver model. Nature. 2014;506(7488):382–6. doi: 10.1038/nature12875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.David AL, McIntosh J, Peebles DM, et al. Recombinant adeno-associated virus-mediated in utero gene transfer gives therapeutic transgene expression in the sheep. Hum Gene Ther. 2011;22(4):419–26. doi: 10.1089/hum.2010.007. [DOI] [PubMed] [Google Scholar]
- 49.Mattar CN, Nathwani AC, Waddington SN, et al. Stable human FIX expression after 0.9G intrauterine gene transfer of self-complementary adeno-associated viral vector 5 and 8 in macaques. Mol Ther. 2011;19(11):1950–60. doi: 10.1038/mt.2011.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McCarty DM, Young SM, Jr, Samulski RJ. Integration of adeno-associated virus (AAV) and recombinant AAV vectors. Annu Rev Genet. 2004;38:819–45. doi: 10.1146/annurev.genet.37.110801.143717. [DOI] [PubMed] [Google Scholar]
- 51.Chandler RJ, LaFave MC, Varshney GK, et al. Vector design influences hepatic genotoxicity after adeno-associated virus gene therapy. J Clin Invest. 2015;125(2):870–80. doi: 10.1172/JCI79213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nichols T, Whitford MH, Arruda VR, et al. Translational data from AAV-mediated gene therapy of hemophilia B in dogs. Hum Gene Ther Clin Dev. 2014 doi: 10.1089/humc.2014.153. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Buchlis G, Podsakoff GM, Radu A, et al. Factor IX expression in skeletal muscle of a severe hemophilia B patient 10 years after AAV-mediated gene transfer. Blood. 2012;119(13):3038–41. doi: 10.1182/blood-2011-09-382317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jiang H, Pierce GF, Ozelo MC, et al. Evidence of multiyear factor IX expression by AAV-mediated gene transfer to skeletal muscle in an individual with severe hemophilia B. Mol Ther. 2006;14(3):452–5. doi: 10.1016/j.ymthe.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 55.Baltimore D, Berg P, Botchan M, et al. Biotechnology. A prudent path forward for genomic engineering and germline gene modification. Science. 2015;348(6230):36–8. doi: 10.1126/science.aab1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schuettrumpf J, Liu JH, Couto LB, et al. Inadvertent germline transmission of AAV2 vector: findings in a rabbit model correlate with those in a human clinical trial. Mol Ther. 2006;13(6):1064–73. doi: 10.1016/j.ymthe.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 57.Favaro P, Downey HD, Zhou JS, et al. Host and vector-dependent effects on the risk of germline transmission of AAV vectors. Mole Ther. 2009;17(6):1022–30. doi: 10.1038/mt.2009.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Favaro P, Finn JD, Siner JI, et al. Safety of liver gene transfer following peripheral intravascular delivery of adeno-associated virus (AAV)-5 and AAV-6 in a large animal model. Hum Gene Ther. 2011;22(7):843–52. doi: 10.1089/hum.2010.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Manno CS, Chew AJ, Hutchison S, et al. AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood. 2003;101(8):2963–72. doi: 10.1182/blood-2002-10-3296. [DOI] [PubMed] [Google Scholar]
- 60.Arruda VR, Hagstrom JN, Deitch J, et al. Posttranslational modifications of recombinant myotube-synthesized human factor IX. Blood. 2001;97(1):130–8. doi: 10.1182/blood.v97.1.130. [DOI] [PubMed] [Google Scholar]
- 61.Xu L, Gao C, Sands MS, et al. Neonatal or hepatocyte growth factor-potentiated adult gene therapy with a retroviral vector results in therapeutic levels of canine factor IX for hemophilia B. Blood. 2003;101(10):3924–32. doi: 10.1182/blood-2002-10-3050. [DOI] [PubMed] [Google Scholar]
- 62.Xu L, Nichols TC, Sarkar R, et al. Absence of a desmopressin response after therapeutic expression of factor VIII in hemophilia A dogs with liver-directed neonatal gene therapy. Proc Natl Acad Sci U S A. 2005;102(17):6080–5. doi: 10.1073/pnas.0409249102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cantore A, Ranzani M, Bartholomae CC, et al. Liver-directed lentiviral gene therapy in a dog model of hemophilia B. Sci Transl Med. 2015;7(277):277ra28. doi: 10.1126/scitranslmed.aaa1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barzel A, Paulk NK, Shi Y, et al. Promoterless gene targeting without nucleases ameliorates haemophilia B in mice. Nature. 2015;517(7534):360–4. doi: 10.1038/nature13864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hacein-Bey-Abina S, von Kalle C, Schmidt M, et al. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N Engl j Med. 2003;348(3):255–6. doi: 10.1056/NEJM200301163480314. [DOI] [PubMed] [Google Scholar]
- 66.Eckhardt CL, van Velzen AS, Peters M, et al. Factor VIII gene (F8) mutation and risk of inhibitor development in nonsevere hemophilia A. Blood. 2013;122(11):1954–62. doi: 10.1182/blood-2013-02-483263. [DOI] [PubMed] [Google Scholar]
- 67.DiMichele D. Inhibitor development in haemophilia B: an orphan disease in need of attention. Br J Haematol. 2007;138(3):305–15. doi: 10.1111/j.1365-2141.2007.06657.x. [DOI] [PubMed] [Google Scholar]
- 68.Gouw SC, van den Berg HM, Oldenburg J, et al. F8 gene mutation type and inhibitor development in patients with severe hemophilia A: systematic review and meta-analysis. Blood. 2012;119(12):2922–34. doi: 10.1182/blood-2011-09-379453. [DOI] [PubMed] [Google Scholar]
- 69.Warrier I, Ewenstein BM, Koerper MA, et al. Factor IX inhibitors and anaphylaxis in hemophilia B. J Pediatr Hematol Oncol. 1997;19(1):23–7. doi: 10.1097/00043426-199701000-00003. [DOI] [PubMed] [Google Scholar]
- 70.Ewenstein BM, Takemoto C, Warrier I, et al. Nephrotic syndrome as a complication of immune tolerance in hemophilia B. Blood. 1997;89(3):1115–16. [PubMed] [Google Scholar]
- 71.Mingozzi F, Liu YL, Dobrzynski E, et al. Induction of immune tolerance to coagulation factor IX antigen by in vivo hepatic gene transfer. J Clin Invest. 2003;111(9):1347–56. doi: 10.1172/JCI16887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mount JD, Herzog RW, Tillson DM, et al. Sustained phenotypic correction of hemophilia B dogs with a factor IX null mutation by liver-directed gene therapy. Blood. 2002;99(8):2670–6. doi: 10.1182/blood.v99.8.2670. [DOI] [PubMed] [Google Scholar]
- 73.Niemeyer GP, Herzog RW, Mount J, et al. Long-term correction of inhibitor-prone hemophilia B dogs treated with liver-directed AAV2-mediated factor IX gene therapy. Blood. 2009;113(4):797–806. doi: 10.1182/blood-2008-10-181479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Finn JD, Ozelo MC, Sabatino DE, et al. Eradication of neutralizing antibodies to factor VIII in canine hemophilia A after liver gene therapy. Blood. 2010;116(26):5842–8. doi: 10.1182/blood-2010-06-288001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mingozzi F, Hasbrouck NC, Basner-Tschakarjan E, et al. Modulation of tolerance to the transgene product in a nonhuman primate model of AAV-mediated gene transfer to liver. Blood. 2007;110(7):2334–41. doi: 10.1182/blood-2007-03-080093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Soucie JM, Richardson LC, Evatt BL, et al. Risk factors for infection with HBV and HCV in a largecohort of hemophiliac males. Transfusion. 2001;41(3):338–43. doi: 10.1046/j.1537-2995.2001.41030338.x. [DOI] [PubMed] [Google Scholar]
- 77.Doering CB, Spencer HT. Replacing bad (F)actors: hemophilia. Hematology Am Soc Hematol Educ Program. 2014;2014(1):461–7. doi: 10.1182/asheducation-2014.1.461. [DOI] [PubMed] [Google Scholar]
- 78.Kuether EL, Schroeder JA, Fahs SA, et al. Lentivirus-mediated platelet gene therapy of murine hemophilia A with pre-existing anti-factor VIII immunity. J Thromb Haemost. 2012;10(8):1570–80. doi: 10.1111/j.1538-7836.2012.04791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Greene TK, Wang C, Hirsch JD, et al. In vivo efficacy of platelet-delivered, high specific activity factor VIII variants. Blood. 2010;116(26):6114–22. doi: 10.1182/blood-2010-06-293308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen Y, Schroeder JA, Kuether EL, et al. Platelet gene therapy by lentiviral gene delivery to hematopoietic stem cells restores hemostasis and induces humoral immune tolerance in FIX(null) mice. Mol Ther. 2014;22(1):169–77. doi: 10.1038/mt.2013.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Du LM, Nurden P, Nurden AT, et al. Platelet-targeted gene therapy with human factor VIII establishes haemostasis in dogs with haemophilia A. Nat Commun. 2013;4:2773. doi: 10.1038/ncomms3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Marder VJ, Aird WC, Bennett JS, et al. Hemostasis and thrombosis: basic principles and clinical practice. 6th edition Lippincott Williams &Wilkins; Philadelphia: PA: 2013. [Google Scholar]
- 83.Lind P, Larsson K, Spira J, et al. Novel forms of B-domain-deleted recombinant factor VIII molecules. Construction and biochemical characterization. Eur J Biochem. 1995;232(1):19–27. doi: 10.1111/j.1432-1033.1995.tb20776.x. [DOI] [PubMed] [Google Scholar]
- 84.Scallan CD, Liu T, Parker AE, et al. Phenotypic correction of a mouse model of hemophilia A using AAV2 vectors encoding the heavy and light chains of FVIII. Blood. 2003;102(12):3919–26. doi: 10.1182/blood-2003-01-0222. [DOI] [PubMed] [Google Scholar]
- 85.Pipe SW. Functional roles of the factor VIII B domain. Haemophilia. 2009;15(6):1187–96. doi: 10.1111/j.1365-2516.2009.02026.x. [DOI] [PubMed] [Google Scholar]
- 86.Fahs SA, Hille MT, Shi Q, et al. A conditional knockout mouse model reveals endothelial cells as the principal and possibly exclusive source of plasma factor VIII. Blood. 2014;123(24):3706–13. doi: 10.1182/blood-2014-02-555151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Everett LA, Cleuren AC, Khoriaty RN, Ginsburg D. Murine coagulation factor VIII is synthesized in endothelial cells. Blood. 2014;123(24):3697–705. doi: 10.1182/blood-2014-02-554501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Siner JI, Iacobelli NP, Sabatino DE, Ivanciu L, Zhou S, Poncz M, et al. Minimal modification in the factor VIII B-domain sequence ameliorates the murine hemophilia A phenotype. Blood. 2013;121(21):4396–403. doi: 10.1182/blood-2012-10-464164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ward NJ, Buckley SM, Waddington SN, et al. Codon optimization of human factor VIII cDNAs leads to high-level expression. Blood. 2011;117(3):798–807. doi: 10.1182/blood-2010-05-282707. [DOI] [PubMed] [Google Scholar]
- 90.McIntosh J, Lenting PJ, Rosales C, et al. Therapeutic levels of FVIII following a single peripheral vein administration of rAAV vector encoding a novel human factor VIII variant. Blood. 2013;121(17):3335–44. doi: 10.1182/blood-2012-10-462200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ide LM, Iwakoshi NN, Gangadharan B, et al. Functional aspects of factor VIII expression after transplantation of genetically-modified hematopoietic stem cells for hemophilia A. J Gene Med. 2010;12(4):333–44. doi: 10.1002/jgm.1442. [DOI] [PubMed] [Google Scholar]
- 92.Ide LM, Gangadharan B, Chiang KY, et al. Hematopoietic stem-cell gene therapy of hemophilia A incorporating a porcine factor VIII transgene and nonmyeloablative conditioning regimens. Blood. 2007;110(8):2855–63. doi: 10.1182/blood-2007-04-082602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Greene TK, Lyde RB, Bailey SC, et al. Apoptotic effects of platelet factor VIII on megakaryopoiesis: implications for a modified human FVIII for platelet-based gene therapy. J Thromb Haemost. 2014;12(12):2102–12. doi: 10.1111/jth.12749. [DOI] [PubMed] [Google Scholar]
- 94.Ozelo MC, Vidal B, Brown C, et al. Omental implantation of BOECs in hemophilia dogs results in circulating FVIII antigen and a complex immune response. Blood. 2014;123(26):4045–53. doi: 10.1182/blood-2013-12-545780. [DOI] [PubMed] [Google Scholar]
- 95.Hay CR, DiMichele DM. International Immune Tolerance Study. The principal results of the international immune tolerance study: a randomized dose comparison. Blood. 2012;119(6):1335–44. doi: 10.1182/blood-2011-08-369132. [DOI] [PubMed] [Google Scholar]
- 96.Bryant LM, Christopher DM, Giles AR, et al. Lessons learned from the clinical development and market authorization of Glybera. Hum Gene Ther Clin Dev. 2013;24(2):55–64. doi: 10.1089/humc.2013.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kotin RM. Large-scale recombinant adeno-associated virus production. Hum Mol Genet. 2011;20(R1):R2–6. doi: 10.1093/hmg/ddr141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hastie E, Samulski RJ. AAV at 50: a golden anniversary of discovery, research, and gene therapy success, a personal perspective. Hum Gene Ther. 2015;26(5):257–65. doi: 10.1089/hum.2015.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wright JF. Adeno-associated viral vector manufacturing: keeping pace with accelerating clinical development. Hum Gene Ther. 2011;22(8):913–14. doi: 10.1089/hum.2011.2514. [DOI] [PubMed] [Google Scholar]
- 100.Ayuso E, Blouin V, Lock M, et al. Manufacturing and characterization of a recombinant adeno-associated virus type 8 reference standard material. Hum Gene Ther. 2014;25(11):977–87. doi: 10.1089/hum.2014.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Darby SC, Kan SW, Spooner RJ, et al. Mortality rates, life expectancy, and causes of death in people with hemophilia A or B in the United Kingdom who were not infected with HIV. Blood. 2007;110(3):815–25. doi: 10.1182/blood-2006-10-050435. [DOI] [PubMed] [Google Scholar]
- 102.Plug I, Peters M, Mauser-Bunschoten EP, et al. Social participation of patients with hemophilia in the Netherlands. Blood. 2008;111(4):1811–15. doi: 10.1182/blood-2007-07-102202. [DOI] [PubMed] [Google Scholar]
- 103.Hough C, Lillicrap D. Gene therapy for hemophilia: an imperative to succeed. J Thromb Haemost. 2005;3(6):1195–205. doi: 10.1111/j.1538-7836.2005.01401.x. [DOI] [PubMed] [Google Scholar]
- 104.Powell JS. Lasting power of new clotting proteins. Hematology Am Soc Hematol Educ Program. 2014;2014(1):355–63. doi: 10.1182/asheducation-2014.1.355. [DOI] [PubMed] [Google Scholar]
- 105.George LA, Camire RM. Profile of efraloctocog alfa and its potential in the treatment of hemophilia A. J Blood Med. 2015;6:131–41. doi: 10.2147/JBM.S54632. [DOI] [PMC free article] [PubMed] [Google Scholar]