Abstract
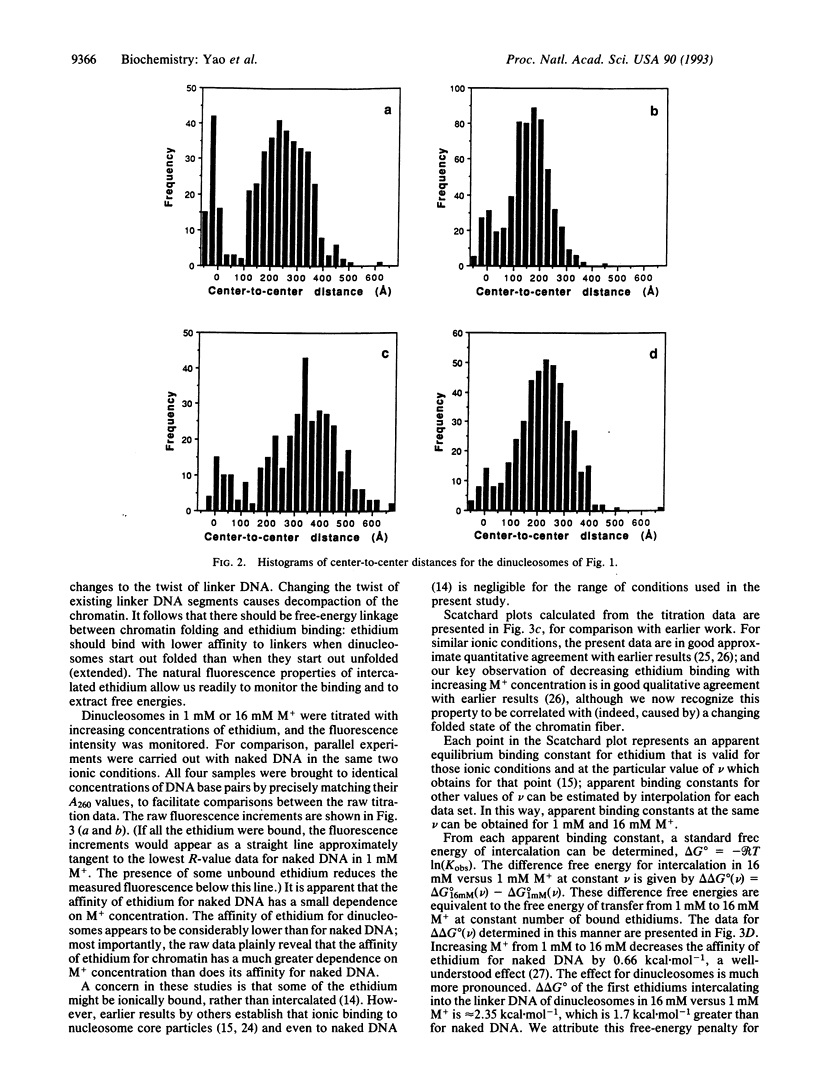
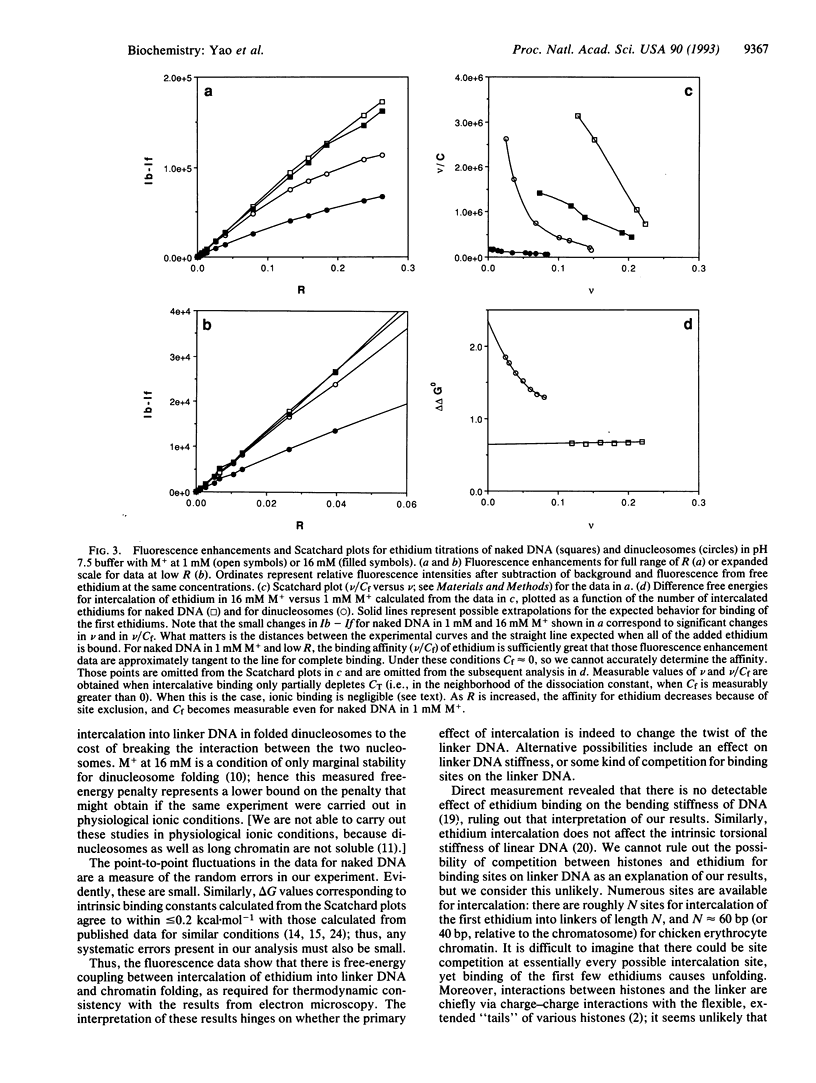
Previous work has shown that nucleosome repeat lengths, and hence linker DNA lengths, are preferentially quantized to a set of values differing by integral multiples of the helical twist of DNA. An explanation was proposed in which this preferential quantitation is due to twist constraints on linker DNA arising from nucleosome-nucleosome interactions in folded chromatin. Here we report the results of a study, using ethidium intercalation, designed to test whether twist constraints do indeed exist. Electron microscopy reveals that ethidium intercalation causes decondensation of dinucleosomes. Direct measurement of the free energy of intercalation by fluorescence spectroscopy reveals competition between chromatin folding and ethidium intercalation. Results from other laboratories establish that these effects of ethidium are due to ethidium-induced changes in the twist of linker DNA, and not to a variety of other possible effects. We conclude that twist constraints on linker DNA do exist. These may explain the observation of preferentially quantized linker DNA lengths. Implications of these results for mechanisms of nucleosome phasing and the mechanisms of drug action are discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Erard M., Das G. C., de Murcia G., Mazen A., Pouyet J., Champagne M., Daune M. Ethidium bromide binding to core particle: comparison with native chromatin. Nucleic Acids Res. 1979 Jul 25;6(10):3231–3253. doi: 10.1093/nar/6.10.3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman P. J. Flexibility of DNA. Annu Rev Biophys Biophys Chem. 1988;17:265–286. doi: 10.1146/annurev.bb.17.060188.001405. [DOI] [PubMed] [Google Scholar]
- Hayes J. J., Bashkin J., Tullius T. D., Wolffe A. P. The histone core exerts a dominant constraint on the structure of DNA in a nucleosome. Biochemistry. 1991 Aug 27;30(34):8434–8440. doi: 10.1021/bi00098a022. [DOI] [PubMed] [Google Scholar]
- Kornberg R. D., Lorch Y. Chromatin structure and transcription. Annu Rev Cell Biol. 1992;8:563–587. doi: 10.1146/annurev.cb.08.110192.003023. [DOI] [PubMed] [Google Scholar]
- Kornberg R. D., Stryer L. Statistical distributions of nucleosomes: nonrandom locations by a stochastic mechanism. Nucleic Acids Res. 1988 Jul 25;16(14A):6677–6690. doi: 10.1093/nar/16.14.6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurray C. T., Small E. W., van Holde K. E. Binding of ethidium to the nucleosome core particle. 2. Internal and external binding modes. Biochemistry. 1991 Jun 11;30(23):5644–5652. doi: 10.1021/bi00237a002. [DOI] [PubMed] [Google Scholar]
- McMurray C. T., van Holde K. E. Binding of ethidium bromide causes dissociation of the nucleosome core particle. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8472–8476. doi: 10.1073/pnas.83.22.8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurray C. T., van Holde K. E. Binding of ethidium to the nucleosome core particle. 1. Binding and dissociation reactions. Biochemistry. 1991 Jun 11;30(23):5631–5643. doi: 10.1021/bi00237a001. [DOI] [PubMed] [Google Scholar]
- Müller W., Crothers D. M. Studies of the binding of actinomycin and related compounds to DNA. J Mol Biol. 1968 Jul 28;35(2):251–290. doi: 10.1016/s0022-2836(68)80024-5. [DOI] [PubMed] [Google Scholar]
- Paoletti J., Magee B. B., Magee P. T. The structure of chromatin: interaction of ethidium bromide with native and denatured chromatin. Biochemistry. 1977 Feb 8;16(3):351–357. doi: 10.1021/bi00622a002. [DOI] [PubMed] [Google Scholar]
- Shrader T. E., Crothers D. M. Effects of DNA sequence and histone-histone interactions on nucleosome placement. J Mol Biol. 1990 Nov 5;216(1):69–84. doi: 10.1016/S0022-2836(05)80061-0. [DOI] [PubMed] [Google Scholar]
- Simpson R. T. Nucleosome positioning: occurrence, mechanisms, and functional consequences. Prog Nucleic Acid Res Mol Biol. 1991;40:143–184. doi: 10.1016/s0079-6603(08)60841-7. [DOI] [PubMed] [Google Scholar]
- Smith S. B., Finzi L., Bustamante C. Direct mechanical measurements of the elasticity of single DNA molecules by using magnetic beads. Science. 1992 Nov 13;258(5085):1122–1126. doi: 10.1126/science.1439819. [DOI] [PubMed] [Google Scholar]
- Sogo J. M., Ness P. J., Widmer R. M., Parish R. W., Koller T. Psoralen-crosslinking of DNA as a probe for the structure of active nucleolar chromatin. J Mol Biol. 1984 Oct 5;178(4):897–919. doi: 10.1016/0022-2836(84)90318-8. [DOI] [PubMed] [Google Scholar]
- Thoma F., Koller T., Klug A. Involvement of histone H1 in the organization of the nucleosome and of the salt-dependent superstructures of chromatin. J Cell Biol. 1979 Nov;83(2 Pt 1):403–427. doi: 10.1083/jcb.83.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widom J. A relationship between the helical twist of DNA and the ordered positioning of nucleosomes in all eukaryotic cells. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):1095–1099. doi: 10.1073/pnas.89.3.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widom J. Physicochemical studies of the folding of the 100 A nucleosome filament into the 300 A filament. Cation dependence. J Mol Biol. 1986 Aug 5;190(3):411–424. doi: 10.1016/0022-2836(86)90012-4. [DOI] [PubMed] [Google Scholar]
- Widom J. Toward a unified model of chromatin folding. Annu Rev Biophys Biophys Chem. 1989;18:365–395. doi: 10.1146/annurev.bb.18.060189.002053. [DOI] [PubMed] [Google Scholar]
- Wu P. G., Fujimoto B. S., Song L., Schurr J. M. Effect of ethidium on the torsion constants of linear and supercoiled DNAs. Biophys Chem. 1991 Dec;41(3):217–236. doi: 10.1016/0301-4622(91)85038-r. [DOI] [PubMed] [Google Scholar]
- Yao J., Lowary P. T., Widom J. Direct detection of linker DNA bending in defined-length oligomers of chromatin. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7603–7607. doi: 10.1073/pnas.87.19.7603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J., Lowary P. T., Widom J. Linker DNA bending induced by the core histones of chromatin. Biochemistry. 1991 Aug 27;30(34):8408–8414. doi: 10.1021/bi00098a019. [DOI] [PubMed] [Google Scholar]