Abstract
Increased cytochrome P450 2E1 (CYP2E1) expression is the main cause of oxidative stress, which exacerbates alcoholic liver diseases (ALDs). Estrogen-related receptor gamma (ERRγ) induces CYP2E1 expression and contributes to enhancing alcohol-induced liver injury. Retinoic acid-related orphan receptor alpha (RORα) has antioxidative functions; however, potential cross-talk between ERRγ and RORα in the regulation of CYP2E1 has not been studied. We report that RORα suppressed ERRγ-mediated CYP2E1 expression. A physical interaction of RORα with ERRγ at the ERRγ−response element in the CYP2E1 promoter was critical in this suppression. At this site, coregulator recruitment of ERRγ was switched from coactivator p300 to the nuclear receptor corepressor 1 in the presence of RORα. Cross-talk between ERRγ and RORα was demonstrated in vivo, in that administration of JC1–40, a RORα activator, significantly decreased both CYP2E1 expression and the signs of liver injury in ethanol-fed mice, and this was accompanied by coregulator switching. Thus, this non-classical RORα pathway switched the transcriptional mode of ERRγ, leading to repression of alcohol-induced CYP2E1 expression, and this finding may provide a new therapeutic strategy against ALDs.
INTRODUCTION
Alcoholic liver disease (ALD) is the common liver disease caused by alcohol abuse, which increases global burden of liver disease-related mortality (1). Chronic alcohol abuse triggers liver injury, which manifests as a broad spectrum of hepatic disorders including steatosis, alcoholic steatohepatitis, fibrosis, cirrhosis and hepatocellular carcinoma (2). Alcohol is converted to acetaldehyde by two oxidative pathways, namely, alcohol dehydrogenase and the microsomal ethanol oxidizing system (3,4). With chronic alcohol consumption, cytochrome P450 2E1 (CYP2E1) is a key microsomal enzyme that induces the generation of reactive oxygen species (ROS) which exacerbate alcohol-induced oxidative stress (4). Feeding a liquid diet containing ethanol to CYP2E1-expressing transgenic mice or to mice infused with an adenovirus encoding CYP2E1 produced higher serum transaminase levels and damaged histological features, further suggesting that CYP2E1 plays a critical role in ALDs (5,6). Therefore, understanding the molecular mechanism of gene expression and modulation of the enzymatic activity of CYP2E1 could provide a potential therapeutic strategy for controlling alcoholic liver injury.
Retinoic acid-related orphan receptor alpha (RORα; NR1F1) regulates diverse target genes associated with energy homeostasis including the regulation of diverse lipid and cholesterol metabolic pathways (7). Cholesterol derivatives, including cholesterol sulfate (CS), act as endogenous ligands that fit in the ligand-binding pocket of RORα, while synthetic RORα agonists such as SR1078 and JC1–40 accomplish the cellular functions of RORα (8–10). Coregulators of transcription factors such as p300 and nuclear receptor corepressor (NCoR) interact with and regulate the transcriptional activity of RORα positively or negatively (11,12). Previously, we reported that RORα and its ligands protect against the progression of non-alcoholic steatohepatitis (NASH) probably through activating AMP-activated protein kinase (AMPK) and by repressing liver X receptor alpha (LXRα)-mediated lipogenesis (8). Further, RORα reduced the formation of ROS and 4-hydroxynonenal (4-HNE), a lipid peroxidation marker, in the hepatocytes and Kupffer cells of mice with NASH induced by a methionine-choline deficient diet (13). Although these studies suggested a potential protective role of RORα in ALDs, its function in the regulation of CYP2E1 has not been studied.
Chronic alcohol abuse increases endocannabinoid levels and results in alcohol-induced hepatic lipid accumulation and oxidative stress via cannabinoid 1 (CB1) receptor activation (14,15). Recently, CYP2E1 was identified as the target of estrogen-related receptor gamma (ERRγ; NR3B3) that was induced by CB1 receptor signaling under exposure to alcohol (16). ERRγ belongs to the NR3B subfamily, which is known to play a role in regulating bioenergetics pathways such as hepatic gluconeogenesis and iron metabolism (17,18). ERRγ recognizes the DNA sequence, 5′–TNAAGGTCA–3′, termed the ERRγ-response element (ERRE) as monomer, homodimer or heterodimer (19). Unlike other classic ligand-dependent nuclear receptors, ERRγ is active constitutively without ligands, and the transcriptional status of ERRγ is mainly regulated by docking of coregulator such as CREB-binding protein/p300, peroxisome proliferator-activated receptor γ coactivator-1α, and nuclear receptor-interacting protein 140 (20–22). Downstream target genes of ERRγ are mainly involved in the metabolism of glucose, lipids and iron, including those encoding phosphoenolpyruvate carboxykinase, glucose-6-phosphatase (G6Pase), lipin-1 and hepcidin (17,18,23). Recently, a specific inverse agonist of ERRγ, GSK5182, was demonstrated to ameliorate chronic alcohol-induced liver injury through inhibition of CYP2E1-mediated ROS production in mice, indicating a new function of ERRγ in the control of alcohol-mediated oxidative stress in the liver (16).
Here, we report that RORα suppressed the CB1 receptor- and the ERRγ-mediated induction of CYP2E1 expression at the transcriptional level. Interestingly, RORα interacted directly with ERRγ to induce the differential recruitment of coregulators at the ERRE site in the upstream promoter of CYP2E1. This process switched the transcriptional mode of ERRγ from an activator to a repressor of CYP2E1 transcription. Finally, administration of the thiourea derivate JC1–40, an activator of RORα, dramatically protected mice from alcohol-induced oxidative stress and liver injury. Together these results suggest that RORα and its ligands might provide a potential therapeutic strategy against ALDs.
MATERIALS AND METHODS
Cell culture and reagents
Hepatocytes were isolated from 8–9 weeks old, male C57BL6/N mouse (Charles River Laboratories, Wilmington, MA) by perfusion of liver using collagenase type IV (Sigma-Aldrich, St Louis, MO), as described previously (13). Hepatocytes were cultured in M199 Medium (Hyclone, Logan, UT) supplemented with 10% fetal bovine serum (FBS), 23 mM HEPES and 10 nM dexamethasone. Chang liver cell line (CCL-13TM), a human hepatoma cell line, was obtained from American Type Culture Collection (ATCC, Rockville, MD), and cultured in Dulbecco's Modified Eagle's Medium (Hyclone) supplemented with 10% FBS. The Chang liver cells possess a similar gene expression profile for hepatocytes markers such as albumin and CYP3A4 with the normal human hepatocytes (24). The cells were grown in an incubator with 5% CO2 and 95% air at 37°C.
Arachidonyl-2′-chloroethylamide (ACEA) and CS were purchased from Sigma-Aldrich. SR1078 were purchased from Tocris Bioscience (Tocris, Bristol, UK). Synthesis and preparation of JC1–40 were previously described (8,25).
Plasmids, si-RNA and recombinant adenoviruses
The mouse CYP2E1 promoter reporters, FLAG-tagged ERRγ, FLAG- and Myc-tagged RORα were described previously (8,16). The eukaryotic expression vectors encoding ERRγ ΔAF2 (aa. 1–445) and RORα ΔAF2 (aa. 1–505) were amplified by PCR and cloned into pCMV-FLAG or pCMV-Myc, respectively. The si-RNA duplex targeting human NCoR1 (5′-AAUGCUACUUCUCGAGGAAACA-3′), mouse RORα (5′-GCAGAGAGACAGCUUGUACGC-3′), mouse ERRγ, and the non-specific si-RNA were synthesized from Shamchully Pharm, Co., Ltd (Korea) (16). Transient transfection of plasmids and si-RNA was carried out using Polyfect (Qiagen, Valencia, CA) or Lipofectamine 2000 (Invitrogen, Carlsbad, CA), as previously described (13,16). The recombinant adenoviruses encoding ERRγ, RORα and green fluorescence protein (GFP), i.e. Ad-ERRγ, Ad-RORα and Ad-GFP, respectively, and their infection were described previously (8,16).
Western blotting, immunoprecipitation and chromatin immunoprecipitation (ChIP) analysis
Western blotting and immunoprecipitation were performed as previously described using specific antibodies against ERRγ (2ZH6812H, R&D System, Minneapolis, MN), RORα (PA1–812, Thermo Scientific, Waltham, MA), CYP2E1 (SC-133491), p300 (SC-585), NCoR1 (SC-1609) (Santa Cruz Biotechnology, Santa Cruz, MA), FLAG (F3165, Sigma-Aldrich), MYC (SC-789, Santa Cruz Biotechnology) or actin (SC-1616, Santa Cruz Biotechnology) (13). Molecular weight information for the protein bands on the blots was provided as a Supplementary Figure S1. ChIP assay was performed as described previously using specific primers (13,16).
Reporter gene analysis
Chang liver cells were transfected with a plasmid mixture containing reporter plasmids, eukaryotic expression vectors, and/or si-RNAs together with pCMV-β-galactosidase using the Polyfect (Qiagen) or Lipofectamine 2000 (Invitrogen), according to the manufacturer's protocol. At the end of experiments, cells were lysed and harvested using luciferase lysis buffer (Promega, Madison, WI). Luciferase activity in whole cell lysates was measured using LB9508 luminometer (Berthold, Bad Wildbad, Germany) and normalized to β-galactosidase activity.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was prepared using Easy-Blue reagents (INtRON Biotechnology, Seoul, Korea) according to the manufacturer's protocol and cDNA was synthesized as previously described (8). qRT-PCR was performed using an ABI StepOnePlusTM Real-time PCR system (Applied Biosystem, Foster City, CA) using specific primers (13,16,26,27). Relative mRNA level of target gene was analyzed by the equation 2−△Ct (△Ct = Ct of target gene minus Ct of β-actin) and presented with a level of control group as 1. Detailed method of qRT-PCR was described previously (13,28).
In situ proximity ligation assays (PLAs)
To detect protein–protein interactions with high selectivity and sensitivity, PLAs were performed using the Duolink II Detection Reagent Red (Sigma) according to manufacturer's protocol. Briefly, cells grown in 8-well chamber slides were transfected with FLAG-ERRγ and MYC-RORα. Cells were fixed and incubated with primary antibodies against FLAG and MYC. The slides were incubated with PLA probes and the subsequent ligation and rolling circle amplification were performed. The PLA signals were visualized by a confocal microscope (Carl Zeiss, New York, NY).
Animals, treatments and histological analysis
Eight-week-old male C57BL/6 mice were gradually habituated to a liquid Lieber-DeCarli liquid diet (Dyets, Bethlehem, PA) with 5% (v/v) ethanol over a period of 2 weeks, then maintained on the 5% ethanol diet (36% ethanol-derived calories) (n = 12) for 5 weeks as described previously (29). Diet consumption was recorded daily and isocaloric amounts of a non-ethanol-containing diet (n = 8) were dispensed to pair-fed animals. After 3 weeks of feeding with a 5% ethanol diet, mice from both the ethanol-fed and pair-fed groups were administered JC1–40 at a dose of 10 mg/kg/day in 0.2 ml 0.5% carboxymethyl cellulose by oral gavage for 2 weeks. All groups of mice maintain a similar body weight within 5% difference after treatment. At the end of treatment, liver tissue was rapidly excised and portions of the liver were stored for further analysis of protein and mRNA, or fixed in 10% formalin for histopathological analysis. All experiments were conducted by blinded and randomized tests according to the guidelines of Seoul National University Institutional Animal Care and Use Committee.
For histological examination, a 3 μm section of paraffin-embedded tissue was stained routinely with hematoxylin and eosin (H&E). Immunohistochemistry was performed using anti-CYP2E1 (Millipore, Darmstadt, Germany) and anti-4-hydroxynonenal (4-HNE) (JalCA, Shizuoka, Japan) antibodies. Activities of glutamate pyruvate transaminase (GPT) and glutamic oxaloacetic transaminase (GOT) were measured using Fuji DRI-CHEM 3500s serum biochemistry analyzer (Fujifilm, Kanagawa, Japan), and amounts of hepatic triglyceride (TG) were measured using the EnzyChromTM Triglyceride Assay Kit (Bio Assay Systems, Hayward, CA).
Statistics
All values were expressed as means ± SD. Statistical analysis was performed using non-parametric Mann–Whitney U test for simple comparisons or Kruskal–Wallis ANOVA for multiple comparisons. P < 0.05 was considered as statistically different. Specified analysis was described in the figure legends.
RESULTS
RORα represses the ERRγ-mediated induction of CYP2E1 expression
CYP2E1 is a key enzyme causing alcohol-induced oxidative stress and liver injury. To illustrate the pathophysiological role of RORα in ALDs, we examined whether RORα affected expression level of CYP2E1 under ethanol exposure. Since alcohol-induced liver injury is associated with CB1 receptor activation, we employed a synthetic CB1 receptor agonist, ACEA, to mimic alcohol-induced CYP2E1 gene expression (14–16). Treatment of RORα agonists such as CS, SR1078 and JC1–40, largely decreased the expression level of CYP2E1 protein as well as transcripts that were induced by ACEA treatment in mouse primary hepatocytes (8,9). Expression level of a known transcriptional activator of CYP2E1, ERRγ, increased after ACEA treatment, but it was not affected by the RORα ligand treatment. Similarly, the CYP2E1 induction caused by adenovirus-mediated ERRγ gene transfer was decreased by treatment with these RORα ligands (Figure 1A) (16). The similar pattern of CYP2E1 repression was observed at both transcript- and protein-levels when RORα was exogenously introduced by infusion of Ad-RORα, suggesting that the ERRγ-induced CYP2E1 gene transcription might be a target of RORα during chronic ethanol consumption (Figure 1B). Our observation that knockdown of either RORα or ERRγ gene transcription using si-RNA decreased the RORα agonist-induced CYP2E1 repression indicates the involvement of RORα in the ERRγ-mediated induction of CYP2E1 transcription (Figure 1C). Knockdown of RORα completely abolished the CS-, JC1–40- or SR1078-induced transcriptional activity of the RORE reporter, indicating that the effect of these compounds was mediated through RORα (Supplementary Figure S2).
Figure 1.
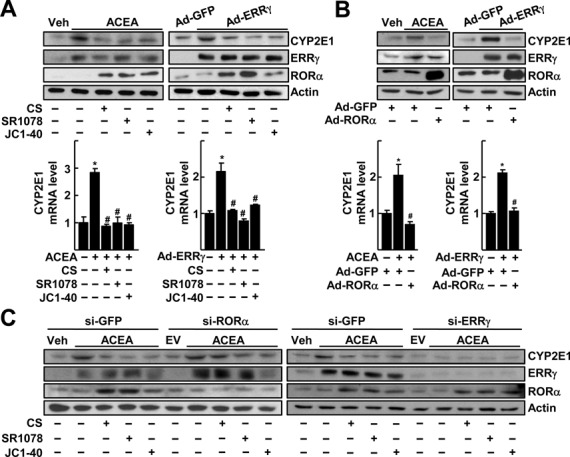
RORα suppresses the ERRγ-mediated induction of CYP2E1 gene expression. (A) Primary cultures of mouse hepatocytes were treated with 20 μM ACEA in the presence or absence of 20 μM CS, 5 μM SR1078 or 20 μM JC1–40 for 24 h. Or the hepatocytes were infected by Ad-GFP or Ad-ERRγ and then underwent the same treatment. The protein (top) and mRNA (bottom) levels were measured by western blotting and qRT-PCR, respectively. (B) Hepatocytes were infected by Ad-GFP or Ad-ERRγ and then treated with 20 μM ACEA for 24 h. Or the hepatocytes were infected by Ad-GFP or Ad-ERRγ together with Ad-GFP or Ad-RORα for 24 h. The protein (top) and mRNA (bottom) levels were measured by western blotting and qRT-PCR, respectively. (C) Hepatocytes were transfected with si-GFP, si-RORα or si-ERRγ for 24 h and then treated with 20 μM ACEA, 20 μM CS, 5 μM SR1078 and/or 20 μM JC1–40 for 24 h as indicated. The protein level was measured by western blotting. The data represent mean ± SD. *P < 0.05 versus vehicle treatment or Ad-GFP infection; #P < 0.05 versus ACEA treatment or Ad-ERRγ infection (n = 3). Representatives of at least three independent experiments with similar results are shown.
Expression of CYP2E1 is controlled by RORα-induced coregulator switch on the ERRE located in the upstream promoter
Previously, we reported an ERRE in the mouse CYP2E1 gene promoter that upregulates the gene expression in response to alcohol exposure (16) (Figure 2A). Therefore, we tested whether the RORα-induced suppression of CYP2E1 gene would be mediated through this ERRE. The activity of a reporter encoding the CYP2E1 gene promoter (–910 to +218 bp) was increased after transient expression of ERRγ, but was significantly reduced after overexpression of RORα or following JC1–40 treatment in Chang liver cells. The activity of a promoter encoding a mutated ERRE was not changed by either ERRγ or RORα, further suggesting the involvement of this ERRE in the RORα-mediated repression of the CYP2E1 promoter (Figure 2B). A reporter encoding the consensus ERRE sequence found in the small heterodimer partner promoter was also largely suppressed in the presence of RORα (Figure 2C) (30). Indeed, ChIP analysis performed on the CYP2E1 promoter region revealed that the levels of histone modifications such as AcH3K9, MeH3K9, and MeH3K27, markers of transcriptional activation or repression, were altered when RORα was expressed by transient overexpression, suggesting that RORα changed transcriptional mode of ERRγ-ERRE from activation to repression (Figure 2D). Since transcriptional coregulators provide epigenetic control mechanisms for transcriptional switch, we tested whether p300 and NCoR1 were involved in the repressive function of ERRγ on the CYP2E1 promoter. Consistently, coactivator p300 bound to the ERRE after treatment with ACEA or following Ad-ERRγ infusion; however, this binding disappeared when cells were treated with RORα agonists or Ad-RORα. In contrast, the DNA binding of NCoR1 at this promoter region was increased by activation of RORα (Figure 2E). Together, these results indicate that RORα represses CYP2E1 gene expression through regulation of the ERRE in upstream promoter by recruitment of NCoR1 instead of p300.
Figure 2.
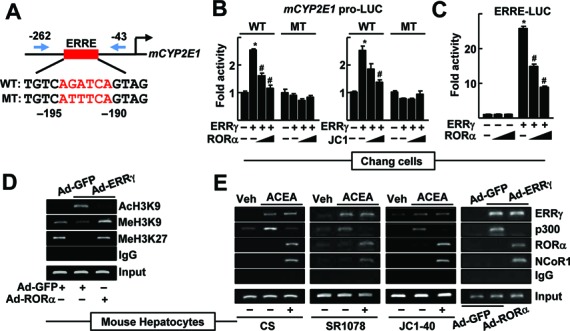
CYP2E1 expression is controlled by RORα through an ERRE in the CYP2E1 promoter. (A) Schematic representation of the mouse CYP2E1 promoter with the wild-type (WT) or the mutant (MT) ERRE. (B) Chang liver cells were transfected with the WT or the MT CYP2E1 promoter-Luc reporter with empty vector, FLAG-ERRγ or FLAG-RORα for 24 h. Or the reporter transfected cells were treated with 10 or 20 μM JC1–40 (JC1) for additional 24 h. (C) Chang liver cells were transfected with the ERRE-Luc reporter with EV, FLAG-ERRγ or FLAG-RORα for 24 h. Luciferase activity that normalized to the corresponding β-galactosidase activity was converted to fold activity with no treatment as 1. The data represent mean ± SD. *P < 0.05 versus EV; #P < 0.05 versus ERRγ (n = 3). (D) The hepatocytes were infected by Ad-GFP or Ad-ERRγ with Ad-RORα for 24 h. (E) The primary cultures of mouse hepatocytes were treated with 20 μM ACEA, or infected by adenovirus encoding GFP or ERRγ. The hepatocytes were treated with 20 μM CS, 5 μM SR1078 or 20 μM JC1–40, or co-infected by Ad-RORα for 24 h as indicated. DNA fragments that contain flanking region of the ERRE on the CYP2E1 promoter were immunoprecipitated with indicated anti-histone antibodies (panel D), anti-ERRγ, anti-p300, anti-RORα or anti-NCoR1 antibodies (panel E) and then amplified by PCR using primers shown in panel A. Representatives of at least three independent experiments with similar results are shown.
Physical interaction of RORα and ERRγ is required for the coregulator switch
To characterize further the cross-talk between ERRγ and RORα, we examined whether these receptors interacted physically. As shown in Figure 3A, a specific interaction of RORα with ERRγ was demonstrated by reciprocal immunoprecipitation using specific antibodies. In situ proximity ligation assays (PLAs) further confirmed the interaction of RORα and ERRγ in the nucleus (Figure 3B). The interaction of RORα and ERRγ was observed in the presence of ACEA, which further increased by CS, but decreased by SR3335, an inverse agonist of RORα (Figure 3C) (31). A deletion mapping study showed that the interaction between RORα and ERRγ was mediated through the LBDs of both receptors (Figure 3D and E). The ERRγ-LBD fused to GAL4 DNA binding domain activated the reporter encoding upstream activating sequences, i.e. Gal4-tk-Luc. However, this reporter activity was largely decreased by coexpression of the full-length as well as the LBD of RORα, but not by the LBD-deleted mutant, indicating that the RORα-induced repression of ERRγ was mediated by a physical interaction of these receptors through their LBDs (Figure 3F).
Figure 3.
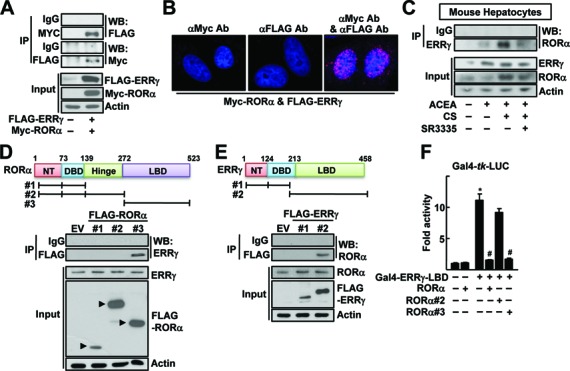
Physical protein interaction of RORα and ERRγ. (A) Whole cell lysates obtained from the Chang liver cells transfected with FLAG-ERRγ and MYC-RORα were immunoprecipitated (IP) and probed using the indicated antibodies by western blotting (WB). The level of proteins in the cell lysates was analyzed by WB as input. (B) Chang liver cells were transfected with FLAG-ERRγ and MYC-RORα for 24 h. Interaction of ERRγ and RORα was visualized with red dots by in situ proximity ligation assay. As a negative control, a single staining with the anti-FLAG and anti-MYC antibodies was performed. DAPI was used to stain the nuclei. (C) The primary cultures of mouse hepatocytes were treated with 20 μM ACEA, 20 μM CS or 10 μM SR3335 for 24 h as indicated. Whole cell lysates obtained from the hepatocytes were IP and probed by the indicated antibodies by WB. The level of proteins in the cell lysates was analyzed by WB as input. (D and E) Chang liver cells were transfected with the FLAG- or MYC-tagged deleted constructs as indicated and whole cell lysates were IP and WB using specific antibodies. The level of proteins in the cell lysates was analyzed by WB as input. NT: N-terminus, DBD: DNA binding domain and LBD: ligand binding domain. (F) Chang liver cells were transfeced with Gal4-tk-Luc reporter and Gal4-ERRγ-LBD in the presence of the FLAG-tagged RORα deletion constructs for 24 h. Luciferase activity that normalized to the corresponding β-galactosidase activity was converted to fold activity with no treatment as 1. The data represent mean ± SD. *P < 0.05 versus EV; #P < 0.05 versus Gal4-ERRγ-LBD (n = 3). Representatives of at least three independent experiments with similar results are shown.
Furthermore, we observed that ERRγ interacted with coactivator p300 when cells were treated with ACEA, whereas cotreatment with CS reduced the ERRγ-p300 interaction. In contrast, CS treatment increased the binding of ERRγ to the corepressor NCoR1 (Figure 4A). The ERRγ-NCoR1 binding disappeared in the absence of RORα, indicating that this binding was mediated by RORα (Figure 4B). Consistently, the RORα-mediated repression of ERRE activity was recovered by overexpression of p300 or by knockdown of NCoR1 (Figure 4C). Indeed, NCoR1 repressed the ERRE activity only when RORα was coexpressed, demonstrating that the repressive function of ERRγ-NCoR1 required RORα (Figure 4D). Since nuclear receptors recruit cofactors through AF2 region, we examined the cofactor switch with the AF2 deleted mutants of ERRγ and RORα. ERRγ interacted with RORα regardless of the presence of AF2 and vice versa. ERRγ interacted with NCoR1 in the presence of RORα, but not of RORα-ΔAF2, suggesting that ERRγ recruited NCoR1 which bound to RORα, since ERRγ did not interact with NCoR1 by itself (Figure 4E, left). Consistently, ERRγ-ΔAF2 interacted with NCoR1 only when wild-type RORα was present (Figure 4E, right). Together, these results indicate that RORα represses the transcriptional function of ERRγ by recruitment of the corepressor NCoR1.
Figure 4.
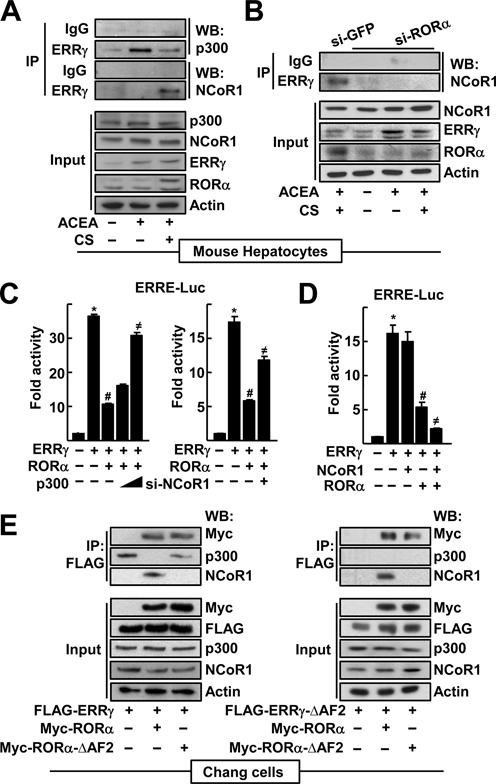
RORα recruits NcoR1 instead of p300 to ERRγ in the CYP2E1 promoter. (A) The primary cultures of mouse hepatocytes were treated with 20 μM ACEA or 20 μM CS for 24 h as indicated. (B) The primary cultures of mouse hepatocytes were transfected with si-GFP or si-RORα, and treated with 20 μM ACEA and/or 20 μM CS as indicated. Whole cell lysates were IP and probed using the indicated antibodies by WB. The level of proteins in the cell lysates was analyzed by WB as input. (C) Chang liver cells were transfected with the ERRE-Luc with empty vector, the expression vectors encoding FLAG-ERRγ, FLAG-RORα or increasing amount of MYC-p300 for 24 h as indicated (left). Or cells were transfected with the ERRE-Luc with the receptor expression vectors in the presence of si-NCoR1 for 48 h as indicated (right). Expression of NCoR1 protein after si-RNA-mediated knock-down was shown in Supplementary Figure S8. (D) Chang liver cells were transfected with the ERRE-Luc with the expression vectors encoding ERRγ, RORα or NCoR1 for 24 h as indicated. Luciferase activity that normalized to the corresponding β-galactosidase activity was converted to fold activity with no treatment as 1. The data represent mean ± SD. *P < 0.05 versus EV; #P < 0.05 versus ERRγ; ≠P < 0.05 versus ERRγ with RORα (n = 3). (E) Whole cell lysates obtained from the Chang liver cells transfected with FLAG-ERRγ, ERRγ-ΔAF2, MYC-RORα and/or RORα-ΔAF2 were IP and probed using the indicated antibodies by WB. The level of proteins in the cell lysates was analyzed by WB as input. Representatives of at least three independent experiments with similar results are shown.
Administration of JC1–40 decreases hepatic CYP2E1 expression and protects against ethanol-induced liver injury in mice
Finally, we tested whether activation of RORα would decrease the expression of CYP2E1 and attenuate the degree of liver injury in mice induced by chronic ethanol exposure. Both the ethanol-fed and pair-fed control groups were administered JC1–40, an activator of RORα, at 10 mg/kg per day for 2 weeks. Chronic ethanol feeding resulted in alcoholic liver injury; the numbers and sizes of hepatic lipid droplets were enhanced and the level of 4-HNE was increased when assessed by H&E staining and immunohistochemistry, respectively (Figure 5A and B). The ratio of liver weight to body weight, the serum levels of GPT and GOT, and the amount of hepatic TG were increased in the ethanol-fed group (Figure 5C–E and Supplementary Figure S3). However, importantly, the administration of JC1–40 dramatically lowered all these liver injury parameters (Figure 5A–E and Supplementary Figure S3).
Figure 5.
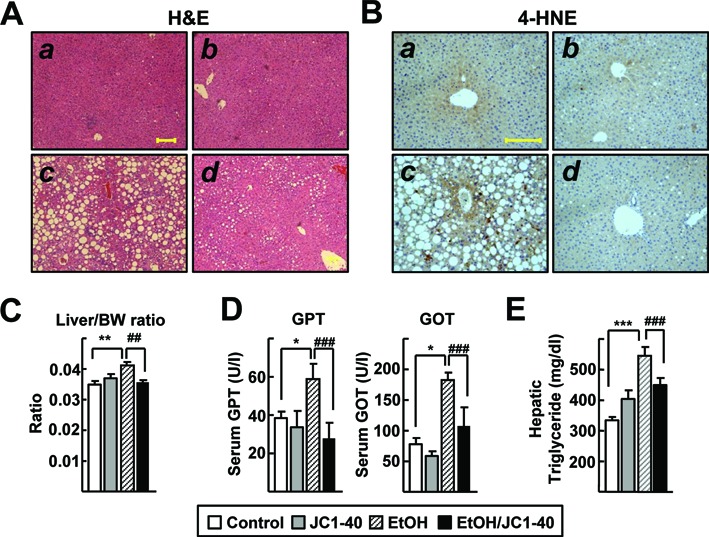
JC1–40 protects against ethanol-induced oxidative liver damage in mice. Eight week-old C57BL/6N mice were fed with the isocaloric pair-fed diet (A and B) or 5% ethanol-containing Lieber-DeCarli liquid diet (C and D) for 5 weeks. After 3 weeks of diet feeding, vehicle (A and C) or JC1–40, 10 mg/kg/day (B and D), was administered daily at doses by oral gavage for 2 weeks. (A) H&E staining of liver sections. Magnification of X100. Yellow bar represents 200 μm. (B) Immunohistochemistry staining of 4-HNE in liver sections. Magnification of X200. Yellow bar represents 200 μm. (C) Liver/body weight ratio was measured at the end of experiments. (D) Serum GPT and GOT enzyme activities were analyzed at the end of experiments. (E) Hepatic triglyceride levels were analyzed at the end of experiment. The data represent mean ± SD. *P < 0.05, **P < 0.01 and ***P < 0.001 versus pair-fed control group (n = 8); #P < 0.05, ##P < 0.01 and ###P < 0.001 versus ethanol-fed with vehicle group (n = 12).
Consistent with the results obtained from in vitro experiments, the hepatic level of protein as well as mRNA for CYP2E1 were increased in the ethanol-fed group, but decreased in the JC1–40-treated group (Figure 6A–C). Also we observed an interaction between ERRγ and RORα, especially in the JC1–40-treated liver tissues of ethanol-fed mice (Figure 6C). Expression level of RORα protein was significantly decreased the ethanol-fed group, whereas it was largely increased in the JC1–40 treated groups (Figure 6C). These results indicate the protective role of RORα, which might be associated with suppression of CYP2E1 expression. Finally, in vivo DNA binding of p300 in the CYP2E1 promoter was significantly increased in livers of the ethanol-fed group, but largely decreased in the JC1–40 administered group. In contrast, the DNA binding of NCoR1 was significantly increased in the JC1–40 treated group (Figure 6D). Together, these results demonstrated the presence of RORα induced coregulator switch at the ERRE site in the CYP2E1 gene promoter in vivo, which was correlated with RORα-induced protection from oxidative stress-induced liver injury after chronic alcohol exposure (Figure 6E).
Figure 6.
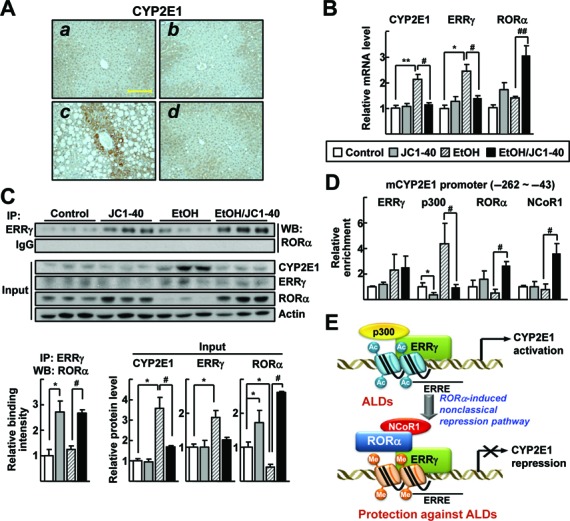
JC1–40 decreases ERRγ and CYP2E1 expression in the ethanol-induced mouse liver disease. Eight week-old C57BL/6N mice were fed with the isocaloric pair-fed diet (A and B) or 5% ethanol-containing Lieber-DeCarli liquid diet (C and D) for 5 weeks. After 3 weeks of diet feeding, vehicle (A and C) or JC1–40, 10 mg/kg/day (B and D), was administered daily at doses by oral gavage for 2 weeks. (A) Immunohistochemistry staining of CYP2E1 in liver sections. Magnification of X200. Yellow bars represent 200 μm. (B) mRNA levels of CYP2E1, ERRγ and RORα in liver tissues were analyzed by qRT-PCR. *P < 0.05, and **P < 0.01 versus pair-fed control group (n = 8); #P < 0.05, and ##P < 0.05 versus ethanol-fed with vehicle group (n = 12). (C) Whole liver tissue lysates were IP with anti-ERRγ antibody and probed using anti-RORα antibody by WB. Protein expression levels of CYP2E1, ERRγ and RORα in liver tissues were analyzed by WB (top) and the indicated band intensities were quantified (bottom). (D) DNA fragments obtained from liver tissues that contain flanking region of the ERRE on the CYP2E1 promoter were immunoprecipitated with the anti-ERRγ, anti-p300, anti-RORα or anti-NCoR1 antibodies and then amplified by qPCR using primers shown in Figure 2E. *P < 0.05 versus pair-fed control group; #P < 0.05 versus ethanol-fed with vehicle group (n = 6). (E) Schematic model for repression of the ERRγ-induced CYP2E1 gene expression by RORα in the hepatocytes under ethanol exposure.
DISCUSSION
Nuclear receptors bind to a specific DNA element in target gene promoters as monomers, homodimers or heterodimers to perform transcriptional regulation (32). To date, ERRγ has been shown to form homodimers or heterodimers with ERRα (33). Here, we showed for the first time that ERRγ binds to RORα on the ERRE half-site, resulting in transcriptional repression of specific target genes such as that encoding CYP2E1 (Figure 6E). Because RORα does not alter the promoter activity of CYP2E1 in the absence of ERRγ, this RORα action mechanism is non-classical and clearly different from the classical pathway involving a specific DNA binding-mediated regulation of target gene transcription (Figure 2B). Especially, NCoR1 was recruited to the ERRE site when RORα repressed ACEA-induced ERRγ activity (Figure 2E and 6D). A similar type of non-classical pathway was reported in the regulation of the LXRα-dependent LXR-response element (LXRE) activation by estrogen receptor alpha (ERα) (34). ERα inhibited transcription of LXRα target genes such as those encoding sterol regulatory element-binding protein 1 and stearoyl CoA desaturase 1 through direct interaction with LXRα, resulting in repression of the LXRα-dependent hepatic lipid accumulation in mice (34). During this process, ERα replaced p300 to NCoR1 at the LXRα bound LXRE in the same way as RORα switches the coregulator in the ERRE. Similarly, RORα suppressed the transcriptional activity of CCAAT/enhancer-binding protein β via physical interaction, causing dissociation of p300 from the relevant transcription factor (35). Previously, we showed that RORα decreased the transcriptional activation of LXRα resulting in disruption of the autoregulatory activation loop of LXRα (8). It would be interesting to determine the target receptors or proteins for the non-classical RORα pathway described here, which might include LXRα.
The potential counteracting function of RORα against ERRγ has been noted in the regulation of the Lipin-1 gene of which the product plays an important role in metabolic processes. ERRγ induced transcriptional activation of Lipin-1 expression in AML12 mouse liver cells (23). Meanwhile, hepatic expression of Lipin-1 was enhanced in the RORαsg/sg mice, which displayed loss of RORα function. This observation suggests that loss of the non-classical function of RORα increased the ERRγ-mediated hepatic Lipin-1 expression in these mice (36). However, in the case of G6Pase, a key enzyme of gluconeogenesis, both ERRγ and RORα act as activators through ERRE and ROR-response element in the promoter region of this gene, respectively (17,37). When we examined regulation of these downstream target genes in our experimental model, Ad-RORα infusion repressed the ERRγ-mediated Lipin-1 induction, whereas it increased the G6Pase level, which is similar to the previous reports (Supplementary Figure S4). These observations suggest that the non-classical RORα pathway might operate for specific target sites in specific target promoters. Further studies on the target genes of the non-classical RORα pathway and on what determines the action mode of RORα in the target sites are warranted.
Interestingly, expression level of RORα protein was significantly downregulated in livers of the ethanol-fed groups in this study (Figure 6B). Hepatic expression level of RORα was lower in patients with ALDs to compare with that of normal, indicating that our finding obtained from mice is potentially relevant to the RORα regulation in human ALDs (38) (Supplementary Figure S5). However, the mechanism of how expression of hepatic RORα is downregulated under ethanol exposure is not known. Previously, RORα was shown to sense cellular stress and expression level of RORα was changed by various types of cellular stress including ultraviolet irradiation and H2O2 treatment (39). Therefore, ethanol-induced cellular stress such as hepatic oxidative stress, endoplasmic reticulum stress response, lipotoxicity and inflammation, may lead to alterations in RORα expression level. Especially, c-JUN, a downstream of stress responsive c-Jun N-terminal kinases (JNK) signaling pathway, prevented RORα binding to RORE and suppressed transcriptional activity of RORα in hepatic steatosis (40). Since RORα expression is induced by its own transcriptional function, hepatic stresses including that activates the JNK signaling may cause disruption of the autoregualtion of RORα expression (8). Further understandings on the molecular mechanism of RORα downregulation under ethanol exposure may help to illustrate pathogenesis of ALDs.
Ethanol feeding increases amount of endocannabinoids such as 2-arachidonylglycerol, which is a CB1R agonist, in the stellate cells (15). The hepatic endocannabinoid system is the major contributor of pathogenesis of ALD since activating CB1 receptor results in induction of CYP2E1 mRNA in the hepatocytes (16). Interestingly, ethanol treatment induced expression of CYP2E1, but not of CB1R and ERRγ in the hepatocytes (Supplementary Figure S6A). Similarly, ethanol did not increase DNA binding of ERRγ or p300 on the CYP2E1 promoter, which is contrast to ACEA (Supplementary Figure S6B). Together these results indicate that the cross-talk between ERRγ and RORα functions mainly in the CB1R-ERRγ axis of the hepatocytes that triggered by paracrine effect of endocannabinoids secreted from the stellate cells.
In clinics, patients with severe ALDs are treated with regimens such as anti-inflammatory corticosteroids and pentoxyfilline (2,41). Unfortunately, however, these pharmacological treatments show insufficient effectiveness and sensitivity (2,41). Here, we showed that treatment with a RORα ligand decreased the ERRγ-induced CYP2E1 expression and contributed to the repression of alcohol-induced oxidative stress and liver injury in ethanol-fed mice. In addition, the human CYP2E1 promoter contains an ERRE, and RORα was found to repress ERRγ-mediated CYP2E1 expression in human Chang liver cells (16) (Supplementary Figure S7). In addition, further, several other lines of evidence have demonstrated that RORα possesses other beneficial functions that could improve the symptoms of ALDs. For example, RORα activates AMPK which plays an important role in regulating sterol regulatory element-binding protein 1-mediated lipid synthesis under conditions of alcohol exposure (8,42). RORα inhibits the transcriptional activity of LXRα causing auto/paracrine activation of monocyte chemoattractant protein 1, an essential chemokine causing alcohol-induced liver injury (8,29,43). Administration of RORα ligands protects against hepatic oxidative stress through the induction of antioxidant enzymes including superoxide dismutase 2 and glutathione peroxidase 1 (13). In conclusion, RORα might possess multitargeting ability against important pathogenic events during the progression of ALDs, and agonists of RORα could contribute to the development of useful new therapeutic strategies to improve the outcomes for affected patients.
Supplementary Material
Acknowledgments
Author Contributions: Y.H., D.K., H.C. and M.L. designed the study and interpreted the results; Y.H., D.K., T.N. and N.K. performed the experiments; and Y.H. and M.L. wrote the manuscript.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
Bio & Medical Technology Development Program [2012M3A9B6055338]; Korea Mouse Phenotyping Project [NRF-2014M3A9D5A01073556]; Basic Science Research Program [2014R1A2A1A10052265 to M.O.L.]; the National Creative Research Initiatives [20110018305 to H.S.C.]. Funding for open access charge: Bio & Medical Technology Development Program [2012M3A9B6055338]; Korea Mouse Phenotyping Project [NRF-2014M3A9D5A01073556]; Basic Science Research Program [2014R1A2A1A10052265 to M.O.L.]; the National Creative Research Initiatives Grant [20110018305 to H.S.C.].
Conflict of interest statement. None declared.
REFERENCES
- 1.Rehm J., Samokhvalov A.V., Shield K.D. Global burden of alcoholic liver diseases. J. Hepatol. 2013;59:160–168. doi: 10.1016/j.jhep.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 2.Gao B., Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572–1585. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lieber C.S. Alcoholic fatty liver: its pathogenesis and mechanism of progression to inflammation and fibrosis. Alcohol. 2004;34:9–19. doi: 10.1016/j.alcohol.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Liu J. Ethanol and liver: recent insights into the mechanisms of ethanol-induced fatty liver. World J. Gastroenterol. 2014;20:14672–14685. doi: 10.3748/wjg.v20.i40.14672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morgan K., French S.W., Morgan T.R. Production of a cytochrome P450 2E1 transgenic mouse and initial evaluation of alcoholic liver damage. Hepatology. 2002;36:122–134. doi: 10.1053/jhep.2002.33720. [DOI] [PubMed] [Google Scholar]
- 6.Bai J., Cederbaum A.I. Adenovirus-mediated expression of CYP2E1 produces liver toxicity in mice. Toxicol. Sci. 2006;91:365–371. doi: 10.1093/toxsci/kfj165. [DOI] [PubMed] [Google Scholar]
- 7.Jetten A.M. Retinoid-related orphan receptors (RORs): critical roles in development, immunity, circadian rhythm, and cellular metabolism. Nucl. Recept. Signal. 2009;7:e003. doi: 10.1621/nrs.07003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim E.J., Yoon Y.S., Hong S., Son H.Y., Na T.Y., Lee M.H., Kang H.J., Park J., Cho W.J., Kim S.G., et al. Retinoic acid receptor-related orphan receptor α-induced activation of adenosine monophosphate-activated protein kinase results in attenuation of hepatic steatosis. Hepatology. 2012;55:1379–1388. doi: 10.1002/hep.25529. [DOI] [PubMed] [Google Scholar]
- 9.Solt L.A., Burris T.P. Action of RORs and their ligands in (patho)physiology. Trends Endocrinol. Metab. 2012;23:619–627. doi: 10.1016/j.tem.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y., Solt L.A., Kojetin D.J., Burris T.P. Regulation of p53 stability and apoptosis by a ROR agonist. PLoS One. 2012;7:e34921. doi: 10.1371/journal.pone.0034921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harding H.P., Atkins G.B., Jaffe A.B., Seo W.J., Lazar M.A. Transcriptional activation and repression by RORalpha, an orphan nuclear receptor required for cerebellar development. Mol. Endocrinol. 1997;11:1737–1746. doi: 10.1210/mend.11.11.0002. [DOI] [PubMed] [Google Scholar]
- 12.Lau P., Bailey P., Dowhan D.H., Muscat G.E. Exogenous expression of a dominant negative RORalpha1 vector in muscle cells impairs differentiation: RORalpha1 directly interacts with p300 and myoD. Nucleic Acids Res. 1997;27:411–420. doi: 10.1093/nar/27.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han Y.H., Kim H.J., Kim E.J., Kim K.S., Hong S., Park H.G., Lee M.O. RORα decreases oxidative stress through the induction of SOD2 and GPx1 expression and thereby protects against nonalcoholic steatohepatitis in mice. Antioxid. Redox. Signal. 2014;21:2083–2094. doi: 10.1089/ars.2013.5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Basavarajappa B.S., Saito M., Cooper T.B., Hungund B.L. Stimulation of cannabinoid receptor agonist 2-arachidonylglycerol by chronic ethanol and its modulation by specific neuromodulators in cerebellar granule neurons. Biochim. Biophys. Acta. 2000;1535:78–86. doi: 10.1016/s0925-4439(00)00085-5. [DOI] [PubMed] [Google Scholar]
- 15.Jeong W.I., Osei-Hyiaman D., Park O., Liu J., Bátkai S., Mukhopadhyay P., Horiguchi N., Harvey-White J., Marsicano G., Lutz B., et al. Paracrine activation of hepatic CB1 receptors by stellate cell-derived endocannabinoids mediates alcoholic fatty liver. Cell. Metab. 2008;7:227–235. doi: 10.1016/j.cmet.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 16.Kim D.K., Kim Y.H., Jang H.H., Park J., Kim J.R., Koh M., Jeong W.I., Koo S.H., Park T.S., Yun C.H., et al. Estrogen-related receptor γ controls hepatic CB1 receptor-mediated CYP2E1 expression and oxidative liver injury by alcohol. Gut. 2013;62:1044–1054. doi: 10.1136/gutjnl-2012-303347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim D.K., Ryu D., Koh M., Lee M.W., Lim D., Kim M.J., Kim Y.H., Cho W.J., Lee C.H., Park S.B., et al. Orphan nuclear receptor estrogen-related receptor γ (ERRγ) is key regulator of hepatic gluconeogenesis. J. Biol. Chem. 2012;287:21628–21639. doi: 10.1074/jbc.M111.315168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim D.K., Jeong J.H., Lee J.M., Kim K.S., Park S.H., Kim Y.D., Koh M., Shin M., Jung Y.S., Kim H.S., et al. Inverse agonist of estrogen-related receptor γ controls Salmonella typhimurium infection by modulating host iron homeostasis. Nat. Med. 2014;20:419–424. doi: 10.1038/nm.3483. [DOI] [PubMed] [Google Scholar]
- 19.Giguère V. Transcriptional control of energy homeostasis by the estrogen-related receptors. Endocr. Rev. 2008;29:677–696. doi: 10.1210/er.2008-0017. [DOI] [PubMed] [Google Scholar]
- 20.Huss J.M., Kopp R.P., Kelly D.P. Peroxisome proliferator-activated receptor coactivator-1alpha (PGC-1alpha) coactivates the cardiac-enriched nuclear receptors estrogen-related receptor-alpha and -gamma. Identification of novel leucine-rich interaction motif within PGC-1alpha. J. Biol. Chem. 2002;277:40265–40274. doi: 10.1074/jbc.M206324200. [DOI] [PubMed] [Google Scholar]
- 21.Klein F.A., Atkinson R.A., Potier N., Moras D., Cavarelli J. Biochemical and NMR mapping of the interface between CREB-binding protein and ligand binding domains of nuclear receptor: beyond the LXXLL motif. J. Biol. Chem. 2005;280:5682–5692. doi: 10.1074/jbc.M411697200. [DOI] [PubMed] [Google Scholar]
- 22.Castet A., Herledan A., Bonnet S., Jalaguier S., Vanacker J.M., Cavaillès V. Receptor-interacting protein 140 differentially regulates estrogen receptor-related receptor transactivation depending on target genes. Mol. Endocrinol. 2006;20:1035–1047. doi: 10.1210/me.2005-0227. [DOI] [PubMed] [Google Scholar]
- 23.Kim D.K., Kim J.R., Koh M., Kim Y.D., Lee J.M., Chanda D., Park S.B., Min J.J., Lee C.H., Park T.S., et al. Estrogen-related receptor γ (ERRγ) is a novel transcriptional regulator of phosphatidic acid phosphatase, LIPIN1, and inhibits hepatic insulin signaling. J. Biol. Chem. 2011;286:38035–38042. doi: 10.1074/jbc.M111.250613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang T., Li C., Zhang L., Li M., Zhou P. A promising hepatocyte-like cell line, CCL-13, exhibits good liver function both in vitro and in an acute liver failure model. Transplant Proc. 2013;45:688–694. doi: 10.1016/j.transproceed.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 25.Park Y., Hong S., Lee M., Jung H., Cho W.J., Kim E.J., Son H.Y., Lee M.O., Park H.G. N-methylthioureas as new agonists of retinoic acid receptor-related orphan receptor. Arch. Pharm. Res. 2012;35:1393–1401. doi: 10.1007/s12272-012-0809-0. [DOI] [PubMed] [Google Scholar]
- 26.Peterson T.R., Sengupta S.S., Harris T.E., Carmack A.E., Kang S.A., Balderas E., Guertin D.A., Madden K.L., Carpenter A.E., Finck B.N., et al. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell. 2011;146:408–420. doi: 10.1016/j.cell.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheng L., Zhou Y., Chen Z., Ren D., Cho K.W., Jiang L., Shen H., Sasaki Y., Rui L. NF-κB–inducing kinase (NIK) promotes hyperglycemia and glucose intolerance in obesity by augmenting glucagon action. Nat. Med. 2012;18:943–949. doi: 10.1038/nm.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bustin S.A., Benes V., Garson J.A., Hellemans J., Huggett J., Kubista M., Mueller R., Nolan T., Pfaffl M.W., Shipley G.L., et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 29.Na T.Y., Han Y.H., Ka N.L., Park H.S., Kang Y.P., Kwon S.W., Lee B.H., Lee M.O. 22-S-Hydroxycholesterol protects against ethanol-induced liver injury by blocking the auto/paracrine activation of MCP-1 mediated by LXRα. J. Pathol. 2015;235:710–720. doi: 10.1002/path.4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanyal S., Kim J.Y., Kim H.J., Takeda J., Lee Y.K., Moore D.D., Choi H.S. Differential regulation of the orphan nuclear receptor small heterodimer partner (SHP) gene promoter by orphan nuclear receptor ERR isoforms. J. Biol. Chem. 2002;277:1739–1748. doi: 10.1074/jbc.M106140200. [DOI] [PubMed] [Google Scholar]
- 31.Kumar N., Kojetin D.J., Solt L.A., Kumar K.G., Nuhant P., Duckett D.R., Cameron M.D., Butler A.A., Roush W.R., Griffin P.R., et al. Identification of SR3335 (ML-176): a synthetic RORα selective inverse agonist. ACS Chem. Biol. 2011;6:218–222. doi: 10.1021/cb1002762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glass C.K. Differential recognition of target genes by nuclear receptor monomers, dimers, and heterodimers. Endocr. Rev. 1994;15:391–407. doi: 10.1210/edrv-15-3-391. [DOI] [PubMed] [Google Scholar]
- 33.Huppunen J., Aarnisalo P. Dimerization modulates the activity of the orphan nuclear receptor ERRgamma. Biochem. Biophys. Res. Commun. 2004;314:964–970. doi: 10.1016/j.bbrc.2003.12.194. [DOI] [PubMed] [Google Scholar]
- 34.Han S.I., Komatsu Y., Murayama A., Steffensen K.R., Nakagawa Y., Nakajima Y., Suzuki M., Oie S., Parini P., Vedin L.L., et al. Estrogen receptor ligands ameliorate fatty liver through a nonclassical estrogen receptor/Liver X receptor pathway in mice. Hepatology. 2014;59:1791–1802. doi: 10.1002/hep.26951. [DOI] [PubMed] [Google Scholar]
- 35.Ohoka N., Kato S., Takahashi Y., Hayashi H., Sato R. The orphan nuclear receptor RORalpha restrains adipocyte differentiation through a reduction of C/EBPbeta activity and perilipin gene expression. Mol. Endocrinol. 2009;23:759–771. doi: 10.1210/me.2008-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lau P., Fitzsimmons R.L., Raichur S., Wang S.C., Lechtken A., Muscat G.E. The orphan nuclear receptor, RORalpha, regulates gene expression that controls lipid metabolism: staggerer (SG/SG) mice are resistant to diet-induced obesity. J. Biol. Chem. 2008;283:18411–18421. doi: 10.1074/jbc.M710526200. [DOI] [PubMed] [Google Scholar]
- 37.Chopra A.R., Louet J.F., Saha P., An J., Demayo F., Xu J., York B., Karpen S., Finegold M., Moore D., et al. Absence of the SRC-2 coactivator results in a glycogenopathy resembling Von Gierke's disease. Science. 2008;322:1395–1399. doi: 10.1126/science.1164847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Affò S., Dominguez M., Lozano J.J., Sancho-Bru P., Rodrigo-Torres D., Morales-Ibanez O., Moreno M., Millán C., Loaeza-del-Castillo A., Altamirano J., et al. Transcriptome analysis identifies TNF superfamily receptors as potential therapeutic targets in alcoholic hepatitis. Gut. 2013;62:452–460. doi: 10.1136/gutjnl-2011-301146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu Y., McAvoy S., Kuhn R., Smith D.I. RORA, a large common fragile site gene, is involved in cellular stress response. Oncogene. 2006;25:2901–2908. doi: 10.1038/sj.onc.1209314. [DOI] [PubMed] [Google Scholar]
- 40.Lu Y., Liu X., Jiao Y., Xiong X., Wang E., Wang X., Zhang Z., Zhang H., Pan L., Guan Y., et al. Periostin promotes liver steatosis and hypertriglyceridemia through downregulation of PPARα. J. Clin. Invest. 2014;124:3501–3513. doi: 10.1172/JCI74438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Williams J.A., Manley S., Ding W.X. New advances in molecular mechanisms and emerging therapeutic targets in alcoholic liver diseases. World J. Gastroenterol. 2014;20:12908–12933. doi: 10.3748/wjg.v20.i36.12908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.You M., Matsumoto M., Pacold C.M., Cho W.K., Crabb D.W. The role of AMP-activated protein kinase in the action of ethanol in the liver. Gastroenterology. 2004;127:1798–1808. doi: 10.1053/j.gastro.2004.09.049. [DOI] [PubMed] [Google Scholar]
- 43.Mandrekar P., Ambade A., Lim A., Szabo G., Catalano D. An essential role for monocyte chemoattractant protein-1 in alcoholic liver injury: regulation of proinflammatory cytokines and hepatic steatosis in mice. Hepatology. 2011;54:2185–2197. doi: 10.1002/hep.24599. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


