Abstract
Although dietary supplement use is common, its assessment is challenging, especially among ethnic minority populations such as Hispanics/Latinos. Using the Hispanic Community Health Study/Study of Latinos (HCHS/SOL) (n = 16,415), this report compares two strategies for capturing dietary supplement use over a 30-day period: a medication-based inventory and a nutrition-based dietary supplement interview. Age-standardized prevalence was calculated across multiple dietary supplement definitions, adjusted with survey/nonresponse weights. The prevalence of dietary supplement use was substantially higher as measured in the dietary supplement interview, compared to the medication inventory: for total dietary supplements (39% vs 26%, respectively), for nonvitamin, nonmineral supplements (24% vs 12%), and for botanicals (9.2% vs 4.5%). Concordance between the two assessments was fair to moderate (Cohen’s kappa: 0.31–0.52). Among women, inclusion of botanical teas increased the prevalence of botanical supplement use from 7% to 15%. Supplement assessment that includes queries about botanical teas yields more information about patient supplement use.
Keywords: dietary supplements, epidemiology, Hispanics/Latinos, measurement methodology
Introduction
Growth in the number of marketed dietary supplements has been marked, increasing from 4,000 products in 1994 to 55,000 in 2009.1 Dietary supplements result in >$20 billion in sales each year with growth of about 6% per year2 and prevalence of dietary supplement use is common in the United States (US) general public.3,4 In national studies, among Hispanics/Latinos, the reported prevalence of any dietary supplement use (34%) in the past 30 days was lower as compared with non-Hispanic whites (59%).3 National studies of botanical and nonvitamin, nonmineral (NVNM) supplement use in the prior year also report lower prevalence among Hispanics/Latinos, 12% compared with 23%,4 but other studies conducted within Hispanic/Latino populations have resulted in much higher estimates: >60% in the past 12 months,5,6 leading to questions about the comparability of these study designs.
Assessment of supplement use is challenging in any population, but is particularly difficult among ethnic minorities and with regard to botanical and other NVNM supplements. Most observational studies rely on self-completed questionnaires7–10 or telephone surveys11 to collect dietary supplement data, asking participants to choose from a list of supplements that may or may not be consistent with cultural traditions. Product examination is considered a criterion standard for supplement assessment,12 but, if not assessed in the home, could miss supplements individuals forget to bring in to the study visit. Moreover, disclosure of supplement use to healthcare providers has been suboptimal, potentially leaving patients open to adverse effects.13 A more systematic approach to supplement assessment is clearly indicated.
The current study utilizes the baseline data of the His-panic Community Health Study/Study of Latinos (HCHS/SOL). HCHS/SOL is unique in its supplement assessments in two ways. The study collected supplement data in a sample of US Hispanics/Latinos using both a dietary interview method and a medication-based (product examination inventory) method to assess supplement use over 30 days. In addition, supplement use was also captured in the 24-hour dietary recall data, reflecting immediate use. This analysis categorized dietary supplement types and measured their prevalence among Hispanics/Latinos of diverse Hispanic backgrounds by a medication inventory (MV) and a dietary supplement interview (DI). The analysis also addresses the prevalence of common supplement ingredients.
Methods
Data source
The HCHS/SOL is a prospective cohort study designed to identify risk factors and disease prevalence among Hispanic/Latino residents of four communities in the US (Miami, Bronx, Chicago, and San Diego). To achieve a representative sample of the target population, a two-stage area household probability design was employed.14,15 HCHS/SOL investigators chose communities to achieve a balanced recruitment of Hispanics/Latinos from across countries of origin and geographic areas of the US.14 Within communities, census tracts were selected based on their proximity to the field center clinics and demographics; study plans called for cross-stratifying by high vs low concentration of Hispanic/Latino residents and high vs low SES.15 The recruitment plan focused on advertising and face-to-face recruitment within neighborhoods and housing blocks, including communities with high concentrations of Hispanics/Latinos.14,15 Hispanic/Latino community involvement was key to the highly successful recruitment strategy.15 This report is based on cross- sectional data from the baseline data collection, 2008–2011.
Study population
The study enrolled 16,415 noninstitutionalized adults who self-identified their background as Cuban, Dominican, Puerto Rican, Mexican, Central American, South American, or other Hispanic/Latino. Of the enrolled, 16,279 had data for the outcomes and were included in the analyses. Individuals within sampled households were screened for eligibility (living in the household, age 18–74, able to attend a clinic visit, and not planning to move within six months).15 Enrolled individuals attended a comprehensive examination visit at the study field center where they reviewed/signed consent documents and participated in study assessments, including demographic, medical, nutrition, and physical activity assessments. Study procedures were approved by human research ethics boards at participating institutions. Human research ethics approval for the current study was waived by the University of North Carolina Institutional Review Board, because the analysis was based solely on secondary, de-identified data.
Categorization of the dietary supplement data
The medication inventory, the dietary supplement interview, and the two 24-hour dietary recalls all contained product name files for a variety of dietary supplements. Dietary supplement products were categorized using an adapted form of the Langual categorization system proposed by Saldanha et al.16 The Langual system involves coding multiple facets of a product, from the general, to the specific. For this study, coded facets included product type (Facet A), physical form (Facet E, with collapsed categories), and ingredients (Facet H).
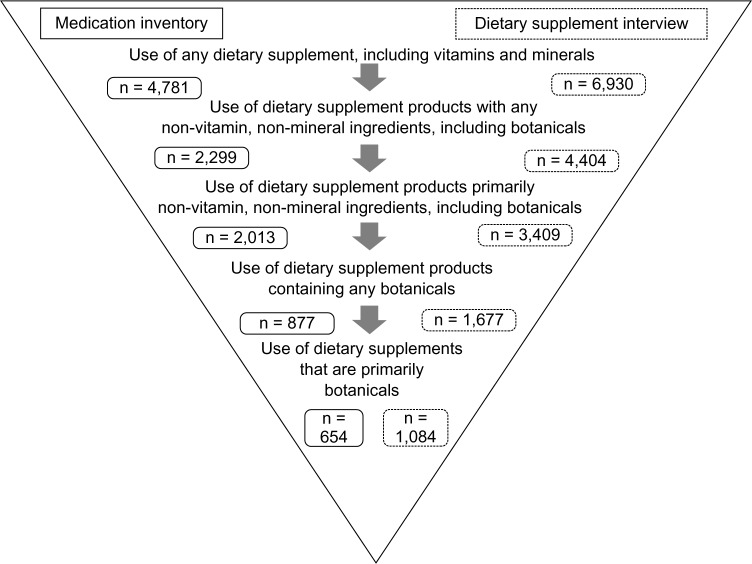
Although the Langual system nicely organizes dietary supplement ingredients, definitions of dietary supplement product types have not yet been standardized. In this study, based on the available product information, combination products that contained ≥50% of the recommended daily allowance (RDA) of a vitamin or mineral, and also contained a botanical or other NVNM ingredient were classified as a dietary supplement with NVNM. Those that contained <50% RDA of a vitamin or mineral were classified as a primarily NVNM product. Botanicals were similarly classified. NVNM products included botanicals, but botanical products excluded other NVNM (eg, glucosamine, coenzyme Q10, omega-3 and omega-6 fatty acids, fiber products, enzymes, probiotics, amino acids, and protein supplements) (Fig. 1). Based on these classifications, supplement users were defined as: (1) users of any dietary supplements; (2) users of supplements containing any NVNM (including botanicals); (3) users of supplements consisting of primarily NVNM ingredients (including botanicals); (4) users of supplements containing any botanical ingredients; (5) users of primarily botanical supplements.
Figure 1.
Dietary supplement assessment protocol.
Notes: Sample is restricted to participants with data for both the medication inventory and the dietary supplement interview. For the medication inventory, participants were asked to bring in all dietary supplements and home remedies taken in the past four weeks. Supplements were recorded in the medications database. For the dietary supplement interview, after providing information about food intakes in the past 24 hours, participants responded to a series of questions about their supplement use in the past 30 days. The dietary supplement interview utilized the dietary supplements assessment module of the Nutrition Data System for Research. Additional botanicals, captured with the 24-hour food recalls, are not reflected in the figure because of a differing time frame.
Measurement variables
Medication inventory
Participants in the HCHS/SOL were asked to bring all medications, dietary supplements, and herbal remedies taken in the four weeks prior to their baseline visit. The medication inventory was designed to therapeutically classify all prescription and over-the-counter medications and supplements, including vitamins, minerals, botanicals, and supplements used by participants during the four weeks preceding examination. The inventory involved scanning any Universal Product Code bar code symbols on medication packaging, pill imprint searches using Facts & Comparisons® Drug Identifier and Ident-A-Drug Reference © when necessary, and automated therapeutic classification of >99% of products based on their generic/brand name using a Master Drug Data Base (Medispan MDDB®) supplemented with Spanish-language brand and generic name equivalents from Lexi-Comp Online™ and OVID © Martindale. For the remaining 1% of products, manual coding was performed by healthcare professionals with expertise in medication and supplement identification. Approximately 10,000 products were identified as dietary supplements. Over 1,600 products could not be coded beyond identification as a dietary supplement because of insufficient information, resulting in missing NVNM data for 630 individuals (4%).
Dietary supplement interview
At the time of the field center clinic visit, immediately following the 24-hour dietary recall, technicians interviewed participants (in Spanish or English, according to their preference) about their recalled dietary supplement use in the 30 days prior to the study visit. The dietary supplements from the 30-day supplement interview were recorded in the Dietary Supplement Assessment Module (DSAM) of the Nutrition Data System for Research database (NDSR). Information for supplements that did not match in the DSAM were updated from the product label (obtained from the manufacturer when possible) and validated against outside resources. DSAM-based product name files were recorded with greater detail than the medication inventory files, enabling refined product coding. The dietary supplement interview data consisted of ∼2,700 different products representing ∼15,000 entries. Calculations of the prevalence of individual botanicals within dietary supplement products depended on the DSAM ingredient-level dataset. Botanical supplement reports were limited to those with a prevalence of at least 0.2%.
Twenty-four-hour dietary recall
The 30-day dietary supplement interview files recorded a few botanical teas. However, botanical teas, other liquid dietary supplement products, and culturally specific botanicals appeared in the two 24-hour dietary recalls enabling the calculation of: (1) estimates of supplement use including botanical teas and (2) estimates also including green tea (Camellia sinensis) and nopal (whole fruit).
Statistical analysis
The distribution of key variables was examined across dietary supplement users, NVNM supplement users, and botanical supplement users, including demographics (age, gender, education, percent of poverty for family size, and year), healthcare access (percent of poverty, insurance status, self-reported lack of access to care), health behaviors (cigarette smoking, diet quality, physical activity), and acculturation measures (language preference, years of residence in the US, born in US). All variables were examined for missing data; analyses were based on 16,060 individuals who had data for both the medication inventory and dietary supplement interview.
Because neither the dietary supplement interview nor the medication inventory could be considered a gold standard measure, comparison of the two was limited to calculation of Cohen’s kappa statistics with positive and negative agreements17 and prevalence and bias-corrected kappa (PABAK).18 Kappa statistics were interpreted following established criteria.19 In addition, to look at the sensitivity of dietary supplement prevalence estimates to various assumptions, estimates were calculated based on: (1) the dietary supplement interview updated with botanical teas from the 24-hour dietary recall; (2) the dietary supplement interview with 24-hour recall data, including nopal and green tea; and (3) the combined dietary supplement interview, medication inventory, and dietary recall.
Results
Characteristics of the study population
Distributions of key characteristics of the study population are presented, classifying participants as users of any dietary supplement (n = 7,658), users of NVNM supplements (including botanicals) (n = 3,916), users of botanical supplements (n = 1,433), and users of no supplements (n = 8,402) based on their reported use in either the dietary supplement interview or the medication inventory (Table 1). Supplement use categories are nested (Fig. 1). Users of supplements that included NVNM ingredients were older (mean age of 46 vs 38 years among nonusers), more likely to be female (55 vs 48%), and more likely to be nonsmokers (65 vs 58%).
Table 1.
Distribution of sample population characteristics by the use of supplement typesa in HCHS/SOL.
| USERS OF ANY SUPPLEMENTSa N = 7,658 |
USERS OF ANY NVNM SUPPLEMENTS N = 3,916 |
USERS OF ANY BOTANICAL SUPPLEMENTS N = 1,433 |
NONUSERS OF DIETARY SUPPLEMENTS N = 8,402 |
||
|---|---|---|---|---|---|
| Nb |
MEAN OR% (95% CI)c |
MEAN OR% (95% CI)c |
MEAN OR% (95% CI)c |
MEAN OR% (95% CI)c |
|
| Mean age | 16,279 | 45.5 (44.8, 46.2) | 46.4 (45.6, 47.1) | 43.8 (42.8, 44.8) | 37.7 (37.2, 38.2) |
| % Female | 9,764 | 56.5 (54.7, 58.3) | 54.8 (52.5, 57.1) | 58.4 (55.0, 61.7) | 48.3 (46.7, 49.8) |
| Mean% poverty threshold | 14,669 | 169 (160, 178) | 182 (170, 193) | 183 (169, 197) | 142 (137, 147) |
| Hispanic/Latino Background (%) | |||||
| Dominican | 1,466 | 9.8 (8.3, 11.6) | 7.6 (6.3, 9.3) | 6.6 (5.2, 8.4) | 9.8 (8.4, 11.3) |
| Central American | 1,718 | 7.7 (6.5, 9.0) | 8.3 (7.0, 10.0) | 8.6 (6.9, 10.6) | 7.0 (6.0, 8.2) |
| Cuban | 2,338 | 18.7 (16.0, 21.7) | 19.4 (16.6, 22.5) | 16.1 (13.4, 19.3) | 22.8 (19.1, 26.8) |
| Mexican | 6,455 | 40.0 (36.5, 43.6) | 41.6 (37.8, 45.6) | 46.9 (42.7, 51.2) | 34.7 (31.5,38.1) |
| Puerto Rican | 2,708 | 14.6 (13.0, 16.3) | 13.3 (11.5, 15.4) | 12.6 (10.4, 15.2) | 17.0 (15.2, 19.0) |
| South American | 1,071 | 5.6 (4.8, 6.5) | 6.0 (5.0, 7.3) | 5.6 (4.4, 7.2) | 4.6 (4.0, 5.3) |
| Other | 523 | 3.7 (3.0, 4.6) | 3.7 (2.8, 4.8) | 3.6 (2.5, 5.1) | 4.1 (3.5, 4.8) |
| Preference for Spanish | 13,009 | 74.8 (72.6, 76.8) | 74.7 (72.2, 77.0) | 75.5 (72.5, 78.1) | 76.6 (74.4, 78.7) |
| Born in US (%) | 2,855 | 21.5 (19.6, 23.5) | 20.7 (18.7,22.8) | 20.7 (18.1, 23.4) | 21.5 (20.0, 23.1) |
| Mean years in US | 16,211 | 21.2 (20.5, 21.9) | 21.1 (20.3,21.9) | 21.1 (20.2, 21.9) | 20.0 (19.1,20.8) |
| Education (%) | |||||
| <HS | 6,091 | 28.5 (26.5, 30.6) | 25.8 (23.5, 28.3) | 25.5 (22.5, 28.7) | 36.2 (34.5,38.1) |
| HS graduate | 4,116 | 26.3 (24.6, 28.0) | 24.9 (22.8, 27.1) | 23.7 (21.1, 26.5) | 29.2 (27.8, 30.6) |
| At least some college | 5,741 | 45.2 (42.8, 47.7) | 49.3 (46.3, 52.2) | 50.8 (47.1, 54.5) | 34.6 (32.9, 36.3) |
| % with no insurance | 7,893 | 47.2 (44.8, 49.7) | 50.0 (47.0, 53.0) | 53.2 (49.4, 57.1) | 50.6 (48.6, 52.7) |
| % unable to get needed healthcare | 2,695 | 15.1 (13.7, 16.6) | 15.6 (14.0, 17.4) | 17.0 (14.8, 19.4) | 15.6 (14.4, 16.8) |
| % meeting physical activity guidelinesd | 10,335 | 68.7 (66.9, 70.5) | 70.4 (68.3, 72.5) | 71.3 (68.2, 74.2) | 64.5 (63.1, 66.0) |
| Mean AHEIe | 16,126 | 48.3 (47.9, 48.7) | 48.9 (48.4, 49.4) | 49.5 (49.0, 50.1) | 47.1 (46.7, 47.5) |
| Cigarette use (%) | |||||
| Never smoked | 9,884 | 64.6 (62.8, 66.4) | 64.8 (62.4, 67.0) | 63.0 (59.5, 66.3) | 57.8 (56.0, 59.5) |
| Former smoker | 3,216 | 18.6 (17.3, 20.1) | 19.8 (18.1, 21.6) | 21.3 (18.9, 24.1) | 17.6 (16.5, 18.7) |
| Current smoker | 3,148 | 16.7 (15.1, 18.5) | 15.5 (13.7, 17.4) | 15.7 (13.2, 18.5) | 24.7 (23.3, 26.1) |
| Self-reported health (%) | |||||
| Fair-poor | 4,834 | 25.5 (23.7, 27.4) | 24.3 (22.2, 26.5) | 23.7 (21.2(26.5) | 26.3 (25.0, 28.0) |
| Good | 7,492 | 45.1 (43.1, 47.0) | 44.1 (41.7, 46.5) | 46.1 (43.0, 49.3) | 46.8 (45.3, 48.4) |
| Very good-excellent | 3,898 | 29.4 (27.5, 31.4) | 31.6 (29.0, 34.3) | 30.2 (27.0, 33.5) | 26.6 (25.2, 28.1) |
| Geographic area (US) (%) | |||||
| Bronx | 4,051 | 25.8 (22.9, 28.9) | 21.2 (18.5, 24.2) | 18.1 (15.3, 21.3) | 29.8 (26.6, 33.1) |
| Chicago | 4,108 | 14.2 (12.3, 16.3) | 14.6 (12.4, 17.1) | 15.2 (12.5, 18.4) | 16.9 (14.8, 19.2) |
| Miami | 4,054 | 28.8 (25.0, 33.0) | 30.8 (26.8, 35.2) | 28.8 (24.8, 33.3) | 31.5 (27.0, 36.4) |
| San Diego | 4,066 | 31.2 (27.1, 35.6) | 33.3 (28.6, 38.4) | 37.8 (32.5, 43.5) | 21.8 (18.8, 25.1) |
Notes:
Supplement users were defined as users by either the medication inventory or the dietary supplement interview. Categories presented for comparison of distributions include only users of any dietary supplement, users of primarily NVNM products (including botanicals), and users of botanical supplements (including fiber).
N, number of participants in sample, rather than the representative population.
Means and percentages reflect sample weights and are age standardized to the US population.
Physical activity data were summarized as meeting or not meeting CDC 2008 guidelines for physical activity.
Nutritional data were summarized with the Alternative Healthy Eating lndex-2010 (AHEI-2010). The AHEI was developed to update prior healthy eating indices based on US Dietary Guidelines with recommendations derived from scientific studies of diet and health outcomes.33,34 The AHEI assigns values for consumption of vegetables, fruit, whole grains, sugar-sweetened beverages, nuts/legumes, red/processed meats, trans fat, long-chain n-3 fatty acids, total polyunsaturated fatty acids, sodium, and alcohol intake with a maximum value of 110.34 Healthy individuals aged 55–80 in the Nurses' Health Study had a mean AHEI-2010 of 53.2.33
Abbreviation: AHEI, alternative healthy eating index.
Users of botanical supplements only had a higher education and greater adherence to a higher quality diet as measured by the 2010 Alternative Healthy Eating Index. They were more likely to be of Mexican background and living in San Diego; they were less likely to have insurance or to have access to needed healthcare.
Concordance of the medication inventory and dietary supplement interview
Prevalence of supplement use as measured by the dietary supplement interview was higher than that for the medication inventory, even for overall dietary supplement use (39.3% vs 25.9%) (Table 2). The absolute differences were smaller, but the proportional difference was greater for NVNM and botanical products: prevalence estimates of products with any NVNM or botanical components in the dietary supplement interview were about twice that of the medication inventory. Positive agreement and overall kappa statistics were consistent with moderate agreement for most comparisons (κ: 0.44–0.52). Kappa for botanical products was only fair (κ: 0.31–0.36), but negative agreement was high, as was prevalence and bias-adjusted kappa (PABAK: 0.81–0.88). Kappa statistics for liquid or powdered products (κ: 0.11–0.13) were low, but negative agreement for these products were high at 0.96–0.99 (PABAK: 0.85–0.95). In fact, negative agreement was high across all categories of supplements (κ: 0.82–0.99). In sensitivity analyses, assigning uncategorized products from the medication inventory to NVNM or botanical supplements, based on their similarity to products that were definitely classified as such, reduced the disparity between the two assessments (Table 2).
Table 2.
Comparison of the 30-day dietary supplement use assessed through the medication inventory and dietary supplement interview in HCHS/SOLa.
| VARIABLE | MEDICATION INVENTORY (MV) | DIETARY SUPPLEMENT INTERVIEW (Dl) | MV+ DI+ | MV+ Dl- | MV-DI+ | MV-Dl- | POSITIVE AGREEMENT | NEGATIVE AGREEMENT | COHEN'S KAPPA | PREVALENCE-AND BIAS-ADJUSTED KAPPAc |
|---|---|---|---|---|---|---|---|---|---|---|
| % (95% Cl)b | % (95% Cl)b | n | n | n | n | (95% CI) | (95% CI) | (95% CI) | ||
| Any dietary supplement (DS) | 25.9 (24.6, 27.1) | 39.3 (37.9, 40.6) | 4053 | 759 | 2910 | 8557 | 0.69 (0.68, 0.70) | 0.82 (0.82, 0.83) | 0.52 (0.51, 0.53) | 0.55 |
| DS with any NVNM component | 12.4 (11.6, 13.2) | 24.4 (23.2, 25.6) | 1733 | 577 | 2,306 | 11,033 | 0.55 (0.53, 0.56) | 0.88 (0.88, 0.89) | 0.44 (0.42, 0.46) | 0.63 |
| DS primarily NVNM | 10.8 (10.1, 11.5) | 18.8 (17.7, 19.9) | 1465 | 544 | 1612 | 12,028 | 0.58 (0.56, 0.59) | 0.92 (0.91, 0.92) | 0.50 (0.48, 0.52) | 0.72 |
| DS with any botanical | 4.5 (4.1, 4.9) | 9.2 (8.4, 10.0) | 417 | 466 | 1009 | 13,737 | 0.36 (0.34, 0.39) | 0.95 (0.95, 0.95) | 0.31 (0.29, 0.34) | 0.81 |
| Primarily botanical products | 3.3 (3.0, 3.7) | 5.7 (5.1, 6.3) | 305 | 354 | 589 | 14,401 | 0.39 (0.36, 0.42) | 0.97 (0.97, 0.97) | 0.36 (0.33, 0.39) | 0.88 |
| Sensitivity Analysis | ||||||||||
| DS with NVNMd | 15.3 (14.3, 16.4) | 24.4 (23.2, 25.6) | 2125 | 804 | 2263 | 11,008 | 0.58 (0.57, 0.59) | 0.88 (0.87, 0.88) | 0.46 (0.45, 0.48) | 0.62 |
| DS with botanicald | 5.1 (4.6, 5.5) | 9.2 (8.4, 10.0) | 485 | 534 | 986 | 13,739 | 0.39 (0.37, 0.41) | 0.95 (0.94, 0.95) | 0.34 (0.31,0.36) | 0.81 |
Notes: Agreement statistics were calculated with DAG_Stat, an Excel-based program for the calculation of agreement statistics.35
Analysis is restricted to subjects with data for both the medication inventory and the dietary supplement interview (n= 16,279). An additional 630 (4%) of participants took supplements for which insufficient detail was available for coding.
Proportions are adjusted by sample and nonresponse weights and are age standardized to the 2010 Census distribution for Hispanics/Latinos.
The PABAK adjusts not only for the prevalence effect (the difference between the positive and negative agreements) but also for differences in the marginal probability of supplement use between the assessments.
Sensitivity analysis reflects coding supplements with insufficient information, but likely to be NVNM and botanical (based on similar products and those with the same medication inventory codes).
Abbreviations: DS, dietary supplements; NVNM, nonvitamin, nonmineral products, including botanicals; MV, medication inventory; DS, dietary supplement interview.
Sensitivity of the prevalence estimates to the measurement strategy
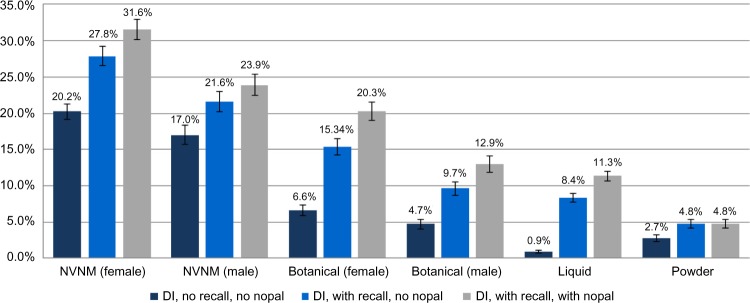
NVNM and botanical prevalence estimates were sensitive to inclusion of botanical teas and other liquid supplements captured in the dietary recalls (Fig. 2). The use of botanical teas (excluding green tea) was relatively common: A total of 6.5% (95% CI: 5.8–7.3) reported a botanical tea or other botanical liquid product on either of the recall visits. The prevalence of use of any nopal was 3.2% (95% CI: 2.7–3.6), and the prevalence of the use of dietary green tea was 3.6% (95% CI: 3.1–4.1). If teas, liquid nopal, and other liquid supplements were included in the estimates as primarily botanical supplements, prevalence increased by 131% in women and 105% in men. Additionally, counting brewed green tea and nopal as botanical supplements increased the prevalence of botanical supplements substantially, by 32% in women and 34% in men. Additionally, counting all fiber products as botanicals increased the prevalence of botanical use by another 9% in women and 11% in men to 22.5% and 14.7%, respectively. The combined dietary and medication information resulted in a prevalence of 43.6% (95% CI: 42.2–45.0) for any dietary supplement, 27.8% (95% CI: 26.5–29.1) for supplements with NVNM, and 11.9% (95% CI: 11.0–12.7) for supplements with any botanical components.
Figure 2.
Prevalence of NVNM supplements considering the addition of supplements captured in the dietary recalls. Prevalence estimates are standardized to the 2010 US Census and weighted with sample and nonresponse weights and stratified by gender: ‘DI no recall no nopal’ references the dietary supplement interview data without dietary recall data. “DI with recall no nopal” references the dietary supplement interview data with the addition of dietary recall data, but excluding raw nopal and green tea. “DI with recall with nopal” references the dietary supplement interview data with dietary recall data, including raw/cooked nopal and green tea.
Note: Only nopal ingested outside recipes were included in these estimates.
Prevalence of individual supplement ingredients
NVNM ingredients included fish oil, glucosamine or related products, lutein-containing products; prevalence of these substances and individual botanical ingredients were calculated from the ingredient files (Table 3). Among the NVNM supplements, omega-3 fatty acids, lutein, and lycopene were the most common, occurring with a prevalence of about 10%. Omega-3 fatty acids were most often consumed as single supplements, while lutein and lycopene were often added to multivitamin products. Other common combination products included those containing glucosamine (3.4%) and lipotropic agents (primarily lecithin and inositol −3.4%).
Table 3.
Prevalence of selected supplement ingredients in the 30-day dietary supplement interview in HCHS/SOL.
| SUPPLEMENT INGREDIENT | N | PREVALENCE | SUPPLEMENT INGREDIENT | N | PREVALENCE |
|---|---|---|---|---|---|
| Vitamins | 5,674 | 32.7 (31.5, 33.9) | |||
| Minerals | 5,484 | 31.7 (30.4, 33.0) | Garlic | 333 | 1.7 (1.4, 2.1) |
| NVNM | Ginger | 111 | 0.65 (0.49, 0.87) | ||
| Amino acids | 562 | 3.1 (2.8, 3.6) | Ginkgo | 454 | 2.6 (2.2, 3.0) |
| Chondroitin | 351 | 1.9 (1.6, 2.3) | Ginseng | 435 | 2.6 (2.3, 3.0) |
| Coenzyme Q10 | 158 | 1.0 (0.7, 1.3) | Graine | 271 | 1.5 (1.3, 1.8) |
| Collagen | 179 | 0.9 (0.7, 1.1) | Grape seed | 176 | 0.90 (0.72, 1.1) |
| Enzyme | 55 | 0.25 (0.18, 0.36) | Green tea | 277 | 1.7 (1.4, 2.0) |
| Fiber | 246 | 1.3 (1.1, 1.5) | Guarana | 54 | 0.29 (0.20, 0.41) |
| Glucosamine | 664 | 3.4 (3.0, 4.0) | Hawthorn | 42 | 0.21 (0.13, 0.34) |
| Lipotropica | 666 | 3.4 (3.1, 3.8) | Holy basil (Tulsi) | 29 | 0.21 (0.11, 0.38) |
| Lutein | 1,574 | 9.5 (8.8, 10.3) | Horseradish | 53 | 0.37 (0.24, 0.58) |
| Lycopene | 1,654 | 10.5 (9.7, 11.4) | Horsetail | 160 | 0.70 (0.54, 0.90) |
| MSM | 271 | 1.4 (1.1, 1.7) | Licorice | 232 | 0.93 (0.77, 1.1) |
| Omega-3b | 1,765 | 9.6 (9.0, 10.4) | Milk thistle | 71 | 0.50 (0.30, 0.84) |
| Omega-6c | 190 | 1.1 (0.83, 1.4) | Mint | 54 | 0.27 (0.19, 0.38) |
| Omega-9d | 143 | 0.87 (0.62, 1.2) | Mushroom | 102 | 0.43 (0.30, 0.63) |
| Probiotics | 120 | 0.55(0.42, 0.72) | Nettle | 45 | 0.37 (0.21, 0.65) |
| Protein | 359 | 2.2 (1.8, 2.6) | Olive leaf | 40 | 0.20 (0.13, 0.32) |
| Botanicals | Parsley | 153 | 0.88 (0.69, 1.1) | ||
| Aloe | 127 | 0.60 (0.45, 0.79) | Pepper (black) | 43 | 0.23 (0.11, 0.40) |
| Astragalus | 48 | 0.29 (0.18, 0.48) | Pine bark | 93 | 0.39 (0.29, 0.53) |
| Bioflavonoids | 36 | 0.28 (0.17, 0.46) | Pumpkin seed | 59 | 0.48 (0.31, 0.73) |
| Black cohosh | 80 | 0.30 (0.22, 0.40) | Rhodiola | 88 | 0.40 (0.29, 0.56) |
| Boswellia | 81 | 0.27 (0.19, 0.37) | Rose hips | 75 | 0.29 (0.21, 0.40) |
| Cascara/Senna | 61 | 0.30 (0.22, 0.42) | Rosemary | 94 | 0.57 (0.42, 0.77) |
| Cayenne | 96 | 0.41 (0.31, 0.54) | Sage | 37 | 0.21 (0.12, 0.36) |
| Cinnamon | 101 | 0.48 (0.36, 0.64) | Saw palmetto | 106 | 0.88 (0.66, 1.2) |
| Chamomile | 54 | 0.23 (0.16, 0.33) | Schisandra | 59 | 0.35 (0.24, 0.50) |
| Dandelion | 75 | 0.38 (0.27, 0.53) | Seaweed | 213 | 1.0 (0.82, 1.3) |
| Dong quai | 52 | 0.22 (0.15, 0.33) | Soy | 100 | 0.49 (0.37, 0.64) |
| Echinacea | 101 | 0.69 (0.52, 0.92) | Spirulina | 157 | 0.86 (0.67, 1.1) |
| Elderberry | 52 | 0.33 (0.21, 0.52) | Turmeric | 79 | 0.37 (0.26, 0.53) |
| Eleuthero | 103 | 0.55 (0.38, 0.81) | Valerian | 44 | 0.22 (0.15, 0.31) |
| Fennel | 54 | 0.28 (0.20, 0.40) | Vegetable extract | 432 | 2.3 (2.0, 2.7) |
| Fo-ti | 58 | 0.21 (0.15, 0.30) | Yerba Mate | 40 | 0.25 (0.17, 0.37) |
| Fruit extracts | 796 | 4.1 (3.7, 4.5) |
Notes: Chamomile, green tea, and nopal are also commonly consumed as foods – not included in these estimates. Prevalence estimates are based on the entire sample rather than within dietary supplement users and are adjusted for age and the complex survey weights.
Lipotropic: lecithin, inositol.
Omega-3: fish oils, flaxseed oil;
Omega-6: linoleic acid, borage, evening primrose oils;
Omega-9: olive oil, oleic acid;
Grain: oats, wheat, corn, quinoa, alfalfa.
Many combination products containing vegetable or fruit extracts were counted as botanicals. Over 2% of study participants consumed a supplement with at least one vegetable extract and over 4% consumed a product containing a fruit extract. Culinary herbs, such as parsley, sage, and oregano were occasional constituents of combination products.
Ginkgo and ginseng were the most commonly reported botanicals (2.6%), followed by green tea extracts and garlic (1.7%). Licorice was also a component of botanical products among about 1% of participants. Chamomile at 0.23% and nopal at 0.13% were less common constituents of dietary supplement products – almost 2% of participants reported consuming chamomile tea in the dietary recall (1.9%; 95% CI: 1.6–2.3).
Discussion
Depending on the assessment (medication inventory and/or dietary supplement interview ± dietary recall data), HCHS/SOL estimated that 4.5%–19% of participants reported taking botanical supplements and 12%–32% reported use of NVNM supplements, including botanicals. Results of this study demonstrate the challenges of accurate dietary supplement assessments, particularly with regard to the botanical and other NVNM products. Estimates based on the medication inventory alone differed substantially from those based on the dietary supplement interview and neither assessment appears to have captured the use completely. Some products were reported only with the medication inventory and the others only with the dietary supplement interview. Botanical estimates in particular were markedly sensitive to varying assumptions about what constitutes a botanical product – including all fiber products as botanicals, a reasonable, but not universal assumption, markedly increased estimates, as did the inclusion of botanical teas and other liquid dietary supplements ascertained in the 24-hour dietary recall files.
Comparability of assessment comparisons
The most accurate self-report measure of dietary supplement use is considered to be a detailed interview with label capture and transcriptions, conducted in the home.12 Other studies have used a label capture system conducted in the clinic for supplement assessment, similar to the medication inventory in HCHS/SOL.20,21 In these studies, participants completed a dietary supplement questionnaire at home prior to the in-person interview, perhaps prompting improved supplement recall.
Kappa statistics in studies comparing questionnaires with label capture have been variable, ranging from 0.46 to 0.92.12,21 Agreement of supplement use as measured by a telephone interview compared to a label capture was somewhat lower– with a kappa as low as 0.14.20 A fourth study compared vitamin and mineral supplement assessment via a self-completed health questionnaire and two types of dietary assessments, a food frequency questionnaire and a 7-day food diary.22 Agreement in these studies comparing the three instruments was substantial (κ: 0.72–0.81).
In contrast, our agreement statistics are somewhat lower, especially for botanical products. Although every effort was made to classify dietary supplement products similarly in the two datasets, some misclassification was likely, given the amount of missing information in the medication inventory. In addition, a kappa statistic is less reliable in a setting of low prevalence, as seen with botanical supplements23 and with imbalances in the marginal totals as was seen in both the botanical and NVNM data.24 However, moderate agreement was seen with the comparisons of overall dietary supplement use and with the comparisons of NVNM supplements and negative agreement statistics were uniformly high.
Low agreement was not surprising, given the very different supplement ascertainment by the two systems. A review of medications is more likely to underestimate dietary supplement use. In clinical situations, accurate capture of dietary supplement use is uncommon: 33% of individuals with chronic disease reported that they had informed their healthcare provider of their supplement use and disclosure rates were lower among Hispanics (22%).13 When asked why they did not disclose their supplement use, patients reported that they did not think that it was important for their providers to know.25 A similar mechanism may be driving the lower prevalence in the medication inventory data in this study.
On the other hand, the dietary supplement interview benefited from both a systematic inquiry and the use of DSAM, a detailed database designed specifically for the capture of dietary supplement data. In addition, the dietary supplement interview followed a detailed interview about food intakes in the past 24 hours. Individuals may have been more likely to recall supplements they regarded as enhancements to their diet after a 24-hour dietary recall.
The value of the medication inventory and the dietary supplement interview independently could be called into question. However, together, and when combined with data from the 24-hour dietary recalls, a more complete picture of supplement use emerges. Comparisons of supplement assessments by these different techniques furnish important information about the impact of study design on reports of supplement prevalence in the Hispanic/Latino literature. Wide variations in botanical supplement prevalence may result from differences in assessment strategies as well as supplement definitions (including vs excluding botanical teas). In addition, because of the high probability of under-reporting, asking about supplement use multiple times is more likely to yield accurate estimates. In a study of home remedies for childhood diarrhea, researchers only learned of the botanical remedies after establishing a trusted relationship with participants.26
Comparing HCHS/SOL dietary supplement prevalence estimates to other studies
Depending on the instrument (inventory vs interview), HCHS/SOL prevalence estimates of botanical dietary supplement use in the last 30 days ranged from 4.5% to 9%, increasing to 15% with the addition of data from the dietary recall (botanical teas and other liquid supplements also consumed as foods). Commonly reported supplements were consistent with patterns in the general US public, rather than those reportedly common among Hispanics/Latinos such as aloe, mint, chamomile, flax, and linden.27 Interestingly, botanicals traditional among Hispanics/Latinos were more often reported with the 24-hour dietary recalls than with either the dietary supplement interview or the medication inventory.
Comparable studies were limited to four; most of them targeted an older population. In a home-based interview of dietary supplement use in the past two weeks with label capture among individuals aged ≥77 years in Texas, about 38% of Hispanics reported using vitamin or mineral supplements and 5% reported using botanicals, most commonly garlic, ginkgo, and saw palmetto.28 Another study employed a similar strategy (home-based interviews, use in the past two weeks) among Mexican Americans aged ≥65 years across the US–Mexican Border States and reported botanical medicine use was about 10%.29 In this latter study, investigators specifically inquired about the use of herbal teas and the most common botanical supplements were consistent with the previously reported favorites among Hispanics/Latinos: chamomile, mint, and aloe.29 In another study, using a telephone interview of supplement and medication use in the past seven days30 in the general US public, the prevalence of NVNM use was 12% among Hispanics; the most common supplements were lutein, ginkgo, garlic, and glucosamine,30 similar to those reported in HCHS/SOL. Another telephone interview (subjects ≥52) reported Hispanic/Latino use of any supplement at 45% and botanicals at 12%.
Limitations and strengths
All HCHS/SOL participants were recruited from urban centers; the dietary supplement habits of Hispanics/Latinos in rural areas may be very different. However, although the target population is limited to the four cities, HCHS/SOL’s design, using probability sampling within diverse regions, is superior to the convenience samples typical of many dietary supplement studies in these populations.
Dietary supplement assessment suffers from the same limitations as other data largely dependent on self-report or inventory methods conducted outside the home. It is unlikely that the medication inventory or the dietary supplement interview alone adequately captured supplement use. In the medication inventory, because supplements were spread across the files, instead of receiving a designation as a supplement, some supplements could have been missed, artificially reducing the prevalence estimates resulting from these data. On the other hand, the dietary supplement interview could have been improved by the use of prompts for supplements common among Hispanics/Latinos (eg, chamomile, mint, and aloe) rather than those common in the general population (Echinacea, ginseng, ginkgo, St. John’s wort). However, the dietary supplement interview data, using the DSAM, achieved much greater detail than has been reported in prior studies. The ability to add botanical teas from the 24-hour dietary recall data filled in some of the missing information from the dietary supplement interview alone. Moreover, the combination of instruments enriched the picture of dietary supplement use in the population.
An additional limitation of self-reported data is that consistency of use is impossible to determine. This limitation also applies to administrative data: even with prescription fill data, it is unclear whether or not individuals are taking the medications they purchase.
Public health significance
The importance of accurate measurement of dietary supplement use cannot be overstated. Although dietary supplements have biologic activity with the potential for adverse reactions and interactions with drugs,31,32 dietary supplements are not regulated with the same level of scrutiny as drugs.31,33 The limited regulation also precludes adequate surveillance of use – estimates of interactions and harmful reactions rely on adverse events reporting from individual users and their healthcare providers, many of which will go unrecognized.1,34 Unlike prescription drugs, tracking use via pharmacy charges is not possible, and supplement recording via medical records is poor,35 particularly among ethnic minorities.36 In an era of electronic health records, an updated, user-friendly dietary supplement database could greatly enhance the ability of clinicians to capture supplement use among patients, perhaps even by enabling patients to input their own supplement and over-the-counter products.
Areas for future research
The reported prevalence of types of dietary supplements (botanical, NVNM) has been variable among Hispanics/Latinos. Some of this variability is related to sampling design (probability vs convenience), but, based on the findings in this report, it is likely that some of the variability reflects the nonstandardized definitions of what constitutes a botanical or even a dietary supplement. Studies that include only purchased dietary supplement products are not counting home remedies such as botanical teas. Because unprocessed botanicals and teas are part of the health practices of many ethnic minority populations, including Hispanics/Latinos, excluding them from estimates may seriously underestimate use. In addition, failing to inquire about the use of a wide range of dietary supplements, including botanical teas, compromises clinical assessments. Development of dietary supplement assessment strategies that are rapid, patient-and provider-friendly, and comprehensive are likely to both enhance patient empowerment and increase patient safety.
Acknowledgments
We thank the participants and staff (particularly Marston Youngblood) of HCHS/SOL for their important contributions. Additional information about HCHS/SOL is available at: http://www.cscc.unc.edu/hchs/.
Footnotes
ACADEMIC EDITOR: Christopher Chang, Editor in Chief
FUNDING: The Hispanic Community Health Study/Study of Latinos was carried out as a collaborative study supported by contracts from the National Heart, Lung, and Blood Institute (NHLBI) to the University of North Carolina (N01-HC65233), University of Miami (N01-HC65234), Albert Einstein College of Medicine (N01-HC65235), Northwestern University (N01-HC65236), and San Diego State University (N01-HC65237). The following Institutes/Centers/Offices contribute to the HCHS/SOL through a transfer of funds to the NHLBI: National Institute on Minority Health and Health Disparities, National Institute on Deafness and Other Communication Disorders, National Institute of Dental and Craniofacial Research, National Institute of Diabetes and Digestive and Kidney Diseases, National Institute of Neurological Disorders and Stroke, NIH Institutions, and Office of Dietary Supplements. The National Center on Complementary and Alternative Medicine provided research training support (Faurot, T32-AT003378). The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: LAY discloses fees received for research from Boehringer-Ingelheim, Eli Lilly and Company, GI Dynamics, Halozyme Therapeutics, Johnson and Johnson, Lexicon Pharmaceuticals Inc, Medtronic, Novo Nordisk Inc, Orexigen Therapeutics Inc, PhaseBio, Sanofi, Takeda Pharmaceuticals USA Inc, ToleRx, NIH, Patient-Centered Research Outcomes Institute (PCORI), Scion Neurostim and Bayer, outside the work presented here. EW discloses grants from the National Institute of Child Health and Human Development, the National Heart, Lung, and Blood Institute, the National Institute of Environmental Health Sciences, the American Heart Association, the Federal Aviation Administration and the National Institute on Aging, and consulting fees from the EPA for work related to the Particulate Matter Integrated Science Assessment, outside the work presented here. Other authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the study: AMSR, KRF, EAW, HMG, GAT, SFC, MLD, JB, REG, LVH. Analyzed the data: KRF. Wrote the first draft of the manuscript: KRF, AMSR. Contributed to the writing of the manuscript: KRF, AMSR, PG, JOR, LAY, EAW, CP. Agreed with manuscript results and conclusions: KRF, AMSR, PG, JOR, LAY, CP, EAW, HMG, DACM, GAT, SFC, MLD, JB, REG, LVH. Jointly developed the structure and arguments for the paper: KRF, AMSR, PG, JOR, LAY, EAW, CP. Made critical revisions and approved the final version: KRF, AMSR, PG, JOR, LAY, CP, EAW, HMG, DACM, GAT, SFC, MLD, JB, REG, LVH. All the authors reviewed and approved the final manuscript.
REFERENCES
- 1.Dietary Supplements: FDA May Have Opportunities to Expand Its Use of Reported Health Problems to Oversee Products. 13–244. Washington DC: Office USGA; 2013. [Google Scholar]
- 2.DaVanzo JE, Heath S, El-Gamil A, Dobson A. The Economic Contribution of the Dietary Supplement Industry: Analyses of the Economic Benefits to the US Economy. Washington, DC: Natural Products Foundation; 2009. [Google Scholar]
- 3.Bailey RL, Gahche JJ, Lentino CV, et al. Dietary supplement use in the United States, 2003–2006. J Nutr. 2011;141(2):261–6. doi: 10.3945/jn.110.133025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008;12:1–23. [PubMed] [Google Scholar]
- 5.Rivera JO, Ortiz M, Lawson ME, Verma KM. Evaluation of the use of complementary and alternative medicine in the largest United States-Mexico border city. Pharmacotherapy. 2002;22(2):256–64. doi: 10.1592/phco.22.3.256.33543. [DOI] [PubMed] [Google Scholar]
- 6.Ortiz BI, Clauson KA. Use of herbs and herbal products by Hispanics in south Florida. J Am Pharm Assoc (2003) 2006;46(2):161–7. doi: 10.1331/154434506776180649. [DOI] [PubMed] [Google Scholar]
- 7.Bair YA, Gold EB, Greendale GA, et al. Ethnic differences in use of complementary and alternative medicine at midlife: longitudinal results from SWAN participants. Am J Public Health. 2002;92(11):1832–40. doi: 10.2105/ajph.92.11.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bright-Gbebry M, Makambi KH, Rohan JP, et al. Use of multivitamins, folic acid and herbal supplements among breast cancer survivors: the black women’s health study. BMC Complement Altern Med. 2011;11:30–30. doi: 10.1186/1472-6882-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Enger SM, Van den Eeden SK, Sternfeld B, et al. California Men’s Health Study (CMHS): a multiethnic cohort in a managed care setting. BMC Public Health. 2006;6:172–2. doi: 10.1186/1471-2458-6-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Refuerzo JS, Blackwell SC, Sokol RJ, et al. Use of over-the-counter medications and herbal remedies in pregnancy. Am J Perinatol. 2005;22(6):321–4. doi: 10.1055/s-2005-873235. [DOI] [PubMed] [Google Scholar]
- 11.Krousel-Wood MA, Muntner P, Joyce CJ, et al. Adverse effects of complementary and alternative medicine on antihypertensive medication adherence: findings from the cohort study of medication adherence among older adults. J Am Geriatr Soc. 2010;58(1):54–61. doi: 10.1111/j.1532-5415.2009.02639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Satia-Abouta J, Patterson RE, King IB, et al. Reliability and validity of self-report of vitamin and mineral supplement use in the vitamins and lifestyle study. Am J Epidemiol. 2003;157(10):944–54. doi: 10.1093/aje/kwg039. [DOI] [PubMed] [Google Scholar]
- 13.Mehta DH, Gardiner PM, Phillips RS, McCarthy EP. Herbal and dietary supplement disclosure to health care providers by individuals with chronic conditions. J Altern Complement Med. 2008;14(10):1263–9. doi: 10.1089/acm.2008.0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sorlie PD, Avilés-Santa LM, Wassertheil-Smoller S, et al. Design and implementation of the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol. 2010;20(8):629–41. doi: 10.1016/j.annepidem.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lavange LM, Kalsbeek WD, Sorlie PD, et al. Sample design and cohort selection in the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol. 2010;20(8):642–9. doi: 10.1016/j.annepidem.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saldanha LG, Dwyer JT, Holden JM, et al. A structured vocabulary for indexing dietary supplements in databases in the United States. J Food Compost Anal. 2012;25(2):226–33. doi: 10.1016/j.jfca.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cicchetti DV, Feinstein AR. High agreement but low kappa: II. Resolving the paradoxes. J Clin Epidemiol. 1990;43(6):551–8. doi: 10.1016/0895-4356(90)90159-m. [DOI] [PubMed] [Google Scholar]
- 18.Byrt T, Bishop J, Carlin JB. Bias, prevalence and kappa. J Clin Epidemiol. 1993;46(5):423–9. doi: 10.1016/0895-4356(93)90018-v. [DOI] [PubMed] [Google Scholar]
- 19.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–74. [PubMed] [Google Scholar]
- 20.Patterson RE, Kristal AR, Levy L, McLerran D, White E. Validity of methods used to assess vitamin and mineral supplement use. Am J Epidemiol. 1998;148(7):643–9. doi: 10.1093/aje/148.7.643. [DOI] [PubMed] [Google Scholar]
- 21.Nahin RL, Fitzpatrick AL, Williamson JD, et al. GEM Study Investigators Use of herbal medicine and other dietary supplements in community-dwelling older people: baseline data from the ginkgo evaluation of memory study. J Am Geriatr Soc. 2006;54(11):1725–35. doi: 10.1111/j.1532-5415.2006.00942.x. [DOI] [PubMed] [Google Scholar]
- 22.Lentjes MA, Welch AA, Luben RN, Khaw KT. Differences in dietary supplement use and secular and seasonal trends assessed using three different instruments in the EPIC-Norfolk population study. J Diet Suppl. 2013;10(2):142–51. doi: 10.3109/19390211.2013.790336. [DOI] [PubMed] [Google Scholar]
- 23.Viera AJ, Garrett JM. Understanding interobserver agreement: the kappa statistic. Fam Med. 2005;37(5):360–3. [PubMed] [Google Scholar]
- 24.Feinstein AR, Cicchetti DV. High agreement but low kappa: I. The problems of two paradoxes. J Clin Epidemiol. 1990;43(6):543–9. doi: 10.1016/0895-4356(90)90158-l. [DOI] [PubMed] [Google Scholar]
- 25.Young LA, Faurot KR, Gaylord SA. Use of and communication about dietary supplements among hospitalized patients. J Gen Intern Med. 2009;24(3):366–9. doi: 10.1007/s11606-008-0890-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andrews TJ, Ybarra V, Matthews LL. For the sake of our children: Hispanic immigrant and migrant families’ use of folk healing and biomedicine. Med Anthropol Q. 2013;27(3):385–413. doi: 10.1111/maq.12048. [DOI] [PubMed] [Google Scholar]
- 27.Ortiz BI, Shields KM, Clauson KA, Clay PG. Complementary and alternative medicine use among Hispanics in the United States. Ann Pharmacother. 2007;41(6):994–1004. doi: 10.1345/aph.1H600. [DOI] [PubMed] [Google Scholar]
- 28.Raji MA. Ethnic differences in herb and vitamin/mineral use in the elderly. Ann Pharmacother. 2005;39(6):1019–23. doi: 10.1345/aph.1E506. [DOI] [PubMed] [Google Scholar]
- 29.Loera JA, Black SA, Markides KS, Espino DV, Goodwin JS. The use of herbal medicine by older Mexican Americans. J Gerontol A Biol Sci Med Sci. 2001;56(11):M714–8. doi: 10.1093/gerona/56.11.m714. [DOI] [PubMed] [Google Scholar]
- 30.Kelly JP, Kaufman DW, Kelley K, Rosenberg L, Mitchell AA. Use of herbal/natural supplements according to racial/ethnic group. J Altern Complement Med. 2006;12(6):555–61. doi: 10.1089/acm.2006.12.555. [DOI] [PubMed] [Google Scholar]
- 31.Dietary Supplements: A Framework for Evaluating Safety. Washington, DC: National Academies Press; 2005. [PubMed] [Google Scholar]
- 32.Qato DM, Alexander GC, Conti RM, Johnson M, Schumm P, Lindau ST. Use of prescription and over-the-counter medications and dietary supplements among older adults in the United States. JAMA. 2008;300(24):2867–78. doi: 10.1001/jama.2008.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hardy CJ. FDA Regulation of Dietary Supplements. Dietary Supplement Intensive. Bethesda, MD: Office of Dietary Supplements, National Institutes of Health; 2010. [Google Scholar]
- 34.Woo JJ. Adverse event monitoring and multivitamin-multimineral dietary supplements. Am J Clin Nutr. 2007;85(1):323S–4S. doi: 10.1093/ajcn/85.1.323S. [DOI] [PubMed] [Google Scholar]
- 35.Cohen RJ, Ek K, Pan CX. Complementary and alternative medicine (CAM) use by older adults: a comparison of self-report and physician chart documentation. J Gerontol A Biol Sci Med Sci. 2002;57(4):M223–7. doi: 10.1093/gerona/57.4.m223. [DOI] [PubMed] [Google Scholar]
- 36.Rivera JO. Herbals and asthma: usage patterns among a border population. Ann Pharmacother. 2003;38(2):220–5. doi: 10.1345/aph.1D319. [DOI] [PubMed] [Google Scholar]