Abstract
Due to the indiscriminate use of antibiotics, resistance to antibiotics has increased remarkably in Staphylococcus aureus. Vancomycin is the final drug to treat the S. aureus infection, but nowadays, resistance to this antibiotic is also increasing. So, the investigation of antibiotic resistance pattern is important. As there is already resistance to vancomycin, there is an urgent need to develop a new kind of antimicrobial to treat S. aureus infection. Eugenol may be the new drug of choice. This study was conducted to evaluate the antibacterial activity of eugenol against vancomycin-resistant S. aureus isolated from clinical pus samples. Thirty six pus samples were included in the study. Samples were isolated, identified and antimicrobial susceptibility tests were performed as per routine laboratory protocol. The antimicrobial activity and mechanisms of killing of eugenol were studied. Out of 36 pus samples, only 20 isolates were confirmed as S. aureus strains and 6 isolates exhibited vancomycin resistance. Eugenol successfully destroyed the vancomycin-resistant strains via reactive oxygen species generation and membrane damage. The prevalence of vancomycin resistance is increased day by day in different countries, and necessary steps to prevent the spread and emergence of resistance should be taken. The findings of the study suggested that eugenol might be used to treat vancomycin-resistant S. aureus.
Keywords: Staphylococcus aureus, VRSA, eugenol, antibacterial, reactive oxygen species
Introduction
Staphylococcus aureus is a normal flora of human beings and other animals. It is also an opportunistic pathogen generally found in human beings in areas such as skin, nose, throat, mouth, and intestinal tract, causing mild-to-life-threatening diseases such as endocarditis, sepsis, soft tissue injury, urinary tract infection, respiratory tract infection, intestinal tract infection, and bloodstream infections.1,2 S. aureus is ubiquitous commensal gram-positive cocci on human skins and the anterior part of the body, but it frequently causes surgical wound infections with high prevalence rate ranging from 4.6% to 54.4% worldwide.3,4 To control S. aureus infection, different antibiotics are used, but recently, several antibiotics are not working against S. aureus infection because they are capable to resist these antibiotics. S. aureus has developed resistance to most classes of antimicrobial agents. Before 1944, penicillin was used to treat staphylococcal infection but, in 1944, first penicillin-resistant S. aureus was isolated. They produce penicillinase enzyme that destroys the penicillin.5 Nowadays, >90% S. aureus strains are resistant to penicillin.6 Later on, a semisynthetic penicillin known as methicillin was used to treat penicillin-resistant S. aureus. In 1962, methicillin was also resistant to S. aureus. In India, the prevalence rate of methicillin-resistant S. aureus (MRSA) is high in hospitals.7 Due to MRSA appeared, a glycopeptide antibiotic known as vancomycin was used to treat MRSA. In 1996, first intermediate resistance to vancomycin was reported,8 and in June 2002, resistance finally emerged first in USA. This resistance appeared because vancomycin-resistant S. aureus (VRSA) contains vanA gene and mecA gene.9 In India, first VRSA was found in Kolkata in 2005.10 To overcome this drug resistance and to treat the S. aureus infection, there is an urgent need to identify the antibiotic resistance pattern of bacteria and to develop a new kind of antimicrobial agent for proper management of S. aureus-infected patients.
Eugenol is a major phytochemical of clove oil and is primarily used as a flavoring agent in food and cosmetic products. Many studies revealed that eugenol shows excellent antimicrobial, antioxidant, and anti-inflammatory activities.11,12 It has been known that different antibiotics can influence the expression of staphylococcal exotoxins. Therefore, the goal of this study was to assess the function of eugenol on VRSA. For this purpose, the S. aureus strains are isolated from pus samples, identified on the basis of physiological or biochemical characteristics according to Bergeys Manual of Systematic Bacteriology,13–15 characterized by traditional biochemical reactions, and observed antibiotic resistance patterns of isolated S. aureus strains against some conventional and traditional antibiotics, including vancomycin, to identify VRSA. In developing countries, phenotypic tests are used in the diagnosis of staphylococcal infections.
Materials and Methods
Culture media, chemicals, and quality control strains
All the culture media, crystal violet, Lugol’s iodine, safranine, N,N,N′,N′-tetramethyl-p-phenylenediamine dihydrochloride, latex agglutination reagent, and antibiotic disks were purchased from HiMedia. Sodium chloride (NaCl), hydrogen peroxide (H2O2), sucrose, potassium dihydrogen phosphate (KH2PO4), and dipotassium hydrogen phosphate (K2HPO4) were procured from Merck Ltd. and SRL Pvt. Ltd. All other chemicals were from Merck Ltd. and SRL Pvt. Ltd. and were the highest grade available.
Different standard bacterial strains such as S. aureus ATCC 25923, Staphylococcus epidermidis ATCC 12228, Escherichia coli ATCC 23509, and Enterococcus faecalis ATCC were obtained from Microbiology Laboratory at Midnapore Medical College and Hospital and Microbiology Laboratory at University of Calcutta. These strains were stored in agar slants at 4°C for further studies and used as reference strains.
Collection, transport, and culture of sample
A total of 36 pus samples were collected from male outpatients, with the age range of 18–40 years who attended nearby hospital, after proper inquiry of their infection history and treatment summary between June 2013 and November 2014. All the patients enrolled in this study signed informed consent and were from low socioeconomic population. Inpatients, previously admitted patients, and postoperative patients were excluded from the study. Samples were collected using autoclaved sterile vials directly by using swab sticks. These samples were then transported to the laboratory within two hours of collection.16 The whole study protocol was approved by the Institutional Ethical Committee, Vidyasagar University, Midnapore, and conducted in accordance with the principles of the Declaration of Helsinki.
Isolation and identification of S. aureus
The samples kept in Luria broth was incubated in a shaking incubator at 37°C for 24 hours. Bacterial cultures were found growing on nutrient agar media. The samples were then purified by single-colony isolation technique on nutrient agar.10 Isolates were subcultured on tryptic soy agar plates containing 5% sheep blood agar and incubated at 37°C for 16–24 hours for characterization studies. On the basis of colony morphology, gram staining, and different biochemical reactions, the organisms were identified as S. aureus.15–25
Antimicrobial susceptibility testing
Antimicrobial susceptibility testing of clinical isolates was done by Kirby–Bauer disk diffusion method as recommended by the Clinical Laboratory Standards Institute guidelines.26,27 Commercially available antibiotic disks (HiMedia) were used for antimicrobial susceptibility testing. Susceptibility of isolates to penicillin G, ampicillin, oxacillin, cefotaxime, gentamycin, streptomycin, tetracycline, erythromycin, chloramphenicol, norfloxacin, amikacin, amoxiclav (ceftazidime/clavulanic acid), imipenem, ciprofloxacin, kanamycin, methicillin, and vancomycin was determined by the disk agar diffusion (DAD) technique. S. aureus ATCC 25923, an all-sensitive reference strain, was used as a quality control strain for the DAD test.
Antibacterial activity of eugenol
Determination of minimum inhibitory concentration
The minimum inhibitory concentration (MIC) was determined by a microdilution method, using Luria broth (HiMedia) according to the National Committee for Clinical Laboratory Standards with some modification.28,29 In brief, 10 µL of bacterial strain (SA-6) containing 2.5 × 105 CFU/mL S. aureus cells were added individually to 1 mL of nutrient broth. Different concentrations (1, 2, 5, 10, 25, 50, 100, and 200 µg/mL) of eugenol (dissolved solution that accurately reflect the amount of eugenol available in solution to act on the microorganisms) were added to the test tubes containing the test strains. After 24 hours of incubation in shaking condition, the MIC values were obtained by checking the turbidity of the bacterial growth. The lowest concentration at which there was no visible turbidity was taken as the MIC of that nanoparticle. The MIC value corresponded to the concentration that inhibited 99% of bacterial growth.
Determination of minimum bactericidal concentration
The minimum bactericidal concentration (MBC) of the eugenol was determined according to the standard method with some modifications.29 This is an extension part of the MIC experiment. The MBC values were determined by subculturing the MIC dilutions onto the sterile agar plates incubated at 37°C for 24 hours. The minimum concentration of the eugenol required for completely killing the tested bacteria was observed and tabulated as MBC level. The MBC value reflects 100% bacterial killing, compared with the positive control (no treatment).
Tolerance level
The tolerance levels of the bacterial strain against eugenol were determined according to the standard method using the following formula:30
Disk agar diffusion
Susceptibility of eugenol to S. aureus strains was determined by the DAD technique according to Bauer et al.26 The test bacterium taken from an overnight culture (inoculated from a single colony) was freshly grown for four hours having 106 CFU/mL were standardized against McFarland standard. With this culture, a bacterial lawn was prepared on Mueller-Hinton agar. Filter paper disks of 6 mm size were used to observe eugenol susceptibility. Water disks were used as control. Filter paper disks were prepared by absorbing 10 µL of drug from 2 mg/mL of eugenol and water, respectively. The diameter of the zone of bacterial growth inhibition surrounding the disk (including the disk) was measured.29
Intracellular reactive oxygen species generation
The intracellular reactive oxygen species (ROS) generation was measured by using 2,7-dichlorofluorescein diacetate (DCFH2-DA).29 The DCFH2-DA passively enters into the cell and reacts with ROS to form the highly fluorescent compound 2,7-dichlorofluorescein. In brief, S. aureus cells were treated with eugenol at their respective MIC concentrations for 24 hours. After treatment schedule, the cell pellet was collected, and a homogeneous suspension was made by phosphate-buffered saline (PBS) upto 1 mL, and then, the cells were incubated with 1 µg/mL DCFH2-DA for 30 minutes at 37°C in the dark condition. The cells were then washed three times and resuspended with fresh PBS. DCF fluorescence was observed by fluorescence microscopy (Nikon Eclipse LV100 POL). All measurements were done in triplicate and best images were represented in the article.
Action of eugenol on cellular morphology
In 1 mL culture medium, S. aureus cells (106 CFU/mL bacterial cells) were treated with eugenol at their respective MIC concentrations and incubated at 37 ± 2°C with shaking at 198 rpm for 24 hours. Control experiment was conducted in the absence of eugenol. At the end of 24 hours, the bacterial cultures were centrifuged and bacterial pellet was fixed with 50 µL of 2.5% glutaraldehyde and washes three times with 1 × PBS. A total of 50 µL PBS was added to this pellet to form a suspension. One drop of fixed pellet was taken on a glass plate and dried. Then, the sample was platinum coated for observation in a scanning electron microscope (SEM, Hitachi S-3000N).29
Results and Discussion
Identification of S. aureus
From the study, it was observed that 55.55% (20) samples were gram positive and 44.44% (16) samples were gram negative. 100% (20) of gram-positive isolates are oxidase positive, catalase positive, coagulase positive, latex agglutination positive, thermonuclease positive, positive mannitol fermentation activity, and positive hemolytic activity and 100% of gram-positive samples were nonmotile in nature (Table 1).
Table 1.
Standard biochemical tests of clinical isolates collected from pus sample.
| SAMPLE NO. | GRAM STAIN | OXIDASE | CATALASE | COAGULASE TEST | MOTILITY | LATEX AGGLUTINATION TEST | THERMONUCLEASE ACTIVITY | HEMOLYSIS ON BLOOD AGAR | GROWTH ON MSA | ISOLATES NAME |
|---|---|---|---|---|---|---|---|---|---|---|
| S1 | +ve | +ve | +ve | +ve | −ve | +ve | +ve | +ve | +ve | SA1 |
| S2 | −ve | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| S3 | −ve | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| S4 | +ve | +ve | +ve | +ve | −ve | +ve | +ve | +ve | +ve | SA2 |
| S5 | +ve | +ve | +ve | +ve | −ve | +ve | +ve | +ve | +ve | SA3 |
| S6 | −ve | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| S7 | +ve | +ve | +ve | +ve | −ve | +ve | +ve | +ve | +ve | SA4 |
| S8 | +ve | +ve | +ve | +ve | −ve | +ve | +ve | +ve | +ve | SA5 |
| S9 | −ve | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| S10 | +ve | +ve | +ve | +ve | −ve | +ve | +ve | +ve | +ve | SA6 |
| S11 | +ve | +ve | +ve | +ve | −ve | +ve | +ve | +ve | +ve | SA7 |
| S12 | +ve | +ve | +ve | +ve | −ve | +ve | +ve | +ve | +ve | SA8 |
| S13 | −ve | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| S14 | −ve | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| S15 | −ve | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| S16 | +ve | +ve | +ve | +ve | −ve | +ve | +ve | +ve | +ve | SA9 |
| S17 | +ve | +ve | +ve | +ve | −ve | +ve | +ve | +ve | +ve | SA10 |
| S18 | −ve | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| S19 | −ve | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| S20 | +ve | +ve | +ve | +ve | −ve | +ve | +ve | +ve | +ve | SA11 |
| S21 | −ve | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| S22 | +ve | +ve | +ve | +ve | −ve | +ve | +ve | +ve | +ve | SA12 |
| S23 | −ve | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| S24 | −ve | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| S25 | +ve | +ve | +ve | +ve | −ve | +ve | +ve | +ve | +ve | SA13 |
| S26 | +ve | +ve | +ve | +ve | −ve | +ve | +ve | +ve | +ve | SA14 |
| S27 | +ve | +ve | +ve | +ve | −ve | +ve | +ve | +ve | +ve | SA15 |
| S28 | −ve | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| S29 | +ve | +ve | +ve | +ve | −ve | +ve | +ve | +ve | +ve | SA16 |
| S30 | +ve | +ve | +ve | +ve | −ve | +ve | +ve | +ve | +ve | SA17 |
| S31 | +ve | +ve | +ve | +ve | −ve | +ve | +ve | +ve | +ve | SA18 |
| S32 | −ve | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| S33 | +ve | +ve | +ve | +ve | −ve | +ve | +ve | +ve | +ve | SA19 |
| S34 | −ve | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| S35 | +ve | +ve | +ve | +ve | −ve | +ve | +ve | +ve | +ve | SA20 |
| S36 | −ve | ND | ND | ND | ND | ND | ND | ND | ND | ND |
Notes: ND, tests are not done; +ve, tests are positive; −ve, tests are negative.
Gram-negative clinical isolates were not involved in this study, only gram-positive isolates were involved as it is commonly known that S. aureus is gram-positive bacteria. Clinical isolates were gram positive, which may be due to the thicker peptidoglycan layers of their cell walls; iodine penetrates the cell wall of the bacteria and alters the blue dye to inhibit its diffusion through the cell wall during the decolorization process.18 They were spherical cells arranged in irregular clusters resembling a bunch of grapes in gram staining. 100% (20) of gram-positive isolates are oxidase positive because of the presence of N,N,N′,N′-tetramethyl-p-phenylenediamine dihydrochloride as an artificial electron acceptor, which takes electron from cytochrome oxidase in the electron transport chain and changes its color to dark blue.19 100% of oxidase-positive isolates were catalase positive and coagulase positive but 100% of oxidase-positive isolates were nonmotile and gave positivity in latex agglutination test. The catalase test is used to detect an organism’s ability to produce catalase enzyme. In a clinical setting, the catalase test can be used to differentiate various gram-positive cocci, such as among the Staphylococci and Streptococci, and confirm the identification of various pathogens. Here, isolates were catalase positive due to the production of catalase enzyme, which catalyzes H2O2, a potent oxidizing agent into water and oxygen.20 S. aureus bacterial strains generally produces different proteins such as α- and γ-hemolysin, enterotoxins A and B, coagulase, and TSST-1, here isolates were coagulase positive due to the production of coagulase enzyme, which reacts with prothrombin to form staphylothrombin that causes blood to clot by converting fibrinogen to fibrin.21 Due to the absence of flagellum, the clinical isolates were nonmotile. Positivity in latex agglutination test was due to the interaction of human antibody attached to the latex particles with protein A bound to the bacterial cell surface or interaction between cell-associated clumping factor and plasma constituents adsorbed to the latex particles.17 100% of gram-positive and oxidase-positive isolates had potent thermonuclease activity, mannitol fermentation activity, and hemolytic activity. Isolates have hemolytic activity due to the production of hemolysin by isolates, which binds with the hemolysin receptor present on the surface of RBCs that favor hemolysis and make the clear zone surrounding the isolates.3 Latex agglutination activity, hemolytic activity, thermonuclease activity, mannitol fermentation activity, and non-motility of clinical isolates suggest that these may be S. aureus. Thus, among 36 clinical samples, 20 isolates (55.55%) were confirmed to be S. aureus strains. Thermonuclease activity of the isolates may be due to the breakdown of DNA present in the media by the production of nuclease enzyme. Nuclease production, coagulase positivity, and hemolytic activity suggested that the strains were pathogenic in type. The clinically isolated S. aureus strains were newly named as SA (S. aureus) from SA1 to SA20.
Antibiotic susceptibility testing
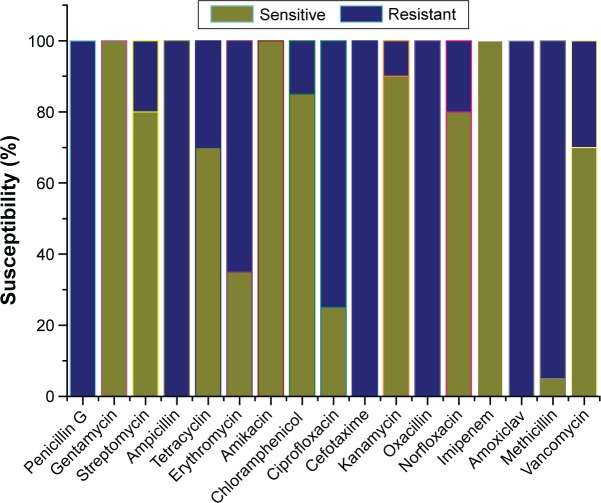
The antibiotic resistance profile of the isolated bacterial strains was revealed by the DAD test (Table 2). The results revealed that out of 20 gram-positive isolated strains, 100% isolated strains were resistant to penicillin G, ampicillin, cefotaxime, oxacillin, and amoxiclav antibiotics and 100% isolated strains were sensitive to gentamycin, amikacin, and imipenem. Of all S. aureus isolated strains, 95% were resistant to methicillin, 75% resistant to ciprofloxacin, 65% resistant to erythromycin, 30% resistant to tetracyclin and vancomycin, 20% resistant to streptomycin and norfloxacin antibiotics, 15% resistant to chloramphenicol, and 10% resistant to kanamycin (Table 2). The bacterial strains with resistance to three or more antibiotics are considered as multidrug-resistant (MDR) strains. From this study, it was revealed that 100% S. aureus isolated strains were MDR strains. They have different types of resistance pattern among MDR strains. Among the 20 MDR S. aureus strains, 5% strains were resistant to 5 antibiotics, 10% strains to 6 antibiotics, 20% strains to 7 antibiotics, 20% strains to 8 antibiotics, 10% strains to 9 antibiotics, 20% to 10 antibiotics, and 15% strains to ≥11 antibiotics. Figure 1 shows the graphical representation of antibiotic susceptibility profile of S. aureus. These S. aureus strains are resistant to β-lactam antibiotics, aminoglycosides, quinolones, macrolides, tetracycline, chloramphenicol, and vancomycin. This resistance may be due to the structural modification by enzymatic action that causes inactivation of the antibiotic; due to altering the outer membrane permeability, the access to target was prevented; antibiotic target site was altered and efflux pumps may be involved, which pumps out the antibiotic and target enzyme bypass or over production.31
Table 2.
Antimicrobial susceptibility testing of 20 isolates of S. aureus.
| ANTIBIOTICS | SENSITIVE NO. (%) | RESISTANT NO. (%) |
|---|---|---|
| Penicillin G | 0/20 (0%) | 20/20 (100%) |
| Gentamycin | 20/20 (100%) | 00/20 (0%) |
| Streptomycin | 16/20 (80%) | 04/20 (20%) |
| Ampicillin | 00/20 (0%) | 20/20 (100%) |
| Tetracyclin | 14/20 (70%) | 06/20 (30%) |
| Erythromycin | 07/20 (35%) | 13/20 (65%) |
| Amikacin | 20/20 (100%) | 00/20 (0%) |
| Chloramphenicol | 17/20 (85%) | 03/20 (15%) |
| Ciprofloxacin | 05/20 (25%) | 15/20 (75%) |
| Cefotaxime | 00/20 (0%) | 20/20 (100%) |
| Kanamycin | 18/20 (90%) | 02/20 (10%) |
| Oxacillin | 00/20 (0%) | 20/20 (100%) |
| Norfloxacin | 16/20 (80%) | 04/20 (20%) |
| Imipenem | 20/20 (100%) | 00/20 (0%) |
| Amoxiclav | 00/20 (0%) | 20/20 (100%) |
| Methicillin | 01/20 (5%) | 19/20 (95%) |
| Vancomycin | 14/20 (70%) | 6/20 (30%) |
Figure 1.
Antibiotic sensitivity pattern of 20 S. aureus strains isolated from pus sample.
Multiple antibiotic resistance (MAR) index of S. aureus shows that 85% strains had an MAR index of ≥0.4 and only 15% strains had an MAR index of <0.4 (Table 3). MAR index > 0.2 indicates the isolates originating from other sources where antibiotics were often used.32,33 However, the MAR values can be viewed as an indication of the extent of microbial exposure to antibiotics used within the community. Treatment of antibiotic-resistant bacteria is a therapeutic problem. Susceptibility pattern is useful to determine the future challenges of effective therapy.
Table 3.
MAR index of S. aureus isolates.
| MAR INDEX | NO. OF ISOLATES | PERCENTAGE (%) |
|---|---|---|
| 0.0 | 00 | 00.00 |
| 0.1 | 00 | 00.00 |
| 0.2 | 01 | 05.00 |
| 0.3 | 02 | 10.00 |
| 0.4 | 08 | 40.00 |
| 0.5 | 06 | 30.00 |
| 0.6 | 01 | 05.00 |
| 0.7 | 02 | 10.00 |
| 0.8 | 00 | 00.00 |
| 0.9 | 00 | 00.00 |
| 1.0 | 00 | 00.00 |
Antibacterial activity of eugenol
Determination of MIC and MBC
Antimicrobial activity of eugenol against isolated S. aureus strains at different concentrations showed a strong dose-dependent antimicrobial activity (Fig. 2). From this study, it was found that, as the concentration of eugenol was increased, microbial growth decreases. Particular phytochemical concentration was noted where no visible growth appears in broth culture, which is considered as MIC concentration. The MIC value of gram-positive S. aureus strain was 100 µg/mL. To avoid the misinterpretations due to the turbidity of insoluble compounds and color of the drug in broth dilution tube, MBC was determined by culturing the MIC dilutions on the sterile agar plates. The particular phytochemical concentration was noted where no visible growth appears on agar plate, which is considered as MBC concentration. The MBC value was 200 µg/mL against S. aureus strain (Fig. 2). From these results, we can suggest that inhibition of bacterial growths or bacterial killing were noted due to the penetration of eugenol into the bacterial cell that inhibits the growth of the bacteria and acts as a bactericidal agent followed by bacteriostatic activity. Eugenol is a monocyclic, oxygenated, aromatic monoterpene like menthol. It shows highly active antimicrobial activity. Several reports on the antimicrobial activity of some monoterpenes showed that the number of double bonds in the structure and acyclic, monocyclic, and/or bicyclic structure has no significant influence on its activity, but in this experience aromatic monoterpenes (carvacrol, eugenol, and thymol) showed the best inhibitory activity.34,35 The gram-positive S. aureus showed similar behaviors against the terpene. These terpenes could penetrate through the exopolysaccharide layer and maintain the inhibitory and bactericidal effects. In the future, eugenol could be used for therapeutical formulations in the replacement of antibiotic to treat diseases caused by resistant S. aureus. The observed results in our study seem to be quite similar to those previously reported. Qiu et al reported that the essential clove oil component including eugenol kills the microbes and inhibits the virulence factor at similar doses.36
Figure 2.
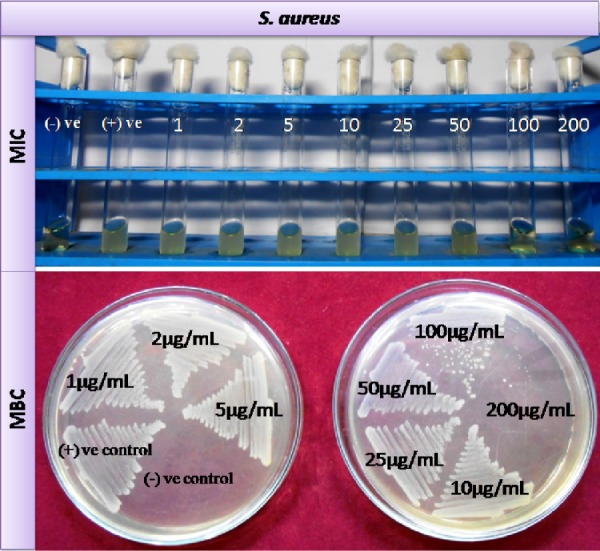
Determination of MIC and MBC values of eugenol for VRSA stain: (A) MIC of eugenol for SA6 (VRSA) isolate was 100 µg/mL and (B) MBC of eugenol for SA6 (VRSA) isolate was 200 µg/mL.
Tolerance level
The MBC/MIC ratio is a parameter that reflects the bactericidal capacity of the analyzed compound. The tolerance level of isolated S. aureus strain toward eugenol was calculated from the respective MIC and MBC values. In S. aureus strain, the tolerance level was 2 when charged with eugenol. Bactericidal agents kill total microbes, whereas bacteriostatic agents simply inhibit the bacterial growth. When MBC/MIC ratio is ≥16 for bacterial strains, the agent is considered bacteriostatic in type, and when this ratio is ≤4, the agent is considered bactericidal.37 MBC is usually identical to or within 1 or 2 doubling dilutions of the MIC; if the MBC exceeds the MIC by 32-fold or more, the microbe is defined as tolerant.29 In our study, eugenol exerted a bactericidal effect against S. aureus strains because the MBC/MIC ratio values were 2.
Disk agar diffusion
This was evident from the study that the diameter of the zone of inhibition obtained significance during the assessment of antibacterial activity. The inhibition zones of S. aureus against eugenol and distilled water as control are shown in Figure 3. In agar diffusion test, the diameter of the inhibition zone of eugenol toward S. aureus is 14 mm. No zone of inhibition was observed in the control disk. A plant-derived phytochemical known as eugenol showed potent function in the inhibition of growth of well-known pathogenic bacteria. This phytomedicine has really proved to be beneficial to minimize the total microbial growth inhibition.
Figure 3.
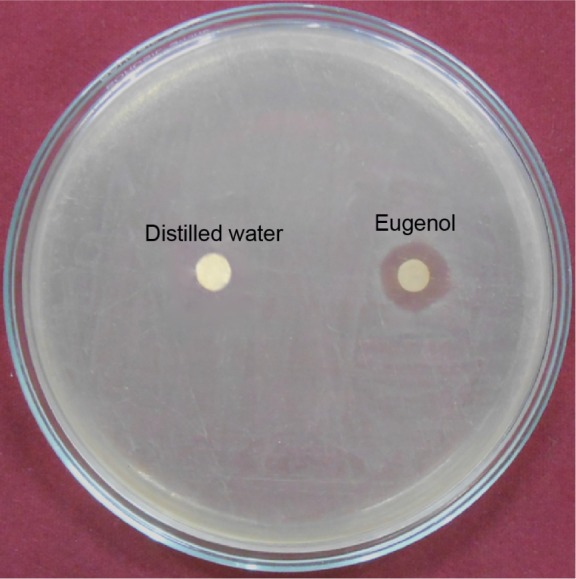
Antimicrobial activity of eugenol against VRSA strain showed by DAD method.
Intracellular ROS generation
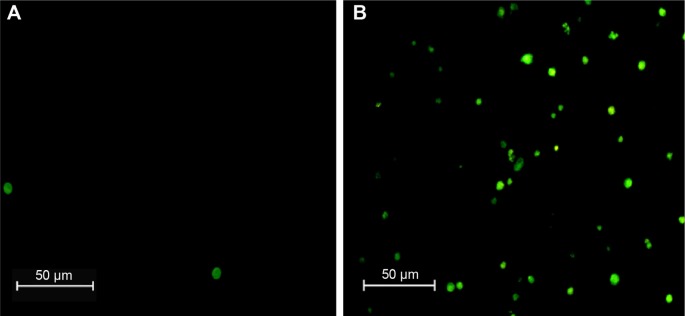
In the S. aureus cell, eugenoltriggered cytotoxicity has been encountered by the generation of ROS. Here, we measure the intracellular ROS; DCFH2-DA was used as intracellular ROS indicator for the eugenoltreated cells. After exposed to the phytochemical, bacterial cells were stained with DCFH2-DA for 30 minutes. Results revealed that the eugenol-treated S. aureus bacteria became DCF+, indicating that ROS were generated and participated in the eugenol-mediated cell death (Fig. 4) without eugenol treatment referred as control where no fluorescent cell was found, indicating no ROS generation. From these results, we believe that the anti S. aureus ability of the eugenol involves the generation of intracellular ROS. Elevation of ROS is the main candidate mediator for bacterial death. The ROS generation was caused by the impeded electronic transport along the respiratory chain in the damaged plasma membrane.38 The underlying mechanisms of ROS production in eugenol-treated cells will be further explored in detail.
Figure 4.
Microscopic images of intracellular ROS generation of VRSA strains: (A) control group and (B) treated group.
Action of eugenol on cellular morphology
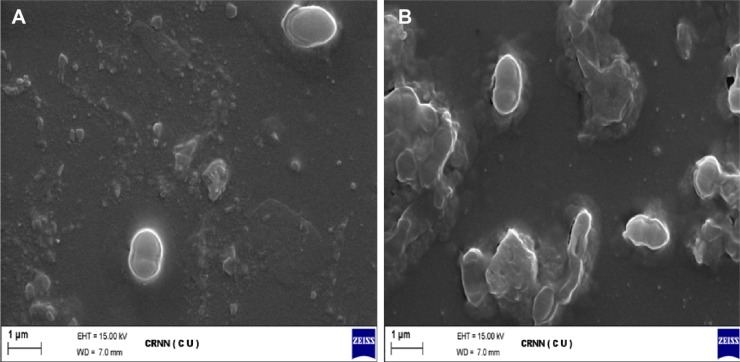
The cellular surface morphology was studied by SEM. The micrograph by SEM of S. aureus cells treated and untreated with eugenol are displayed in Figure 5. In SEM, eugenol was observed in the membrane of the bacteria as well as in the interior of the bacteria. SEM images explore the distribution and the exact location of the drug as well as the structural morphology of the bacterial cells after and before treatment with eugenol. Results showed that the surface of control bacterial cells (untreated cells; Fig. 5A) was smooth, intact, and showed typical characters of surface, while the treated cells (Fig. 5B) were damaged severely. Some cells showed large leakage, others misshapen and fragmentary, many pits and gaps appeared in the images, and their membrane was fragmented. The mechanism by which eugenol is able to penetrate the bacteria is not understood completely, but studies suggest that when bacterial cells were treated with eugenol, changes took place in its membrane morphology that produced a significant increase in its permeability, affecting proper transport through the plasma membrane, leaving the bacterial cells incapable of properly regulating transport through the plasma membrane, and resulting into cell death. These findings suggest the possible antibacterial mechanisms by which eugenol inhibits bacterial growth, as well as cellular responses. Eugenol entered into the cell and produced ROS, thus inhibiting the growth of cells. Simultaneously, eugenol may affect some cellular components to induce the collapse of membrane, resulting in cell decomposition and death eventually.29 However, it can be anticipated that eugenol by acting on cellular membrane and ROS generation will cause the disruption of cell membrane, including the DNA damage impairing the cell death.39
Figure 5.
Action of eugenol on S. aureus cells observed by SEM: (A) S. aureus control and (B) eugenol-treated S. aureus strains.
Summary and Conclusion
The MDR S. aureus is a cause of concern to the clinicians as well as the microbiologist, particularly the vancomycin-resistant S. aureus. Sensitivity profile of the bacteria is essential for the perfect choice of antimicrobial agents for appropriate empirical treatment. In the present study, it was confirmed that several S. aureus strains are already resistant to the latest drug vancomycin; eugenol effectively kills the vancomycin-resistant S. aureus strains via the production of ROS generation and membrane damage. So, we suggest the use of eugenol as the drug of choice for vancomycin-resistant S. aureus causing life-threatening infections. The phytochemical eugenol should be used as a reserve drug only in cases of vancomycin-resistant strains. More research is needed to understand the mechanism of action and increase the effectiveness of eugenol for the use of eugenol as antibiotics.
Acknowledgments
The authors express gratefulness to the USIC, Vidyasagar University, Midnapore and CRNN, University of Calcutta for providing the facilities to execute these studies.
Footnotes
ACADEMIC EDITOR: Douglas MacPherson, Editor in Chief
PEER REVIEW: Seven peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1696 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no external funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert single-blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Provenance: the authors were invited to submit this paper.
Author Contributions
Conceived and designed the experiments: SR and SKDey. Analyzed the data: BD and DM. Wrote the first draft of the manuscript: BD and SKDash. Contributed to the writing of the manuscript: SC, ST, DPD. Agree with Manuscript results and conclusions: BD, SKDash, SR and SKDey. Jointly developed the structure and arguments for the paper: BD, SKDash, SR and SKDey. Made critical revisions and approved final version: BD, SKDash, SR and SKDey. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Jarvis WR. Infection control and changing health care delivery systems. Emerg Infect Dis. 2001;7:170–173. doi: 10.3201/eid0702.010202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coltman K. Urinary tract infections: new thoughts on an old subject. Practitioner. 1981;223:351–355. [PubMed] [Google Scholar]
- 3.Bannerman TL. Staphylococcus, Micrococcus, and other catalasepositive cocci that grow aerobically. In: Murray PR, Baron EJ, Jorgensen JH, Pfaller MA, Yolken RH, editors. Manual of Clinical Microbiology. Vol. 8. Washington, DC: ASM Press; 2003. pp. 384–404. [Google Scholar]
- 4.Giacometti A, Cirion O, Schimizzi AM, et al. Epidemiology and microbiology of surgical wound infections. J Clin Microbiol. 2000;38:918–922. doi: 10.1128/jcm.38.2.918-922.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kirby WMM. Extraction of a highly potent penicillin inactivator from penicillin resistant staphylococci. Science. 1944;99:452–453. doi: 10.1126/science.99.2579.452. [DOI] [PubMed] [Google Scholar]
- 6.Neu HC. The crisis in antibiotic resistance. Science. 1992;257:1064–1073. doi: 10.1126/science.257.5073.1064. [DOI] [PubMed] [Google Scholar]
- 7.Rajaduraipandi K, Mani KR, Panneerselvam K, Mani M, Bhaskar M, Manikandan P. Prevalence and antimicrobial susceptibility pattern of methicillin resistant Staphylococcus aureus: a multicentre study. Indian J Med Microbiol. 2006;24(1):34–38. doi: 10.4103/0255-0857.19892. [DOI] [PubMed] [Google Scholar]
- 8.Hiramatsu K, Hanaki H, Ino T, Yabuta K, Oguri T, Tenover FC. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J Antimicrob Chemother. 1997;40:135–136. doi: 10.1093/jac/40.1.135. [DOI] [PubMed] [Google Scholar]
- 9.Sievert DM, Boulton ML, Stoltman G, et al. Staphylococcus aureus resistant to vancomycin—United States, 2002. MMWR Morb Mortal Wkly Rep. 2002;51:565–567. [PubMed] [Google Scholar]
- 10.Saha B, Singh AK, Ghosh A, Bal M. Identification and characterization of a vancomycin resistant Staphylococcus aureus isolated from Kolkata (South Asia) J Med Microbiol. 2008;57:72–79. doi: 10.1099/jmm.0.47144-0. [DOI] [PubMed] [Google Scholar]
- 11.Gill AO, Holley RA. Mechanisms of bactericidal action of cinnamaldehyde against Listeria monocytogenes and of eugenol against L. monocytogenes and Lactobacillus sakei. Appl Environ Microbiol. 2004;70:5750–5755. doi: 10.1128/AEM.70.10.5750-5755.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagababu E, Lakshmaiah N. Inhibition of xanthine oxidasexanthine-iron mediated lipid peroxidation by eugenol in liposomes. Mol Cell Biochem. 1997;166:65–71. doi: 10.1023/a:1006878315029. [DOI] [PubMed] [Google Scholar]
- 13.Mugalu JNM, Kiguli S, Kaddu-Mulindwa DH. Aetiology, risk factors and immediate outcome of bacteriologically confirmed neonatal septicaemia in Mulago Hospital, Uganda. Afr Health Sci. 2006;6:120–126. doi: 10.5555/afhs.2006.6.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martineau F, Picard FJ, Grenier L, Roy PH, Ouellette M, Bergeron MG. Multiplex PCR assays for the detection of clinically relevant antibiotic resistance genes in staphylococci isolated from patients infected after cardiac surgery. J Antimicrob Chemother. 2000;46:527–534. doi: 10.1093/jac/46.4.527. [DOI] [PubMed] [Google Scholar]
- 15.Palleroni NJ, Genus I. Pseudomonas Migula 1894. In: Krieg NR, Holt JG, editors. Bergey’s Manual of Systematic Bacteriology. Vol. 2. Baltimore, MD: Williams & Wilkins; 1984. pp. 141–199. [Google Scholar]
- 16.Dash SK, Chakraborty SP, Mandal D, Roy S. Isolation and characterization of multi drug resistant uropathogenic Escherichia coli from urine sample of Urinary tract infected patients. Int J Life Sci Pharma Res. 2012;2:25–39. [Google Scholar]
- 17.Doern VG. Evaluation of a commercial latex agglutination test for identification of Staphylococcus aureus. J Clin Microbiol. 1982;15:416–418. doi: 10.1128/jcm.15.3.416-418.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duguid JP. Staining methods. In: Collee JG, Fraser AG, Marmion BP, Simmons A, editors. Mackie & McCartney Practical Medical Microbiology. Vol. 14. Edinburgh: Churchill Livingstone; 1996. pp. 793–812. [Google Scholar]
- 19.Snell JJS, Brown DFJ, Roberts C, editors. Quality Assurance Principles and Practice in the Microbiology Laboratory. London: Public Health Laboratory Service; 1999. pp. 147–148. [Google Scholar]
- 20.MacFaddin JF, editor. Biochemical Tests for Identification of Medical Bacteria. Vol. 3. Philadelphia, PA: Lippincott Williams & Wilkins; 2000. pp. 451–453. [Google Scholar]
- 21.Baird-Parker AD, Hill LR, Kloos WE, et al. Identification of staphylococci. Int J Syst Bacteriol. 1976;26:333–334. [Google Scholar]
- 22.Essers L, Radebold K. Rapid and reliable identification of Staphylococcus aureus by a latex agglutination test. J Clin Microbiol. 1980;12:641–643. doi: 10.1128/jcm.12.5.641-643.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tittsler RP, Sandholzer LA. The use of semi-solid agar for the detection of bacterial motility. J Bacteriol. 1936;31:575–580. doi: 10.1128/jb.31.6.575-580.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lachica RVF, Genigeorgis C, Hoeprich PD. Metachromatic agar-diffusion methods for detecting staphylococcal nuclease activity. Appl Microbiol. 1971;21:585–587. doi: 10.1128/am.21.4.585-587.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baird D, Collee JG, Fraser AG, Marmion BP, Simmons A, editors. Mackie and McCartney Practical Medical Microbiology. Vol. 14. New York, NY: Churchill Livingstone; 1996. Staphylococcus: cluster forming gram positive cocci; pp. 245–261. [Google Scholar]
- 26.Bauer AW, Kirby WMM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45:493–496. [PubMed] [Google Scholar]
- 27.Acar JF, Lorian V, editors. Antibiotics in Laboratory Medicine. Baltimore, MD: Williams & Wilkins; 1980. The disc susceptibility test; pp. 24–25. [Google Scholar]
- 28.NCCLS . Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically. 5th ed. Vol. 17. Wayne, PA: NCCLS; 2000. Approved standard M7-A5. [Google Scholar]
- 29.Das B, Dash SK, Mandal D, et al. Green synthesized silver nanoparticles destroy multi drug resistant bacteria via reactive oxygen species mediated membrane damage. Arabian J Chemistry. 2015. http://dx.doi.org/10.1016/j.arabjc.2015.08.008.
- 30.May J, Shannon K, King A. Glycopeptide tolerance in Staphylococcus aureus. J Antimicrob Chemother. 2006;42:189–197. doi: 10.1093/jac/42.2.189. [DOI] [PubMed] [Google Scholar]
- 31.Chakraborty SP, KarMahapatra S, Bal M, Roy S. Isolation and identification of vancomycin resistant Staphylococcus aureus from post operative pus sample. Al Ameen J Med Sci. 2011;4:152–168. [Google Scholar]
- 32.Krumpernam PH. Multiple antibiotic resistance indexing Escherichia coli to identify risk sources of faecal contamination of foods. Appl Environ Microbiol. 1983;46:165–170. doi: 10.1128/aem.46.1.165-170.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paul S, Bezbarauh RL, Roy MK, Ghosh AC. Multiple antibiotic resistance (MAR) index and its reversion in Pseudomonas aeruginosa. Lett Appl Microbiol. 2002;24:169–171. doi: 10.1046/j.1472-765x.1997.00364.x. [DOI] [PubMed] [Google Scholar]
- 34.Zygadlo JA, Maestri DM, Lamarque AL, et al. Essential oil variability of Minthostachys verticillata. Biochem System Ecol. 1996;24:319–323. [Google Scholar]
- 35.Zygadlo JA, Juliáni HR. Bioactivity of essential oil components. Curr Topic Phytochem. 2000;3:204–214. [Google Scholar]
- 36.Qiu J, Feng H, Lu J, et al. Eugenol reduces the expression of virulence-related exoproteins in Staphylococcus aureus. Appl Environ Microbiol. 2010;76:5846–5851. doi: 10.1128/AEM.00704-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woods GL, Washington JA. The clinician and the microbiology laboratory. In: Mandell G, Bennett J, Dolin R, editors. Mandell, Douglas and Bennett’s Principles and Practice of Infectious Diseases. Philadelphia, PA: Churchill Livingstone; 1995. pp. 169–199. [Google Scholar]
- 38.Su H, Chou C, Hung D, et al. The disruption of bacterial membrane integrity through ROS generation induced by nanohybrids of silver and clay. Biomaterials. 2009;30:5979–5987. doi: 10.1016/j.biomaterials.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 39.Apolónio J, Faleiro ML, Miguel MG, Neto L. No induction of antimicrobial resistance in Staphylococcus aureus and Listeria monocytogenes during continuous exposure to eugenol and citral. FEMS Microbiol Lett. 2014;354:92–101. doi: 10.1111/1574-6968.12440. [DOI] [PubMed] [Google Scholar]