Abstract
Background
Because loss of cervical lordosis leads to disrupted biomechanics, the natural lordotic curvature is considered to be an ideal posture for the cervical spine. The vertebral arteries proceed in the transverse foramen of each cervical vertebra. Considering that the vertebral arteries travel in close anatomical relationship to the cervical spine, we speculated that the loss of cervical lordosis may affect vertebral artery hemodynamics. The aim of this study was to compare the vertebral artery values between subjects with and without loss of cervical lordosis.
Material/Methods
Thirty patients with loss of cervical lordosis and 30 controls matched for age, sex, and body mass index were included in the study. Sixty vertebral arteries in patients with loss of cervical lordosis and 60 in controls without loss of cervical lordosis were evaluated by Doppler ultrasonography. Vertebral artery hemodynamics, including lumen diameter, flow volume, peak systolic velocity, end-diastolic velocity, and resistive index, were measured, and determined values were statistically compared between the patient and the control groups.
Results
The means of diameter (p=0.003), flow volume (p=0.002), and peak systolic velocity (p=0.014) in patients were significantly lower as compared to controls. However, there was no significant difference between the 2 groups in terms of the end-diastolic velocity (p=0.276) and resistive index (p=0.536) parameters.
Conclusions
The present study revealed a significant association between loss of cervical lordosis and decreased vertebral artery hemodynamics, including diameter, flow volume, and peak systolic velocity. Further studies are required to confirm these findings and to investigate their possible clinical implications.
MeSH Keywords: Lordosis; Ultrasonography, Doppler; Vertebral Artery
Background
The natural curvature of the cervical spine is lordotic (Figure 1) [1]. This natural lordotic curvature of the cervical spine is considered to be an ideal posture in terms of biomechanical principles [2]. Loss of cervical lordosis (Figure 2) causes disrupted biomechanics, triggering a degenerative process in the cervical spine [1,2]. Therefore, unfavorable clinical outcomes can be expected in persons with this disorder. The loss of cervical lordosis is associated with neck, upper thoracic, and shoulder pain, tension and cervicogenic headaches, and poorer health-related quality of life outcomes [1,3,4]. However, the possible underlying pathophysiologic mechanisms responsible for these negative clinical outcomes remain obscure.
Figure 1.
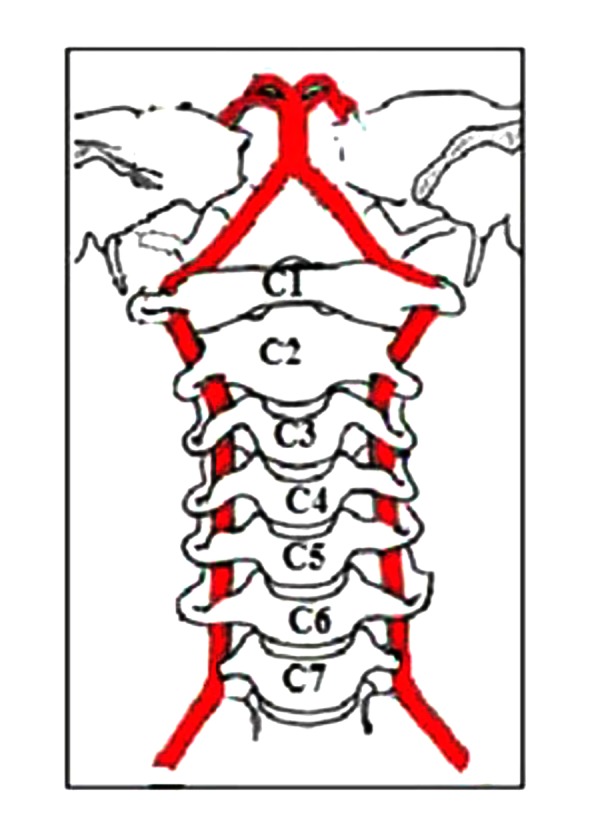
The course of the vertebral arteries through the transverse foramina on both sides of the vertebral bodies.
Figure 2.
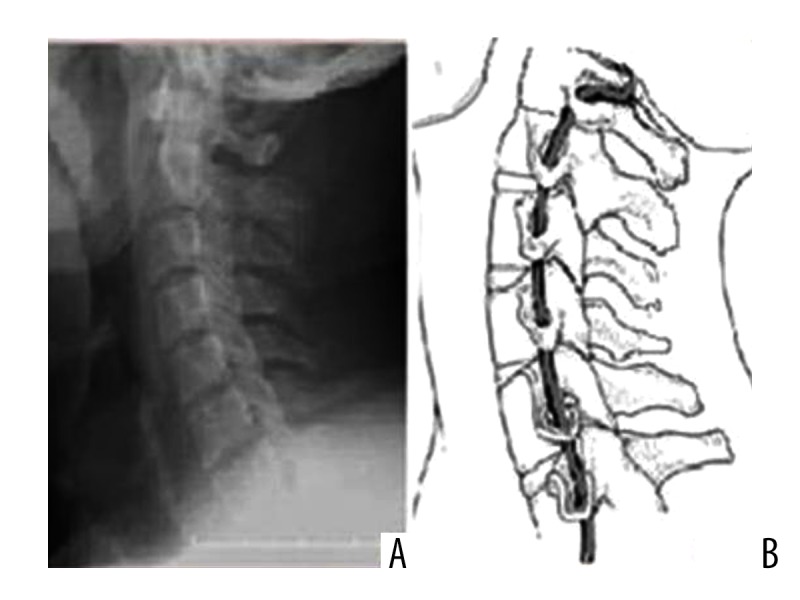
Normal cervical lordosis (A), and normal cervical course of the vertebral artery (B).
The vertebral arteries branch from the subclavian arteries, 1 on each side of the body, then proceed superiorly, in the transverse foramen of each cervical vertebra and merge to form the single midline basilar artery (Figures 1–3) [5]. Based on this close anatomical relationship between the vertebral arteries and the cervical spine, we hypothesized that loss of cervical lordosis may also affect vertebral artery hemodynamics. Although a few studies have investigated the effect of cervical spondylosis on vertebral artery flow [6,7], to the best of our knowledge, no previous study has focused on the status of vertebral artery parameters specifically in patients with loss of cervical lordosis.
Figure 3.
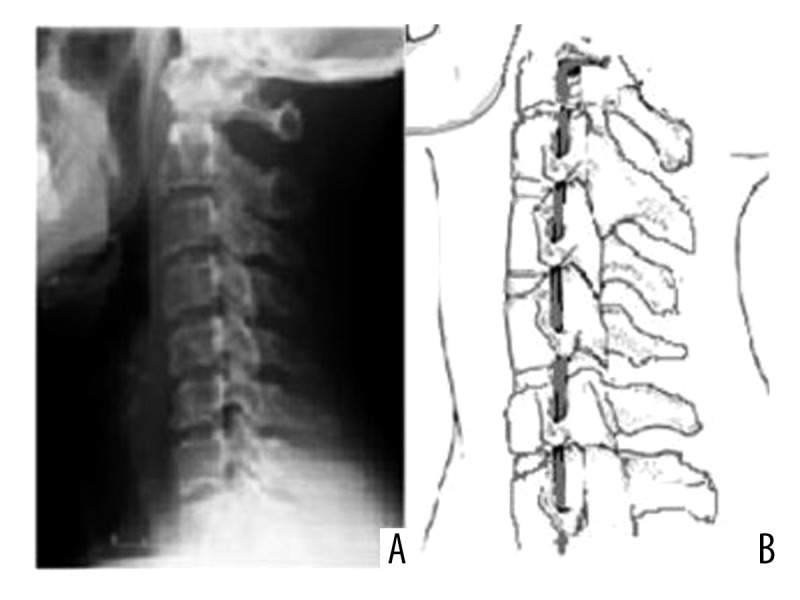
Loss of cervical lordosis (A), and stretched vertebral artery course (B).
Therefore, the purpose of this comparative case-control study was to evaluate the values of the vertebral arteries in patients with loss of cervical lordosis compared to strictly matched control subjects without loss of cervical lordosis.
Material and Methods
After receiving approval for this study from the local ethics committee and obtaining the participants’ informed consent, 30 patients with loss of cervical lordosis and 30 age-, sex-, and body mass index (BMI)-matched controls with normal cervical lordosis were recruited from the physical medicine and rehabilitation outpatient clinic between February 2015 and September 2015.
The cervical lordosis was assessed on lateral cervical radiographs according to the posterior tangent method: the lordosis was defined as the angle between the posterior margins of the vertebral bodies C2 and C7 [8]. It has been reported that this technique has a good inter- and intra-observer reliability, with a smaller standard error of measurement than the 4-line Cobb methods [9]. Exclusion criteria were presence of hypoplastic vertebral artery, obesity (BMI ≥30), psychiatric disorders (e.g., depression and anxiety), alcohol or cigarette use, age younger than 18 or older than 45 years, cervical spondylosis, cervical rib, block vertebra, cardiovascular disorders (e.g., valvular heart disease), acute or chronic infections, rheumatic diseases, hypo- or hyperthyroidism, or any other systemic disorders.
Radiological assessment
Doppler ultrasonographic examinations were done by the same staff radiologist (M.D.B.), who was blinded to status of cervical lordosis and has 7 years of experience with the Philips HD-11 ultrasound device (Bothell, Washington, USA), using the standard 7.5 MHz linear probe. Vertebral artery hemodynamics were measured in the supine position with the head rotated 45 degrees to the contralateral side after 15 min of rest. The routine measurements included careful examinations of the bilateral vertebral arteries in all participants; therefore, 120 arteries were examined (60 in patient group, 60 in control group). The evaluation parameters included lumen diameter (LD), peak systolic velocity (PSV), and end-diastolic velocity (EDV) and were measured at the C5–6 intertransverse segment of the vertebral artery. All the parameters except arterial lumen diameter were calculated automatically by the device’s software (Figure 4). All measurements were repeated 3 times and the mean values were calculated. In addition to these, the resistive index (RI) was calculated. The RI value is the ratio of the difference between the PSV and the EDV divided by PSV value: RI=(PSV–EDV)/PSV, and provides information about arterial impedance [10].
Figure 4.
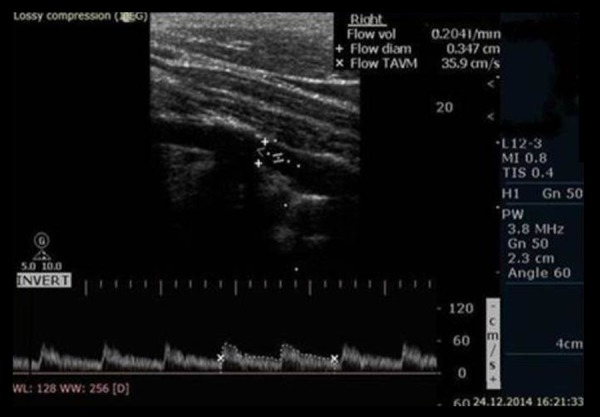
Vertebral artery spectral Doppler ultrasound waveforms in a 33-year-old woman with 204 ml/min flow volume.
Statistical methods
Statistical analyses were performed using the SPSS version 16.0 for Windows software program (SPSS Inc., Chicago, IL, USA). The one-sample Kolmogorov-Smirnov normality test was used to determine whether the data were normally distributed, and descriptive data are presented as mean ± standard deviation because the data were distributed normally. The Student’s t-test was used to compare the means of continuous variables between the 2 groups. A p value of less than 0.05 was considered to be statistically significant.
Results
Participants’ demographic characteristics are presented in Table 1. There were no significant differences between patients with loss of cervical lordosis and control subjects with respect to age, sex, or BMI (for all, p>0.05).
Table 1.
Group characteristics for age, sex, and BMI.
| Loss of cervical lordosis (n=30) | Healthy controls (n=30) | P value | |
|---|---|---|---|
| Age (years) | 31.83±8.53 | 29.67±7.97 | 0.314 |
| Gender (F/M) | 20/10 | 20/10 | |
| Weight (kg) | 66.20±8.53 | 64.03±6.53 | 0.274 |
| Height (m) | 1.67±0.06 | 1.65±0.04 | 0.172 |
| BMI (kg/m2) | 23.76±2.95 | 23.50±2.55 | 0.724 |
Data expressed as mean ± standard deviation and the number of cases; F/M – female/male; BMI – Body Mass Index.
The results of the comparison analysis are shown in Table 2. The means of all 5 parameters evaluated were lower in the patients as compared to the control group. There was a significant difference in LD (p=0.003), FV (p=0.002), and PSV (p=0.014), but no significant differences in EDV (p=0.276) and RI (p=0.536) values were observed between patients and controls.
Table 2.
Comparisons of the vertebral artery parameters between the 2 groups.
| VAs in patients (n=60) | VAs in controls (n=60) | P value | |
|---|---|---|---|
| LD (mm) | 3.25±0.51 | 3.51±0.41 | 0.003 |
| FV (ml/min) | 176±61.68 | 208±49.75 | 0.002 |
| PSV (cm/s) | 56.85±13.09 | 62.68±12.61 | 0.014 |
| EDV (cm/s) | 21.52±7.33 | 22.78±5.16 | 0.276 |
| RI | 0.62±0.09 | 0.63±0.08 | 0.536 |
Data expressed as mean ± standard deviation. VAs – vertebral arteries; LD – lumen diameter; FV – flow volume; PSV – peak systolic velocity; EDV – end-diastolic velocity; RI, (PSV-EDV)/PSV – resistive index.
Discussion
This comparative case-control study was performed to test the hypothesis that loss of cervical lordosis may be associated with vertebral artery hemodynamics. The results of the study revealed that vertebral artery parameters, including LD, FV, and PSV, were significantly different between groups, with patients with loss of cervical lordosis showing lower values in comparison to controls. However, the 2 groups were statistically similar in terms of EDV and RI values. To the best of our knowledge, there are no published data investigating the effect of loss of cervical lordosis on vertebral artery parameters. Our findings demonstrate preliminary evidence that loss of cervical lordosis may play a role in the development of changes related to vertebral artery hemodynamics.
As mentioned above, the normal cervical spine has a lordotic curve (Figure 1) [1,2]. Abnormalities of this natural curvature, such as loss of cervical lordosis or cervical kyphosis, are associated with pain, disability, and poor health-related quality of life [3,11,12]. These negative results related to deviations from the natural curvature may originate from disrupted biomechanics, because axial load is shifted from posterior to anterior column as lordosis is lost, and the increased compressive forces triggers and accelerates the degenerative process [1,2]. In addition to these bony structure changes in the cervical spine, when the natural cervical curvature is disturbed, the normal distribution of neck muscle loads is also disrupted and a larger workload is placed on the supportive soft tissues across cervical segments to maintain biomechanical integrity [2]. Moreover, since the vertebral arteries pass vertically upward through the foramina of the cervical transverse processes, when the cervical curve flattens, they are also in danger of being stretched or compressed (Figure 2) [13–15]. Therefore, a relationship between loss of cervical lordosis and vertebral artery hemodynamics is an expected and logical finding. However, the possible effects of loss of cervical lordosis on vertebral artery hemodynamics and their clinical outcomes are completely unknown. Because the vertebral arteries are the major source of blood supply to the cervical spinal cord and brain stem, the possible factors affecting these vessels warrant investigation.
Although the measurement of vertebral artery hemodynamics has not been investigated specifically in patients with loss of cervical lordosis in any studies performed to date, there are several studies on vertebral artery hemodynamic changes in patients with cervical spondylosis and in healthy subjects [6,7,16,17], showing the strong association of cervical spondylosis with decreased vertebral artery flow. Therefore, we restricted our sample to individuals aged 18–45 years, and we included the participants without cervical spondylosis, thereby aiming to eliminate the effects of cervical spondylosis on the vertebral arteries.
In contrast, Bayrak et al. [6] found no correlation between degeneration scores, cervical curve measurements, and Doppler values of vertebral arteries. However, they evaluated vertebral arteries in patients with the head in the neutral position. In our study, we evaluated vertebral arteries in patients and healthy controls with the head rotated 45 degrees to the contralateral side. Because the vertebral artery passes through the bony transverse foramina, it is vulnerable to stenosis with head rotation caused by stretching and compression [13–15]. Therefore, our results suggest that with the rotation of the flattened cervical spine, stretching and compression increases, causing marked luminal narrowing and reducing blood flow through the vertebral arteries.
While the clinical impact of loss of cervical lordosis is not well documented, some studies have revealed that this disorder is associated with cervicodorsal pain, headaches, and poor life quality [1,3,4]. However, pathophysiologic mechanisms underlying these negative clinical outcomes are unclear. Our results may be helpful in assessing underlying pathophysiological mechanisms, and are therefore critical for a better understanding of the potential clinical implications. In addition, investigating and identifying vascular and extraspinal changes in cervical deformity may be important considerations for preoperative surgical decision-making and postoperative outcomes. Further studies are needed to investigate or determine the clinical implications of this, including rates of transient ischemic attack/strokes, aneurysm rupture, and risks of vertebral artery injury in trauma. Moreover, a study on how manipulation of these narrowed vertebral arteries with reduced blood flow during deformity correction can further predispose to vascular injury/stroke would also be interesting.
One limitation of our study is that we did not assess the potential clinical outcomes of the study findings. Another limitation is that only young participants (age 18–45 years) were included in the study; therefore, our results cannot be generalized to older people.
Conclusions
The results of this study indicate that loss of cervical lordosis is associated with decreased vertebral artery values in LM, FV, and PSV. Further studies need to be done to confirm these observations and to elucidate their possible clinical implications.
Footnotes
Source of support: Departmental sources
Statements
Funding: No grants or funding to disclose.
Informed consent: Informed consent was obtained from all individual participants included in the study.
Ethics approval: This study was approved by the Ethics Committee of the Faculty of Medicine, Yüzüncü Yıl University School of Medicine.
All procedures performed in this study involving human participants were in accordance with the ethics standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethics standards.
Competing interests: We declare that we have no conflicts of interest.
References
- 1.Tan LA, Straus DC, Traynelis VC. Cervical interfacet spacers and maintenance of cervical lordosis. J Neurosurg Spine. 2015;22(5):466–69. doi: 10.3171/2014.10.SPINE14192. [DOI] [PubMed] [Google Scholar]
- 2.Ferrara LA. The biomechanics of cervical spondylosis. Adv Orthop. 2012;2012:493605. doi: 10.1155/2012/493605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McAviney J, Schulz D, Bock R, et al. Determining the relationship between cervical lordosis and neck complaints. J Manipulative Physiol Ther. 2005;28(3):187–93. doi: 10.1016/j.jmpt.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 4.Morningstar MW, Strauchman MN, Weeks DA. Spinal manipulation and anterior headweighting for the correction of forward head posture andcervical hypolordosis: A pilot study. J Chiropr Med. 2003;2(2):51–54. doi: 10.1016/S0899-3467(07)60042-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cacciola F, Phalke U, Goel A. Vertebral artery in relationship to C1–C2 vertebrae: an anatomical study. Neurol India. 2004;52(2):178–84. [PubMed] [Google Scholar]
- 6.Bayrak IK, Durmus D, Bayrak AO, et al. Effect of cervical spondylosis on vertebral arterial flow and its association with vertigo. Clin Rheumatol. 2009;28(1):59–64. doi: 10.1007/s10067-008-0983-0. [DOI] [PubMed] [Google Scholar]
- 7.Machaly SA, Senna MK, Sadek AG. Vertigo is associated with advanced degenerative changes in patients with cervical spondylosis. Clin Rheumatol. 2011;30(12):1527–34. doi: 10.1007/s10067-011-1770-x. [DOI] [PubMed] [Google Scholar]
- 8.Erkan S, Yercan HS, Okcu G, Ozalp RT. The influence of sagittal cervical profile, gender and age on the thoracic kyphosis. Acta Orthop Belg. 2010;76(5):675–80. [PubMed] [Google Scholar]
- 9.Harrison DE, Harrison DD, Cailliet R, et al. Cobb method or Harrison posterior tangent method: which to choose for lateral cervical radiographic analysis. Spine (Phila Pa 1976) 2000;25(16):2072–78. doi: 10.1097/00007632-200008150-00011. [DOI] [PubMed] [Google Scholar]
- 10.Pekkafalı MZ, Kara K. Doppler ultrasound measurements of renal functional reserve in healthy subjects. Med Ultrason. 2015;17(4):464–68. doi: 10.11152/mu.2013.2066.174.dop. [DOI] [PubMed] [Google Scholar]
- 11.Gum JL, Glassman SD, Douglas LR, Carreon LY. Correlation between cervical spine sagittal alignment and clinical outcome after anterior cervical discectomy and fusion. Am J Orthop. 2012;41(6):E81–84. [PubMed] [Google Scholar]
- 12.Scheer JK, Tang JA, Smith JS, et al. International Spine Study Group. Cervical spine alignment, sagittal deformity, and clinical implications: A review. J Neurosurg Spine. 2013;19(2):141–59. doi: 10.3171/2013.4.SPINE12838. [DOI] [PubMed] [Google Scholar]
- 13.Buchanan CC, McLaughlin N, Lu DC, Martin NA. Rotational vertebral artery occlusion secondary to adjacent-level degeneration following anterior cervical discectomy and fusion. J Neurosurg Spine. 2014;20(6):714–21. doi: 10.3171/2014.3.SPINE13452. [DOI] [PubMed] [Google Scholar]
- 14.Fleming JB, Vora TK, Harrigan MR. Rare case of bilateral vertebral artery stenosis caused by C4–5 spondylotic changes manifesting with bilateral bow hunter’s syndrome. World Neurosurg. 2013;79(5–6):799.E1–5. doi: 10.1016/j.wneu.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 15.Yamaoka Y, Ichikawa Y, Morita A. Evaluation of rotational vertebral artery occlusion using ultrasound facilitates the detection of arterial dissection in the atlas loop. J Neuroimaging. 2014;25(4):647–51. doi: 10.1111/jon.12174. [DOI] [PubMed] [Google Scholar]
- 16.Mitchell JA. Changes in vertebral artery blood flow following normal rotation of the cervical spine. J Manipulative Physiol Ther. 2003;26(6):347–51. doi: 10.1016/S0161-4754(03)00074-5. [DOI] [PubMed] [Google Scholar]
- 17.Olszewski J, Majak J, Pietkiewicz P, et al. The association between positional vertebral and basilar artery flow lesion and prevalence of vertigo in patients with cervical spondylosis. Otolaryngol Head Neck Surg. 2006;134(4):680–84. doi: 10.1016/j.otohns.2005.11.023. [DOI] [PubMed] [Google Scholar]


