Abstract
Background:
Water dropwort (Oenanthe javanica) as a popular traditional medicine in Asia shows various biological properties including antioxidant activity. In this study, we firstly examined the neuroprotective effect of Oenanthe javanica extract (OJE) in the hippocampal cornus ammonis 1 region (CA1 region) of the gerbil subjected to transient cerebral ischemia.
Methods:
Gerbils were established by the occlusion of common carotid arteries for 5 min. The neuroprotective effect of OJE was estimated by cresyl violet staining. In addition, 4 antioxidants (copper, zinc superoxide dismutase [SOD], manganese SOD, catalase, and glutathione peroxidase) immunoreactivities were investigated by immunohistochemistry.
Results:
Pyramidal neurons in the CA1 region showed neuronal death at 5 days postischemia; at this point in time, all antioxidants immunoreactivities disappeared in CA1 pyramidal neurons and showed in many nonpyramidal cells. Treatment with 200 mg/kg, not 100 mg/kg, OJE protected CA1 pyramidal neurons from ischemic damage. In addition, 200 mg/kg OJE treatment increased or maintained antioxidants immunoreactivities. Especially, among the antioxidants, glutathione peroxidase immunoreactivity was effectively increased in the CA1 pyramidal neurons of the OJE-treated sham-operated and ischemia-operated groups.
Conclusion:
Our present results indicate that treatment with OJE can protect neurons from transient ischemic damage and that the neuroprotective effect may be closely associated with increased or maintained intracellular antioxidant enzymes by OJE.
Keywords: Antioxidant Enzymes, Hippocampal Cornus Ammonis 1 Region, Neuroprotection, Oenanthe Javanica Extract, Transient Cerebral Ischemia
INTRODUCTION
Cerebral ischemia leads to extensive and irreversible neuronal damage in various brain regions including hippocampus.[1,2,3] Especially, the hippocampus, which is highly susceptible to ischemia, and pyramidal neurons in the hippocampal cornus ammonis 1 region (CA1 region) are most sensitive to ischemic damage.[4] The neuronal loss of pyramidal neurons in the CA1 region commonly represents 3–4 days after initial ischemic insult and thus this unique process is termed “delayed neuronal death”.[5,6] Mechanisms of neuronal death following cerebral ischemia are awfully complex; there have been many theories regarding the mechanisms including glutamate excitotoxicity, oxidative stress, and inflammation.[7,8,9] However, exact mechanism of ischemia-induced neuronal death has not been fully established yet.
Water dropwort (Oenanthe javanica [O. javanica]), a perennial member of the Oenanthe genus in Apiaceae family, is mainly cultivated in marshy areas of Asia and has been widely used in Korea as a very popular traditional medicine for the treatment of diseases including jaundice, hypertension, abdominal pain, fever, leucorrhea, mumps, and urinary infections.[10] Indeed, many researchers have shown that O. javanica has various pharmacological and biological activities such as anti-inflammatory,[11] antioxidant,[12] and anti-hepatitis B virus activities.[13] Furthermore, a recent study reported that persicarin isolated from O. javanica displayed neuroprotective activity against glutamate-induced neurotoxicity in primary cultured rat cortical cells.[14] However, no studies regarding effects of O. javanica against cerebral ischemic damage have been reported yet.
Therefore, in this study, we examined the neuroprotective effect of O. javanica extract (OJE) in the CA1 region of a gerbil model of 5 min transient cerebral ischemia and investigated the effect of OJE on ischemic CA1 region.
METHODS
Experimental animals
Six-month-old male Mongolian gerbils (Meriones unguiculatus) were obtained from the Experimental Animal Center, Kangwon National University, Chunchon, South Korea and housed according to the procedures for animal handling and care adhered to guidelines that are in compliance with the current international laws and policies (Guide for the Care and Use of Laboratory Animals, The National Academies Press, 8th ed., 2011), and they were approved by the Institutional Animal Care and Use Committee at Kangwon University.
Preparation and treatment of Oenanthe javanica extract
O. javanica were collected during 1-week in Kangwon Province (South Korea), in March 2013 and kept in a deep freezer (−70°C). O. javanica was extracted with 10 vol (v/w) of 70% ethanol at 70°C for 4 h, and extraction was repeated 3 times. The extracts were filtered through Whatman Filter Paper (No. 2), concentrated with a vacuum evaporator, and completely dried with a freeze-drier. The extraction yield was 14.5%.
To elucidate the neuroprotective effects of OJE against ischemic damage, the gerbils were divided into four groups: (1) Vehicle (saline)-treated sham-group (vehicle-sham-group), (2) vehicle-treated ischemia-group (vehicle-ischemia-group), (3) 100 mg/kg OJE-treated ischemia-group (100 mg/kg OJE-ischemia-group), (4) 200 mg/kg OJE-treated ischemia-group (200 mg/kg OJE-ischemia-group). OJE was dissolved in saline, and OJE or saline was orally administered once a day for 7 days before ischemic surgery: Last treatment was at 30 min before the surgery.
Induction of transient cerebral ischemia
Transient cerebral ischemia was developed following our previous method.[15,16] In brief, the animals were anesthetized with a mixture of 2.5% isoflurane (Baxter, Deerfield, IL, USA) in 33% oxygen and 67% nitrous oxide. Bilateral common carotid arteries were occluded for 5 min using nontraumatic aneurysm clips (Yasargil FE 723K, Aesculap, Tuttlingen, Germany). The complete interruption of blood flow was confirmed by observing the central artery in retinae using an ophthalmoscope (HEINE K180®, Heine Optotechnik, Herrsching, Germany). The body (rectal) temperature under free-regulating or normothermic (37 ± 0.5°C) conditions was monitored with a rectal temperature probe (TR-100; Fine Science Tools, Foster City, CA, USA) and maintained using a thermometric blanket before, during and after the surgery until the animals completely recovered from anesthesia. Thereafter, the animals were kept on the thermal incubator (Mirae Medical Industry, Seoul, South Korea) to maintain the body temperature of animals until the animals were euthanized. Sham-operated animals were subjected to the same surgical procedures with no occlusion.
Cresyl violet staining
According to our previous method,[17] cresyl violet (CV) staining was carried out. In brief, the vehicle-sham-, vehicle-ischemia-, OJE-sham-, and OJE-ischemia-groups (n = 7 at each point in time) were anesthetized with sodium pentobarbital at the designated times (2 days and 5 days after ischemia-reperfusion) and perfused transcardially with 4% paraformaldehyde in 0.1 M phosphate-buffer (pH 7.4). The brain tissues were serially sectioned on a cryostat (Leica, Wetzlar, Germany) into 30-μm coronal sections. The sections were stained with 1% CV acetate (Sigma, St. Louis, MO, USA) solution. After dehydration, the sections were mounted with Canada Balsam (Kanto, Tokyo, Japan).
Immunohistochemistry for antioxidant enzymes
Immunohistochemistry was done according to our previous method[18] with sheep anti-copper, zinc superoxide dismutase (SOD1, 1:1000, Calbiochem, Darmstadt, Germany), sheep anti-manganese (SOD2, 1:1000, Calbiochem), rabbit anti-catalase (CAT, 1:800, Calbiochem), and mouse anti-glutathione peroxidase (Gpx, 1:800, Calbiochem). In brief, the sections were incubated with primary antibodies overnight at 4°C and exposed to biotinylated goat anti-sheep IgG, donkey anti-rabbit IgG, goat anti-mouse IgG, and streptavidin peroxidase complex (Vector, Burlingame, CA, USA). And they were visualized with 3,3’-diaminobenzidine in 0.1 M Tris HCL buffer.
SOD1, SOD2, CAT, and Gpx immunoreactivity was measured from 8 sections per animal as follows. Images of SOD1-, SOD2-, CAT-, and Gpx-immunoreactive structures were taken from each layer through an AxioM1 light microscope (Carl Zeiss, Oberkochen, Germany) equipped with a digital camera (Axiocam, Carl Zeiss, Oberkochen, Germany) connected to a PC monitor (Samsung, Korea). Semi-quantification of their immunoreactivity was evaluated with digital image analysis software (MetaMorph 4.01; Universal Imaging Corporation, Downingtown, PA, USA). The mean immunoreactivity was measured by a 0–255 gray scale system (white to dark signal corresponded from 255 to 0). Based on this approach, the level of immunoreactivity was scaled as −, ±, +, ++ or +++ representing negative (gray scale value: ≥200), weakly positive (gray scale value: 150–199), moderate (gray scale value: 100–149), strong (gray scale value: 50–99), or very strong (gray scale value: ≤49), respectively.
RESULTS
Cresyl violet-positive cells
CV-positive ells were well observed in all hippocampal subregions in the vehicle-sham-group [Figure 1A and 1a]. However, in the vehicle-ischemia-group, CV-positive pyramidal cells were hardly found in the stratum pyramidale of the CA1 region 5 days after ischemia-reperfusion [Figure 1B and 1b].
Figure 1.
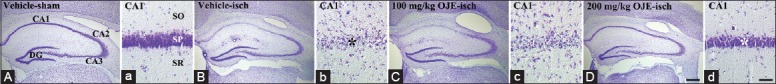
Cresyl violet staining in the hippocampus of the vehicle-sham- (A and a), vehicle-ischemia- (B and b), 100 mg/kg Oenanthe javanica extract-ischemia- (C and c) and 200 mg/kg Oenanthe javanica extract-ischemia- (D and d) groups 5 days after postischemia. In the vehicle-ischemia-group, cresyl violet-positive pyramidal cells are hardly observed in the stratum pyramidale (stratum pyramidale, black asterisk) of the hippocampal CA1 region. Abundant pyramidal cells (white asterisk) are stained with cresyl violet in the stratum pyramidale of the 200 mg/kg Oenanthe javanica extract-ischemia-group. CA: Cornus ammonis; DG: Dentate gyrus; SO: Stratum oriens; SR: Stratum radiatum. Scale bar = 400 μm (A, B, C and D) and 60 μm (a, b, c and d).
In the 100 mg/kg OJE-ischemia-group, the distribution pattern of CV-positive cells was similar to that in the vehicle-ischemia-group [Figure 1C and 1c]. However, in the 200 mg/kg OJE-ischemia-group, abundant CV-positive pyramidal cells were observed in the stratum pyramidale of the CA1 region 5 days after ischemia-reperfusion [Figure 1D and 1d].
Superoxide dismutase 1 and 2 immunoreactivity
In this study, change patterns of SOD1 and 2 were generally similar. Moderate SOD1 and 2 immunoreactivity was observed in the stratum pyramidale of the CA1 region of the vehicle-sham-group [Figure 2a and 2g]. In the vehicle-ischemia-groups, SOD1 and 2 immunoreactivity was not significantly changed in the striatum pyramidale at 2 days postischemia [Table 1 and Figure 2c and 2i]; however, at 5 days postischemia, SOD1 and 2 immunoreactivity was significantly decreased in the striatum pyramidale and found in many nonpyramidal cells [Table 1 and Figure 2e and 2k].
Figure 2.
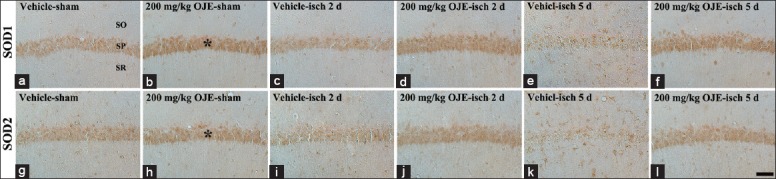
Superoxide dismutase 1 and 2 immunohistochemistry in the hippocampal cornus ammonis 1 region of the vehicle-sham- (a and g), vehicle-ischemia- (c, e, i and k), 200 mg/kg Oenanthe javanica extract-sham- (b and h), and 200 mg/kg Oenanthe javanica extract-ischemia- (d, f, j and l) groups. In the vehicle-ischemia-group, superoxide dismutase 1 and 2 immunoreactivity is significantly decreased in the stratum pyramidale 5 days postischemia. In the 200 mg/kg Oenanthe javanica extract-sham-group, superoxide dismutase 1 and 2 immunoreactivity in the stratum pyramidale (asterisks) is higher than that in the vehicle-sham-group, and the immunoreactivity is maintained. SOD: Superoxide dismutase; SO: Stratum oriens; SR: Stratum radiatum. Scale bar = 60 μm.
Table 1.
Semi-quantification of immunoreactivities of antioxidant enzymes in the hippocampal CA1 region of the vehicle-ischemia- and OJE-ischemia-group
| Antibodies | Group | Category | Time after ischemia/reperfusion | ||
|---|---|---|---|---|---|
| Sham | 2 days | 5 days | |||
| SOD1 | Vehicle | CSP | + | + | ± |
| CSOR | ± | ± | + | ||
| OJE | CSP | ++ | ++ | ++ | |
| CSOR | + | + | + | ||
| SOD2 | Vehicle | CSP | + | + | ± |
| CSOR | ± | ± | + | ||
| OJE | CSP | ++ | ++ | ++ | |
| CSOR | + | + | + | ||
| CAT | Vehicle | CSP | ++ | ++ | ± |
| CSOR | ± | ± | ++ | ||
| OJE | CSP | ++ | ++ | ++ | |
| CSOR | ± | ± | ± | ||
| Gpx | Vehicle | CSP | ++ | + | − |
| CSOR | + | + | ++ | ||
| OJE | CSP | +++ | +++ | +++ | |
| CSOR | + | + | + | ||
The levels of immunoreactivity were defined as five grades, negative (−), weakly positive (±), moderate (+), strong (++) and very strong (+++). CSP: Cells in stratum pyramidale; CSOR: Cells in stratum oriens and radiatum; SOD: Superoxide dismutase; CAT: Catalase; Gpx: Glutathione peroxidase; OJE: Oenanthe javanica extract; CA1: Cornus ammonis 1.
In the 200 mg/kg OJE-sham-group, SOD1 and 2 immunoreactivity in the stratum pyramidale was higher than that in the vehicle-sham-group [Table 1 and Figure 2b and 2h], and, in the 200 mg/kg OJE-ischemia-groups, the immunoreactivity in the stratum pyramidale was maintained until 5 days after ischemia-reperfusion [Table 1 and Figure 2d, 2f, 2j and 2l].
Catalases immunoreactivity
Strong CAT immunoreactivity was detected in the stratum pyramidale of the CA1 region of the vehicle-sham-group [Figure 3a]. In the vehicle-ischemia-groups, CAT immunoreactivity was slightly decreased in the stratum pyramidale at 2 days postischemia [Table 1 and Figure 3c]. At 5 days postischemia, CAT immunoreactivity in the stratum pyramidale was significantly decreased, and many nonpyramidal cells showed CAT immunoreactivity [Table 1 and Figure 3e].
Figure 3.
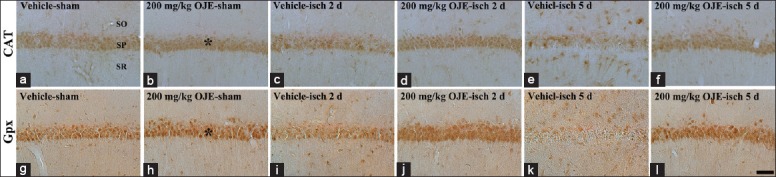
Catalase and glutathione peroxidase immunohistochemistry in the hippocampal cornus ammonis 1 region of the vehicle-sham- (a and g), vehicle-ischemia- (c, e, i and k), 200 mg/kg Oenanthe javanica extract-sham- (b and h), and 200 mg/kg Oenanthe javanica extract-ischemia- (d, f, j and l) groups. Catalase and glutathione peroxidase immunoreactivity is markedly decreased in the stratum pyramidale of the vehicle-ischemia-group 5 days after postischemia; however, in the 200 mg/kg Oenanthe javanica extract-sham- and ischemia-groups, catalase and glutathione peroxidase immunoreactivity in the stratum pyramidale (asterisks) is similar and higher compared with that in the vehicle-sham-group, respectively. CAT: Catalase; Gpx: Glutathione peroxidase; SO: Stratum oriens; SR: Stratum radiatum. Scale bar = 60 μm.
In the 200 mg/kg OJE-sham- and ischemia-groups, CAT immunoreactivity in the stratum pyramidale of the CA1 region was similar to that in the vehicle-sham-group [Table 1 and Figure 3b, 3d and 3f].
Glutathione peroxidase immunoreactivity
In the vehicle-sham-group, strong Gpx immunoreactivity was detected in the stratum pyramidale of the CA1 region [Figure 3g]. Gpx immunoreactivity was decreased in the vehicle-ischemia-groups and, 5 days after ischemia-reperfusion, Gpx immunoreactivity was dramatically decreased in the striatum pyramidale, and its immunoreactivity was shown in many nonpyramidal cells [Table 1 and Figure 3i and 3k].
In the 200 mg/kg OJE-sham-group, Gpx immunoreactivity was very strong in the stratum pyramidale [Table 1 and Figure 3h]. In the stratum pyramidale of the 200 mg/kg OJE-ischemia-groups, very strong Gpx immunoreactivity was sustained after ischemia-reperfusion [Table 1 and Figure 3j and 3l].
DISCUSSION
Many studies have reported that traditional medicinal plant extracts show therapeutic effects against cerebral ischemic insults.[19,20,21] However, the neuroprotective effect of OJE against cerebral ischemic insults has not been studied yet, even though O. javanica has multiple biological activities.
In this study, we firstly examined the neuroprotective effect of OJE against ischemic damage in the gerbil hippocampus induced by 5-min transient cerebral ischemia using CV staining and found that OJE protected the pyramidal neurons of the CA1 region from cerebral ischemic damage. This result is related with a previous study that showed that persicarin isolated from O. javanica protected cultured rat cortical cells from glutamate-induced neurotoxicity.[14] To the best of our knowledge, this study is the first report that showed the neuroprotective effects of OJE in an animal model of cerebral ischemia.
It has been well accepted that cerebral ischemia induces oxidative stress caused by the excessive generation of reactive oxygen species (ROS) and that amassed ROS results in the adverse modification of cellular components such as DNA, protein and lipids, which can impair cellular function and cause neuronal death.[22,23] To counteract oxidative stress, the intracellular antioxidant enzyme system, including SODs, CAT, and Gpx, converts ROS into less noxious compounds.[24,25,26] Extensive experiments have shown that antioxidants have protective potential against ischemic damage.[27,28,29,30]
In this study, to elucidate the neuroprotective mechanism of OJE against ischemia-induced neuronal damage, we examined changes in SODs, CAT, and Gpx in the ischemic CA1 region and found that SODs, CAT, and Gpx immunoreactivities in the OJE-sham-group were significantly higher than those in the vehicle-sham-group. This finding is similar with our recent study that reported that treatment with OJE significantly increased expressions of intracellular antioxidant enzymes in the normal rat kidney.[31] In addition, we found that SODs, CAT, and Gpx immunoreactivities in the OJE-treated-groups were continuously maintained after ischemia-reperfusion, although their immunoreactivities in the vehicle-ischemia-group were significantly decreased 5 days after ischemia-reperfusion.
Recently, it was reported that OJE had protective effect against H2O2 -induced oxidative damage through antioxidant activity in HePG2 cells.[32] In addition, we demonstrated that the maintenance or increase of intracellular antioxidant enzymes by the administration of some traditional medicinal plant extracts is closely associated with protective effects against ischemia-induced neuronal death in the gerbil hippocampal CA1 region.[17,33,34] Therefore, it could be postulated that higher and maintained expressions of intracellular antioxidant enzymes in ischemic neurons after treatment with some extracts may be related with neuroprotective effects against ischemic damage.
In brief, our results show, for the first time, that treatment with OJE protected CA1 pyramidal neurons from ischemic damage induced by transient cerebral ischemia and suggest that the neuroprotection of OJE is related with increased or maintained intracellular antioxidant enzymes by OJE.
Financial support and sponsorship
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (NO2011-0022812), and by the Bio-Synergy Research Project (NRF-2014M3A9C4066454) of the Ministry of Science, ICT, and Future Planning through the National Research Foundation.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yi Cui
REFERENCES
- 1.Globus MY, Busto R, Martinez E, Valdés I, Dietrich WD, Ginsberg MD. Comparative effect of transient global ischemia on extracellular levels of glutamate, glycine, and gamma-aminobutyric acid in vulnerable and nonvulnerable brain regions in the rat. J Neurochem. 1991;57:470–8. doi: 10.1111/j.1471-4159.1991.tb03775.x. [DOI] [PubMed] [Google Scholar]
- 2.Lin CS, Polsky K, Nadler JV, Crain BJ. Selective neocortical and thalamic cell death in the gerbil after transient ischemia. Neuroscience. 1990;35:289–99. doi: 10.1016/0306-4522(90)90083-g. [DOI] [PubMed] [Google Scholar]
- 3.Park CW, Lee JC, Ahn JH, Lee DH, Cho GS, Yan BC, et al. Neuronal damage using fluoro-Jade B histofluorescence and gliosis in the gerbil septum submitted to various durations of cerebral ischemia. Cell Mol Neurobiol. 2013;33:991–1001. doi: 10.1007/s10571-013-9967-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmidt-Kastner R, Freund TF. Selective vulnerability of the hippocampus in brain ischemia. Neuroscience. 1991;40:599–636. doi: 10.1016/0306-4522(91)90001-5. [DOI] [PubMed] [Google Scholar]
- 5.Kirino T. Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res. 1982;239:57–69. doi: 10.1016/0006-8993(82)90833-2. [DOI] [PubMed] [Google Scholar]
- 6.Pulsinelli WA, Brierley JB, Plum F. Temporal profile of neuronal damage in a model of transient forebrain ischemia. Ann Neurol. 1982;11:491–8. doi: 10.1002/ana.410110509. [DOI] [PubMed] [Google Scholar]
- 7.Benakis C, Bonny C, Hirt L. JNK inhibition and inflammation after cerebral ischemia. Brain Behav Immun. 2010;24:800–11. doi: 10.1016/j.bbi.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Lai TW, Zhang S, Wang YT. Excitotoxicity and stroke: Identifying novel targets for neuroprotection. Prog Neurobiol. 2014;115:157–88. doi: 10.1016/j.pneurobio.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Saito A, Maier CM, Narasimhan P, Nishi T, Song YS, Yu F, et al. Oxidative stress and neuronal death/survival signaling in cerebral ischemia. Mol Neurobiol. 2005;31:105–16. doi: 10.1385/MN:31:1-3:105. [DOI] [PubMed] [Google Scholar]
- 10.Park JC, Yu YB, Lee JH. Isolation of steroids and flavonoids from the herb of Oenanthe javanica DC. Korean J Pharmacogn. 1993;24:244–6. [Google Scholar]
- 11.Kim TH, Ku SK, Bae JS. Persicarin is anti-inflammatory mediator against HMGB1-induced inflammatory responses in HUVECs and in CLP-induced sepsis mice. J Cell Physiol. 2013;228:696–703. doi: 10.1002/jcp.24214. [DOI] [PubMed] [Google Scholar]
- 12.Kwon D, Yoon S, Carter O, Bailey GS, Dashwood RH. Antioxidant and antigenotoxic activities of Angelica keiskei, Oenanthe javanica and Brassica oleracea in the Salmonella mutagenicity assay and in HCT116 human colon cancer cells. Biofactors. 2006;26:231–44. doi: 10.1002/biof.5520260402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han YQ, Huang ZM, Yang XB, Liu HZ, Wu GX. In vivo and in vitro anti-hepatitis B virus activity of total phenolics from Oenanthe javanica. J Ethnopharmacol. 2008;118:148–53. doi: 10.1016/j.jep.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 14.Ma CJ, Lee KY, Jeong EJ, Kim SH, Park J, Choi YH, et al. Persicarin from water dropwort (Oenanthe javanica) protects primary cultured rat cortical cells from glutamate-induced neurotoxicity. Phytother Res. 2010;24:913–8. doi: 10.1002/ptr.3065. [DOI] [PubMed] [Google Scholar]
- 15.Lee CH, Park JH, Yoo KY, Choi JH, Hwang IK, Ryu PD, et al. Pre- and post-treatments with escitalopram protect against experimental ischemic neuronal damage via regulation of BDNF expression and oxidative stress. Exp Neurol. 2011;229:450–9. doi: 10.1016/j.expneurol.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 16.Park JH, Park Ok, Cho JH, Chen BH, Kim IH, Ahn JH, et al. Anti-inflammatory effect of tanshinone I in neuroprotection against cerebral ischemia-reperfusion injury in the gerbil hippocampus. Neurochem Res. 2014;39:1300–12. doi: 10.1007/s11064-014-1312-4. [DOI] [PubMed] [Google Scholar]
- 17.Park JH, Joo HS, Yoo KY, Shin BN, Kim IH, Lee CH, et al. Extract from Terminalia chebula seeds protect against experimental ischemic neuronal damage via maintaining SODs and BDNF levels. Neurochem Res. 2011;36:2043–50. doi: 10.1007/s11064-011-0528-9. [DOI] [PubMed] [Google Scholar]
- 18.Yan BC, Park JH, Lee CH, Yoo KY, Choi JH, Lee YJ, et al. Increases of antioxidants are related to more delayed neuronal death in the hippocampal CA1 region of the young gerbil induced by transient cerebral ischemia. Brain Res. 2011;1425:142–54. doi: 10.1016/j.brainres.2011.09.063. [DOI] [PubMed] [Google Scholar]
- 19.Safwen K, Selima S, Mohamed E, Ferid L, Pascal C, Mohamed A, et al. Protective effect of grape seed and skin extract on cerebral ischemia in rat: Implication of transition metals. Int J Stroke. 2015;10:415–24. doi: 10.1111/ijs.12391. [DOI] [PubMed] [Google Scholar]
- 20.Saleem S, Zhuang H, Biswal S, Christen Y, Doré S. Ginkgo biloba extract neuroprotective action is dependent on heme oxygenase 1 in ischemic reperfusion brain injury. Stroke. 2008;39:3389–96. doi: 10.1161/STROKEAHA.108.523480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoo KY, Hwang IK, Kim JD, Kang IJ, Park J, Yi JS, et al. Antiinflammatory effect of the ethanol extract of Berberis koreana in a gerbil model of cerebral ischemia/reperfusion. Phytother Res. 2008;22:1527–32. doi: 10.1002/ptr.2527. [DOI] [PubMed] [Google Scholar]
- 22.Lee JC, Kim IH, Park JH, Ahn JH, Cho JH, Cho GS, et al. Ischemic preconditioning protects hippocampal pyramidal neurons from transient ischemic injury via the attenuation of oxidative damage through upregulating heme oxygenase-1. Free Radic Biol Med. 2015;79:78–90. doi: 10.1016/j.freeradbiomed.2014.11.022. [DOI] [PubMed] [Google Scholar]
- 23.Sugawara T, Chan PH. Reactive oxygen radicals and pathogenesis of neuronal death after cerebral ischemia. Antioxid Redox Signal. 2003;5:597–607. doi: 10.1089/152308603770310266. [DOI] [PubMed] [Google Scholar]
- 24.Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab. 2001;21:2–14. doi: 10.1097/00004647-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Crack PJ, Taylor JM, Flentjar NJ, de Haan J, Hertzog P, Iannello RC, et al. Increased infarct size and exacerbated apoptosis in the glutathione peroxidase-1 (Gpx-1) knockout mouse brain in response to ischemia/reperfusion injury. J Neurochem. 2001;78:1389–99. doi: 10.1046/j.1471-4159.2001.00535.x. [DOI] [PubMed] [Google Scholar]
- 26.Hall ED, Braughler JM. Central nervous system trauma and stroke. II. Physiological and pharmacological evidence for involvement of oxygen radicals and lipid peroxidation. Free Radic Biol Med. 1989;6:303–13. doi: 10.1016/0891-5849(89)90057-9. [DOI] [PubMed] [Google Scholar]
- 27.Aras AB, Guven M, Akman T, Ozkan A, Sen HM, Duz U, et al. Neuroprotective effects of daidzein on focal cerebral ischemia injury in rats. Neural Regen Res. 2015;10:146–52. doi: 10.4103/1673-5374.150724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujimura M, Tominaga T, Chan PH. Neuroprotective effect of an antioxidant in ischemic brain injury: Involvement of neuronal apoptosis. Neurocrit Care. 2005;2:59–66. doi: 10.1385/NCC:2:1:059. [DOI] [PubMed] [Google Scholar]
- 29.Wu JX, Zhang LY, Chen YL, Yu SS, Zhao Y, Zhao J. Curcumin pretreatment and post-treatment both improve the antioxidative ability of neurons with oxygen-glucose deprivation. Neural Regen Res. 2015;10:481–9. doi: 10.4103/1673-5374.153700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang H, Zhang JJ, Mei YW, Sun SG, Tong ET. Effects of immediate and delayed mild hypothermia on endogenous antioxidant enzymes and energy metabolites following global cerebral ischemia. Chin Med J. 2011;124:2764–6. [PubMed] [Google Scholar]
- 31.Tae HJ, Park JH, Cho JH, Kim IH, Ahn JH, Lee JC, et al. Oenanthe javanica extract increases immunoreactivities of antioxidant enzymes in the rat kidney. Chin Med J. 2014;127:3758–63. [PubMed] [Google Scholar]
- 32.Choi H, You Y, Hwang K, Lee J, Chun J, Chung JW, et al. Isolation and identification of compound from dropwort (Oenanthe javanica) with protective potential against oxidative stress in HepG2 cells. Food Sci Biotechnol. 2011;20:1743–6. [Google Scholar]
- 33.Li H, Park JH, Lee JC, Yoo KY, Hwang IK, Lee CH, et al. Neuroprotective effects of Alpinia katsumadai against experimental ischemic damage via control of oxidative stress. Pharm Biol. 2013;51:197–205. doi: 10.3109/13880209.2012.716853. [DOI] [PubMed] [Google Scholar]
- 34.Yoo KY, Li H, Hwang IK, Choi JH, Lee CH, Kwon DY, et al. Zizyphus attenuates ischemic damage in the gerbil hippocampus via its antioxidant effect. J Med Food. 2010;13:557–63. doi: 10.1089/jmf.2009.1254. [DOI] [PubMed] [Google Scholar]


