Chronic obstructive pulmonary disease (COPD) is a leading cause of morbidity and mortality worldwide and results in an economic and social burden that is both substantial and increasing. By the year 2020, COPD will be the third leading cause of mortality and the fifth leading cause of disability worldwide.[1] In a population based study conducted at multiple international sites, approximately 10% of participants 40 years of age or older were found to have airflow obstruction of at least moderate severity according to spirometric criteria.[2] In China, the overall prevalence of COPD in individuals 40 years of age or older was 8.2%.[3] COPD is a slowly progressive respiratory disease, which, although preventable and treatable, is not curable. The final years for patients with advanced COPD are characterized by progressive functional decline, frequent exacerbations, poor quality of life, increasing dependency on informal caregivers and on the health care system.[4] According to the literature, 5-year survival from diagnosis is estimated to be 78% in men and 72% in women with mild disease, but only 30% in men and 24% in women with advanced COPD.[5]
Our current standard clinical management to COPD mainly focuses on the underlying pathophysiology of disease (treating bronchoconstriction, reducing hyperinflation and airway inflammation) within which patients receive episodic care aimed at treating and preventing acute exacerbations. The optimal management of symptoms in patients with advanced COPD or end-stage COPD is an often neglected aspect under this disease management model. While the reality is that for patients who have advanced COPD the symptom burden is substantial, that maximal traditional therapy for advanced COPD produces only modest relief of symptoms, leaving these patients with significantly reduced health-related quality of life.[6,7,8] Dyspnea as the predominant symptom is often poorly controlled and ultimately incapacitating. High quality symptom-focused interventional strategies and palliative care services in our current models of care are less accessible than they are for people with cancer.[6,9,10]
The present review will focus on the patient population with advanced COPD or end-stage COPD, who have been paid less attention by the physicians and investigators previously, and summarize the literature to find innovative and integrated management approaches for this population.
DEFINITION OF ADVANCED CHRONIC OBSTRUCTIVE PULMONARY DISEASE
Generally, advanced COPD refers to the later stage of the condition, in which the symptoms are poorly responsive to treatment and continually worsen over time. The US National Hospice and Palliative Care Organization proposed a very specific definition of end-stage pulmonary disease, with the aim of identifying patients with advanced lung disease eligible for hospice care[11,12] [Table 1]. But the aims are not COPD specific and arbitrarily define the last 6 months of life as end-stage disease, a rather short period. Klimathianaki et al.[13] and Viegi et al.[14] defined the end-stage COPD based on clinical features, such as very severe airflow limitation, severely limited and declining performance status, advanced age, presence of multiple comorbidities, and severe systemic manifestations/complications of COPD [Table 1]. Based on a combined and comprehensive assessment strategy, the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guideline classified the COPD patients into four groups (A, B, C, and D), with the patients in D group the most severe and advanced state of illness [Table 1].
Table 1.
Definitions of advanced COPD
| Items | Criteria |
|---|---|
| Definition of end-stage pulmonary disease (life expectancy of 6 months or less) by the US National Hospice and Palliative Care Organization | |
| Severe chronic lung disease | |
| Disabling dyspnea | Dyspnea at rest, poorly or unresponsive to bronchodilators, resulting in decreased functional capacity (e.g., bed-to-chair existence), fatigue, and cough. Objective evidence: FEV1 < 30% pred after bronchodilator (not necessary to obtain) |
| Disease progression | Increasing visits to the emergency department or hospitalizations for pulmonary infections and/or respiratory failure or increasing clinician home visits before initial certification. Objective evidence: serial decrease of FEV1 > 40 ml/year (not necessary to obtain) |
| Hypoxemia at rest on room air | PO2 ≤55 mmHg or oxygen saturation ≤88% on supplemental oxygen determined either by arterial blood gases or oxygen saturation monitors or hypercapnia, as evidenced by PCO2 ≥50 mmHg |
| Right heart failure | Secondary to pulmonary disease (cor pulmonale) (e.g., not secondary to left heart disease or valvulopathy) |
| Unintentional progressive weight loss | >10% of body weight over the preceding 6 months |
| Resting tachycardia | >100/min |
| Definition of end-stage COPD by clinical features | |
| Airflow limitation | Very severe (FEV1 <30% pred) |
| Performance status | Severely limited and declining |
| Other criteria (at least one) | Advanced age; Presence of multiple comorbidities; Severe systemic manifestations/complications of COPD (e.g. chronic respiratory failure, body composition alterations, peripheral muscle dysfunction, respiratory muscle dysfunction, osteoporosis, pulmonary hypertension, cardiac impairment, fluid retention/edema) |
| Group D patients defined by GOLD | |
| Characteristic | High risk, more symptoms (compared with group A, B and C patients) |
| Spirometric classification | GOLD 3 or 4 (Severe or Very Severe airflow limitation) |
| Exacerbations per year | ≥2 |
| mMRC or CAT | mMRC grade ≥ 2 or CAT score ≥ 10 |
FEV1: Forced expiratory volume in 1 s; COPD: Chronic obstructive pulmonary disease; GOLD: Global Initiative for Chronic Obstructive Lung Disease; mMRC: Modified British Medical Research Council questionnaire; CAT: Chronic Obstructive Pulmonary Disease Assessment Test.
SYMPTOM BURDEN OF ADVANCED CHRONIC OBSTRUCTIVE PULMONARY DISEASE
Dyspnea: The predominant symptom in advanced chronic obstructive pulmonary disease
For patients with COPD, dyspnea is the most prevalent and distressing symptom, and its severity and magnitude increases as the disease progresses, leading to significant disability and a negative effect on the quality of life. Refractory dyspnea is a common and difficult symptom to treat in patients with advanced COPD. In a comparison of symptoms experienced by patients with COPD and lung cancer, patients with cancer reported higher levels of pain, but dyspnea was more frequently reported in the patients with COPD,[15,16] and dyspnea is also more severe in COPD patients. For those who live with advanced COPD, dyspnea is a significant source of disability and profoundly affects the quality of life to the extent that patients become isolated and describe themselves as existing rather than living.[17] In one study, 41% of patients who died of COPD left the house less than once per month or never in their last year of life. In a multicenter study, relief of symptoms such as dyspnea was a top priority targeted for improvement in the care of patients hospitalized with COPD.[18] However, dyspnea is currently the symptom least well palliated by traditional approaches.[19] The prevention, relief, reduction, and soothing of dyspnea symptoms should be an important integral component of standard care for COPD.
Other common symptoms in advanced chronic obstructive pulmonary disease
In addition to dyspnea, patients with COPD also suffer from a considerable range of additional symptoms. Anxiety and depression are common in advanced COPD, resulting from the dyspnea, disability, and isolation caused by the disease.[20,21] In a cross-sectional study of 109 patients with very severe COPD (median forced expiratory volume in 1 s [FEV1] 34% predicted and most on long-term oxygen therapy [LTOT] for more than 1 year), 57% demonstrated significant depressive symptoms.[22] Studies have documented that generalized anxiety disorder and panic disorder are more prevalent in patients with COPD than in the general population.[23] Anxiety and depression have been associated with the higher rates of both COPD exacerbations and mortality.[24,25,26] Fatigue or weakness is also reported, as a consequence of dyspnea.[27] Other symptoms include pain, insomnia and anorexia, weight loss, cough, constipation, and incontinence.[7,9,19,28,29,30] These symptoms are often uncontrolled at the end-stage of the disease.
The severity of these other symptoms, in addition to dyspnea, contributed to the deceased's overall quality of life for patients with advanced COPD. Compelling data demonstrated that patients who live with advanced COPD suffer from a lower quality of life than patients with lung cancer.[6,31] When compared to patients with advanced lung cancer, patients with advanced COPD were found to have significantly worse activities of daily living and worse physical, social, and emotional functioning. Patients with advanced COPD were also more likely to be suffering from clinically relevant depression and anxiety (90%) than the patients with lung cancer (52%).[31] Although COPD patients have such high symptom burden, existing model of care, as mentioned at the beginning, for these patients is reactive and mainly focuses on acute exacerbations.
There is evidence that patients with advanced COPD, suffering on average from 7 to 11 symptoms, have high palliative care needs, and concerns about symptom relief, quality of life, satisfaction with care, disease education, and use of care facilities.[28,32,33,34] In SUPPORT study, when compared with patients with lung cancer, patients with COPD were much more likely to die in the Intensive Care Unit, on mechanical ventilation, and with dyspnea.[15] These differences occurred despite most patients with COPD preferring treatment focused on comfort rather than on prolonging life. Additional studies have documented the significant burden of symptoms and poor quality of palliative care among patients with advanced COPD.[28]
In summary, palliative care which focuses on controlling symptom, relieving suffering and optimizing the quality of life should be better integrated into the current standard clinical management for advanced COPD.
MANAGEMENT STRATEGIES OF ADVANCED CHRONIC OBSTRUCTIVE PULMONARY DISEASE
Conventional pharmacologic interventions
Many patients with advanced COPD obtain symptomatic relief from the use of inhaled bronchodilators. Both short-acting β2-agonists and short-acting anticholinergics are equally effective in improving dyspnea and exertional tolerance in patients with COPD. Long-acting bronchodilators are indicated in patients with advanced COPD. Even though both long-acting β2-agonists (LABA) and long-acting anticholinergics are effective, tiotropium may be superior to LABA, especially in patients with very severe COPD.[35,36] Inhaled corticosteroids are also widely prescribed for advanced COPD. Currently, inhaled corticosteroid plus long-acting β2-agonist or long-acting anticholinergic is the recommended first choice for advanced COPD patients worldwide. A combination of all three classes of drugs (inhaled tri-corticosteroid/long-acting β2-agonist/long-acting anticholinergic) improves lung function and quality of life[37,38] and may further reduce exacerbations.[39] A phosphodiesterase-4 inhibitor, theophylline, or carbocysteine can be used in addition to a long-acting bronchodilator or if long-acting inhaled bronchodilators are unavailable or unaffordable.
While these conventional interventions are helpful, as COPD progresses, the symptoms of advanced COPD become less palliated, especially dyspnea, and other therapies beyond the conventional pharmacological approaches are needed to improve health-related quality of life among these patients.
Adjunctive nonpharmacological approaches
Pulmonary rehabilitation
As airflow obstruction progresses, patients with COPD typically become increasingly sedentary, which leads to muscular and cardiovascular deconditioning. Increasing physical disability contributes to social isolation and depression, which are highly prevalent among patients with advanced COPD. The primary goal of pulmonary rehabilitation is to reduce symptoms, improve the quality of life, and increase physical and emotional participation in daily activities. The components of pulmonary rehabilitation vary widely but a comprehensive program includes exercise training, smoking cessation, nutritional therapy, and self-management education (breathing strategies, use of supplemental oxygen, pharmacologic therapy, and panic control).
Pulmonary rehabilitation has been carefully evaluated in a large number of randomized, controlled trials (RCTs), which generally involved patients with advanced disease according to spirometric criteria (FEV1/forced vital capacity <0.70; FEV1, 30–49% of predicted value), and shown to reduce the perceived intensity of dyspnea, reduce anxiety, and depression associated with COPD improve exercise capacity and health-related quality of life, and even improve survival in some studies.[40,41] Benefits have been reported from rehabilitation programs conducted in inpatient, outpatient, and home settings. Although patients with advanced stages of COPD usually have limited exercise tolerance, pulmonary rehabilitation has still been shown to be effective in this group.[42] In a multicenter study of more than 1000 COPD patients, pulmonary rehabilitation was also found to be effective among patients with chronic respiratory failure.[43]
Oxygen therapy and ventilatory support
LTOT is often prescribed in the later stages of the disease to improve survival for those people with COPD, who are significantly hypoxemic. Two RCTs, published almost 30 years previously, established the significant survival benefit of LTOT when used for at least 15 h/d in hypoxemic COPD patients.[44,45] Oxygen therapy may also provide a symptomatic benefit by reducing dyspnea when administered at rest to hypoxemic patients with advanced COPD. However, little evidence from controlled studies supports its utility for dyspnea in the absence of hypoxemia. The combination of noninvasive ventilation with LTOT may be of some use in a selected subset of patients, particularly in those with pronounced daytime hypercapnia in improving survival but not the quality of life.[46]
SURGICAL OPTIONS
Lung volume reduction surgery, bronchoscopic lung volume reduction, lung transplantation, and bullectomy are surgical options for appropriately selected patients with advanced COPD. However, selection of ideal patients is critical for all these surgical treatments.
Innovative approaches to advanced chronic obstructive pulmonary disease: The dyspnea ladder and opioids
Dyspnea is the most common symptom in end-stage COPD, 98% of patients who died of COPD were breathless all the time or some of the time in their last year of life.[19] If dyspnea persists despite optimal therapy with pharmacologic and nonpharmacologic interventions in patients with end-stage COPD, some innovative approaches should be developed. In this context, some investigator proposed the concept of dyspnea ladder and used opioids to palliated dyspnea and other symptoms of advanced COPD.
Actually, it has been almost 20 years since opioids were first reported to be associated with a reduction of dyspnea symptoms in COPD.[47] Opioids have multiple mechanisms of action for symptom relief, including reductions in ventilation, oxygen consumption, sensitivity to hypercapnia, the central perception of dyspnea (similar to the reduction in the central perception of pain), and anxiety associated with dyspnea.[48] An increasing body of evidence supports the use and safety of oral and parenteral opioids for refractory dyspnea in patients with advanced COPD. A systematic review evaluated the use of opioids for dyspnea in 18 double-blind, randomized, placebo-controlled trials, in which 11/18 studies included only COPD patients. The systematic review confirmed overall beneficial effects of both oral and parenteral opioids on dyspnea, a conclusion supported by subsequent reports.[48,49,50] While evidence of nebulized opioids’ efficacy is conflicting, and there is insufficient data to conclude whether nebulized opioids are effective.[48]
Several recent evidenced-based clinical guidelines recommend that opioids should be considered on an individualized basis for relieving dyspnea in advanced COPD in addition to adequate treatment of the underlying disease [Table 2].[1,51,52,53,54] Rocker et al. proposed a “Dyspnea Ladder”,[55] following the example of the World Health Organization analgesic ladder in the management of cancer pain, which is later modified and adopted by the Canadian Thoracic Society in a guideline of managing dyspnea in patients with advanced COPD [Figure 1].[54] The clinical practice guideline emphasizes a stepwise approach to palliation of refractory dyspnea using conventional therapies, nonpharmacological approaches, carefully initiated and titrated opioids, and it also provides a detailed pathway for prescribing opioid therapy in patients with advanced COPD [Table 2]. In summary, opioids should be dosed and titrated for the individual patient for relief of dyspnea, with due consideration of patient history, comorbid conditions, and risk for respiratory depression. A dyspnea scale should be used to guide dose adjustment with the dual goals of providing adequate dyspnea relief and minimizing the sedative effects. Common opioids side effects are drowsiness, nausea, vomiting, dizziness, and constipation, but there is no evidence indicating that opioids use is associated with deleterious effects on arterial blood gases or oxygen saturation in patients with COPD.[48] Clinically significant respiratory depression is also uncommon with the doses used to treat dyspnea, even in elderly patients. There are no data to suggest that the use of opioids for the management of breathlessness is associated with a reduction in a patient's life expectancy.[52,56]
Table 2.
Guidelines recommendation of opioids for relief of dyspnea in patients with advanced pulmonary disease
| Guideline | Year | Recommendation of opioids for relief of dyspnea |
|---|---|---|
| An Official American Thoracic Society Clinical Policy Statement: Palliative care for patients with respiratory diseases and critical illnesses[51] | 2008 | Opioids and anxiolytics are the primary pharmacologic treatments of dyspnea for adults and children. |
| Opioids can be given orally, subcutaneously, or intravenously | ||
| Starting dosages of opioids in opioid-naïve adult patients with moderate to severe dyspnea: Oxycodone 5–10 mg (oral); methadone 2.5–10 mg (IV), 5–10 mg (oral); morphine 2–10 mg (IV), 5–10 mg (oral); hydromorphone 0.3–1.5 mg (IV), 2–4 mg (oral); fentanyl 50–100 mg (IV) | ||
| These dosing recommendations do not apply to patients who have previously used opioids because dosages for such patients will be higher and must be individualized | ||
| The correct dose and interval for opioid administration in all patients are those that relieve dyspnea or pain without intolerable adverse effects. There is no upper limit that is, the dose should be increased as needed to produce the desired effect or until intolerable side effects occur. Reassessment of the drug’s effects on the patient and titration of the opioid are the mainstays of successful management | ||
| American College of Chest Physicians Consensus Statement on the Management of Dyspnea in Patients with Advanced Lung or Heart Disease[52] | 2010 | Opioid medications for relief of dyspnea |
| Oral and/or parenteral opioids can provide relief of dyspnea | ||
| Opioids should be dosed and titrated for the individual patient with consideration of multiple factors (e.g., renal, hepatic, pulmonary function, and current and past opioid use) for relief of dyspnea | ||
| Respiratory depression is a widely held concern with the use of opioids for the relief of dyspnea | ||
| An Official American Thoracic Society Statement: Update on the Mechanisms, Assessment, and Management of Dyspnea[53] | 2012 | Opioids have been the most widely studied agent in the treatment of dyspnea |
| Short-term administration reduces breathlessness in patients with a variety of conditions, including advanced COPD, interstitial lung disease, cancer, and chronic heart failure. However, evidence of long-term efficacy is limited and conflicting | ||
| Opioids are associated with frequent side effects, particularly constipation, but clinically significant respiratory depression is uncommon with the doses used to treat dyspnea, even in elderly patients | ||
| Randomized controlled trials have not found nebulized opioids to be associated with fewer side effects than oral or parenteral opioids | ||
| Managing dyspnea in patients with advanced chronic obstructive pulmonary disease: A Canadian Thoracic Society clinical practice guideline[54] | 2011 | We recommend that oral (but not nebulized) opioids be used for the treatment of refractory dyspnea in the individual patient with advanced COPD |
| Initiate opioid therapy with oral immediate-release morphine syrup –titrate slowly at weekly intervals over a 4–6 weeks period | ||
| Start therapy with morphine 0.5 mg orally twice daily for 2 days, and then increase to 0.5 mg orally every 4 h while awake for the remainder of week 1. If tolerated and indicated, increase to morphine 1.0 mg orally every 4 h while awake in week 2, increasing by 1.0 mg/week or 25% dosage increments/week until the lowest effective dose that appropriately manages the dyspnea is achieved | ||
| Once a stable dosage is achieved (i.e., no significant dose change for 2 weeks and dyspnea managed), a sustained-release preparation at a comparable daily dose could be considered for substitution | ||
| If patients experience significant opioid-related side effects such as nausea or confusion, substitution of an equipotent dose of oral hydromorphine could be considered (1 mg hydromorphine = 5 mg morphine) | ||
| Stool softeners and laxatives should be routinely offered to prevent opioid-associated constipation | ||
| Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease[1] | 2015 | Oral and parenteral opioids are effective for treating dyspnea in COPD patients with very severe disease |
| There is insufficient data to conclude whether nebulized opioids are effective | ||
| However, some clinical studies suggest that morphine used to control dyspnea may have serious adverse effects and its benefits may be limited to a few sensitive subjects |
IV: Intravenous; COPD: Chronic obstructive pulmonary disease.
Figure 1.
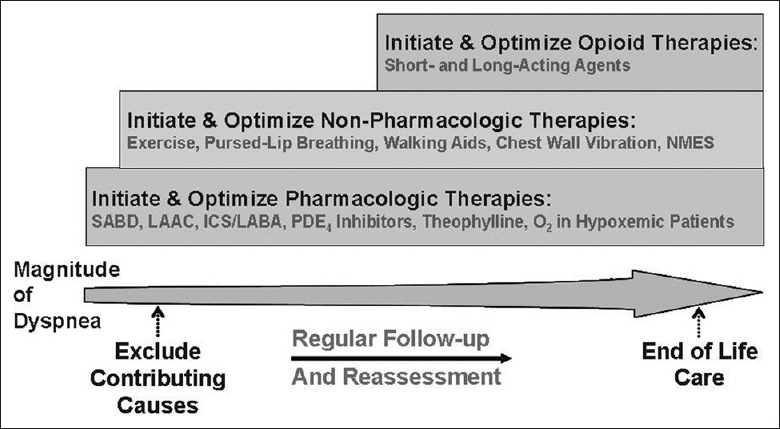
Comprehensive approach to the management of dyspnea in patients with the advanced chronic obstructive pulmonary disease. ICS: Inhaled corticosteroids; LAAC: Long-acting anticholinergics; LABA: Long-acting β2-agonists; NMES: Neuromuscular electrical stimulation; O2: Oxygen; PDE4: Phosphodiesterase-4; SABD: Short-acting bronchodilators (from reference 66).
Despite the evidence supporting their benefit and the recommendation by those clinical guidelines, opioids are infrequently prescribed in clinical practice, especially in China. There is an important need to address barriers of prescribing opioids for clinicians. These barriers are prominent and include insufficient uptake of evidence-based practice guidelines, lack of education regarding opioid prescription, pharmacokinetics, titration, and fears of further respiratory depression and other significant side effects, as well as concerns and attitudes about addiction and dependence. Meanwhile, well designed RCTs are still needed to demonstrate the efficiency and safety of opioids use in Chinese population with advanced COPD, and the optimal initiation dose, dosing interval, titration schedule, and delivery route in this population are also waiting to be addressed.
Palliative care, end-of-life care and hospice care for advanced chronic obstructive pulmonary disease
The disease trajectory in COPD is usually marked by a gradual decline in health status and increasing symptoms, punctuated by acute exacerbations that are associated with an increased risk of death.[57] Palliative care, end-of-life care, and hospice care are important components in the management of all patients with advanced COPD and have been shown to improve the quality of life, reduce symptoms burden, and even prolong survival for some patients. The 2013 updated GOLD guideline also added this part into the management approaches for COPD patients.
Palliative care is a broadest term and includes (but is not limited to) both end-of-life care (care for those who are actively dying), as well as hospice care (a model for delivery of end-of-life care for patients who are terminally ill and predicted to have <6 months to live). The goal of palliative care is to prevent and relieve suffering and to support the best possible quality of life for patients and their families, regardless of the stage of disease or the need for other therapies.[58] Although originally conceived and practiced as end-of-life care, palliative care may be applied to all stages of illness, whether terminal or not.
For patients with the most advanced and terminal illness, hospice services, and end-of-life care may provide additional benefit. Hospice care is defined as the support and care for patients and their families in the last phase of an incurable disease so that they may live as fully and comfortably as possible. In the Western developed countries, hospice services may provide these services within the patient's home or in hospice beds in dedicated hospice units or other institutions such as hospitals or nursing homes. Guidelines and/or criteria for selecting patients with noncancer diseases like COPD for access to hospice services are also available,[11,51,59] such as the guideline proposed by the US National Hospice and Palliative Care Organization (mentioned early in this review). According to this guideline, COPD patients with disabling dyspnea at rest that is poorly responsive to bronchodilators and progression of advanced disease demonstrated by increasing hospitalizations or emergency department visits are eligible for hospice services.
Although a number of studies show that the system burden in patients with advanced COPD is higher, and the health-related quality of life is poorer than the patients with lung cancer, patients with advanced COPD are less likely to receive such palliative care than patients with lung cancer.[4,31,59,60] There are several reasons for this. First, patient-clinician communication about palliative and end-of-life care is infrequent and often of poor quality; many clinicians have discomfort when discuss with their patients about end-of-life care. Secondly, the uncertainty in predicting prognosis for patients with advanced COPD makes a communication about end-of-life care more difficult. Additional barriers preventing advanced COPD patients receiving adequate palliative care include lack of palliative care related knowledge and skills for clinicians, and issues about the medical insurance policy. Recent studies provide insight and guidance into ways to improve communication between clinicians and patients with advanced COPD and their families about end-of-life care and thereby improve the quality of palliative and end-of-life care the patients receive.[61,62,63] The profiles and criteria proposed by these studies to identify patients with COPD who is at high-risk for dying and could benefit from palliative care services,[51,55] are also very helpful to improve the current situation. In China, the professional organizations and palliative care specialists aiming at palliative care, end-of-life care, and hospice care are sparse. Since the reality is hard to change within a short time period, clinicians who care for patients with COPD, should be trained in and capable of, providing a set of recommended basic competencies in palliative care, and should help their patients identify available palliative care resources within their community.
In conclusion, our healthcare systems are increasingly faced with the need to care for an aging population which is significantly burdened with chronic illnesses and frailty. As the prevalence of COPD rises, more patients and families will live with the burdens of advanced COPD than ever before. As patients with advanced COPD approach the end of their disease trajectory, many suffer from unrelenting dyspnea despite optimal conventional pharmacological and nonpharmacological therapies, which largely contribute to the decrease in quality of life of advanced COPD patients. More innovative and effective approaches are needed to achieve symptom control in advanced stages of COPD and opioids have potential value in this context. Traditional healthcare approaches to COPD, based on the biomedical model, have been directed more toward the underlying pathophysiology of disease and less toward patient-defined problems and palliative care aiming at controlling symptom and relieving suffering. The move toward a high quality evidence-based innovative and integrated model of care, including both intensive medical treatments focused on increasing survival and holistic and palliative approaches focusing on optimizing quality of life, to COPD will better help patients and their families.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yi Cui
REFERENCES
- 1.Global Strategy for the Diagnosis. Management and Prevention of Chronic Obstructive Pulmonary Disease, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2015. Available from: http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis-management.html .
- 2.Buist AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, Mannino DM, et al. International variation in the prevalence of COPD (the BOLD Study): A population-based prevalence study. Lancet. 2007;370:741–50. doi: 10.1016/S0140-6736(07)61377-4. [DOI] [PubMed] [Google Scholar]
- 3.Zhong N, Wang C, Yao W, Chen P, Kang J, Huang S, et al. Prevalence of chronic obstructive pulmonary disease in China: A large, population-based survey. Am J Respir Crit Care Med. 2007;176:753–60. doi: 10.1164/rccm.200612-1749OC. [DOI] [PubMed] [Google Scholar]
- 4.Au DH, Udris EM, Fihn SD, McDonell MB, Curtis JR. Differences in health care utilization at the end of life among patients with chronic obstructive pulmonary disease and patients with lung cancer. Arch Intern Med. 2006;166:326–31. doi: 10.1001/archinte.166.3.326. [DOI] [PubMed] [Google Scholar]
- 5.Soriano JB, Maier WC, Egger P, Visick G, Thakrar B, Sykes J, et al. Recent trends in physician diagnosed COPD in women and men in the UK. Thorax. 2000;55:789–94. doi: 10.1136/thorax.55.9.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weingaertner V, Scheve C, Gerdes V, Schwarz-Eywill M, Prenzel R, Bausewein C, et al. Breathlessness, functional status, distress, and palliative care needs over time in patients with advanced chronic obstructive pulmonary disease or lung cancer: A cohort study. J Pain Symptom Manage. 2014;48:569–81.e1. doi: 10.1016/j.jpainsymman.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 7.Janssen DJ, Spruit MA, Uszko-Lencer NH, Schols JM, Wouters EF. Symptoms, comorbidities, and health care in advanced chronic obstructive pulmonary disease or chronic heart failure. J Palliat Med. 2011;14:735–43. doi: 10.1089/jpm.2010.0479. [DOI] [PubMed] [Google Scholar]
- 8.Okutan O, Tas D, Demirer E, Kartaloglu Z. Evaluation of quality of life with the chronic obstructive pulmonary disease assessment test in chronic obstructive pulmonary disease and the effect of dyspnea on disease-specific quality of life in these patients. Yonsei Med J. 2013;54:1214–9. doi: 10.3349/ymj.2013.54.5.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bausewein C, Booth S, Gysels M, Kühnbach R, Haberland B, Higginson IJ. Understanding breathlessness: Cross-sectional comparison of symptom burden and palliative care needs in chronic obstructive pulmonary disease and cancer. J Palliat Med. 2010;13:1109–18. doi: 10.1089/jpm.2010.0068. [DOI] [PubMed] [Google Scholar]
- 10.Goodridge D, Lawson J, Duggleby W, Marciniuk D, Rennie D, Stang M. Health care utilization of patients with chronic obstructive pulmonary disease and lung cancer in the last 12 months of life. Respir Med. 2008;102:885–91. doi: 10.1016/j.rmed.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Medical guidelines for determining prognosis in selected non-cancer diseases. The National Hospice Organization. Hosp J. 1996;11:47–63. doi: 10.1080/0742-969x.1996.11882820. [DOI] [PubMed] [Google Scholar]
- 12.Cahaba Government Benefit Administrators Midwest. Local Coverage Determination for Hospice-determining Terminal Status (L13653) [Last modified on 2008 Jan 03; Last accessed on 2008 Mar 11]. Available from: http://www.cms.hhs.gov/mcd/viewlcd.asp?lcd_id=13653 and lcd_version=28 and show=all .
- 13.Klimathianaki M, Mitrouska I, Georgopoulos D. Siafakas NM, editor. Management of end-stage chronic obstructive pulmonary disease. Management of Chronic Obstructive Pulmonary Disease. Eur Respir Mon. 2006;38:430–50. [Google Scholar]
- 14.Viegi G, Pistelli F, Sherrill DL, Maio S, Baldacci S, Carrozzi L. Definition, epidemiology and natural history of COPD. Eur Respir J. 2007;30:993–1013. doi: 10.1183/09031936.00082507. [DOI] [PubMed] [Google Scholar]
- 15.Claessens MT, Lynn J, Zhong Z, Desbiens NA, Phillips RS, Wu AW, et al. Dying with lung cancer or chronic obstructive pulmonary disease: Insights from SUPPORT. Study to understand prognoses and preferences for outcomes and risks of treatments. J Am Geriatr Soc. 2000;48 5 Suppl:S146–53. doi: 10.1111/j.1532-5415.2000.tb03124.x. [DOI] [PubMed] [Google Scholar]
- 16.Habraken JM, ter Riet G, Gore JM, Greenstone MA, Weersink EJ, Bindels PJ, et al. Health-related quality of life in end-stage COPD and lung cancer patients. J Pain Symptom Manage. 2009;37:973–81. doi: 10.1016/j.jpainsymman.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 17.Rocker G, Horton R, Currow D, Goodridge D, Young J, Booth S. Palliation of dyspnoea in advanced COPD: Revisiting a role for opioids. Thorax. 2009;64:910–5. doi: 10.1136/thx.2009.116699. [DOI] [PubMed] [Google Scholar]
- 18.Rocker GM, Dodek PM, Heyland DK. Canadian Researchers at the End of Life Network. Toward optimal end-of-life care for patients with advanced chronic obstructive pulmonary disease: Insights from a multicentre study. Can Respir J. 2008;15:249–54. doi: 10.1155/2008/369162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elkington H, White P, Addington-Hall J, Higgs R, Edmonds P. The healthcare needs of chronic obstructive pulmonary disease patients in the last year of life. Palliat Med. 2005;19:485–91. doi: 10.1191/0269216305pm1056oa. [DOI] [PubMed] [Google Scholar]
- 20.Schane RE, Walter LC, Dinno A, Covinsky KE, Woodruff PG. Prevalence and risk factors for depressive symptoms in persons with chronic obstructive pulmonary disease. J Gen Intern Med. 2008;23:1757–62. doi: 10.1007/s11606-008-0749-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Warmenhoven F, Bor H, Lucassen P, Vissers K, van Weel C, Prins J, et al. Depressive disorder in the last phase of life in patients with cardiovascular disease, cancer, and COPD: Data from a 20-year follow-up period in general practice. Br J Gen Pract. 2013;63:e303–8. doi: 10.3399/bjgp13X667150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maurer J, Rebbapragada V, Borson S, Goldstein R, Kunik ME, Yohannes AM, et al. Anxiety and depression in COPD: Current understanding, unanswered questions, and research needs. Chest. 2008;134 4 Suppl:43S–56S. doi: 10.1378/chest.08-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pothirat C, Chaiwong W, Phetsuk N, Pisalthanapuna S, Chetsadaphan N, Inchai J. Major affective disorders in chronic obstructive pulmonary disease compared with other chronic respiratory diseases. Int J Chron Obstruct Pulmon Dis. 2015;10:1583–90. doi: 10.2147/COPD.S86742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu W, Collet JP, Shapiro S, Lin Y, Yang T, Platt RW, et al. Independent effect of depression and anxiety on chronic obstructive pulmonary disease exacerbations and hospitalizations. Am J Respir Crit Care Med. 2008;178:913–20. doi: 10.1164/rccm.200804-619OC. [DOI] [PubMed] [Google Scholar]
- 25.de Voogd JN, Wempe JB, Koëter GH, Postema K, van Sonderen E, Ranchor AV, et al. Depressive symptoms as predictors of mortality in patients with COPD. Chest. 2009;135:619–25. doi: 10.1378/chest.08-0078. [DOI] [PubMed] [Google Scholar]
- 26.Eisner MD, Blanc PD, Yelin EH, Katz PP, Sanchez G, Iribarren C, et al. Influence of anxiety on health outcomes in COPD. Thorax. 2010;65:229–34. doi: 10.1136/thx.2009.126201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sillen MJ, Franssen FM, Delbressine JM, Vaes AW, Wouters EF, Spruit MA. Efficacy of lower-limb muscle training modalities in severely dyspnoeic individuals with COPD and quadriceps muscle weakness: Results from the DICES trial. Thorax. 2014;69:525–31. doi: 10.1136/thoraxjnl-2013-204388. [DOI] [PubMed] [Google Scholar]
- 28.Elkington H, White P, Addington-Hall J, Higgs R, Pettinari C. The last year of life of COPD: A qualitative study of symptoms and services. Respir Med. 2004;98:439–45. doi: 10.1016/j.rmed.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 29.Jones I, Kirby A, Ormiston P, Loomba Y, Chan KK, Rout J, et al. The needs of patients dying of chronic obstructive pulmonary disease in the community. Fam Pract. 2004;21:310–3. doi: 10.1093/fampra/cmh317. [DOI] [PubMed] [Google Scholar]
- 30.Solano JP, Gomes B, Higginson IJ. A comparison of symptom prevalence in far advanced cancer, AIDS, heart disease, chronic obstructive pulmonary disease and renal disease. J Pain Symptom Manage. 2006;31:58–69. doi: 10.1016/j.jpainsymman.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 31.Gore JM, Brophy CJ, Greenstone MA. How well do we care for patients with end stage chronic obstructive pulmonary disease (COPD)? A comparison of palliative care and quality of life in COPD and lung cancer. Thorax. 2000;55:1000–6. doi: 10.1136/thorax.55.12.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.White P, White S, Edmonds P, Gysels M, Moxham J, Seed P, et al. Palliative care or end-of-life care in advanced chronic obstructive pulmonary disease: A prospective community survey. Br J Gen Pract. 2011;61:e362–70. doi: 10.3399/bjgp11X578043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Habraken JM, Willems DL, de Kort SJ, Bindels PJ. Health care needs in end-stage COPD: A structured literature review. Patient Educ Couns. 2007;68:121–30. doi: 10.1016/j.pec.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 34.Blinderman CD, Homel P, Billings JA, Tennstedt S, Portenoy RK. Symptom distress and quality of life in patients with advanced chronic obstructive pulmonary disease. J Pain Symptom Manage. 2009;38:115–23. doi: 10.1016/j.jpainsymman.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 35.Brusasco V, Hodder R, Miravitlles M, Korducki L, Towse L, Kesten S. Health outcomes following treatment for six months with once daily tiotropium compared with twice daily salmeterol in patients with COPD. Thorax. 2003;58:399–404. doi: 10.1136/thorax.58.5.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vogelmeier C, Hederer B, Glaab T, Schmidt H, Rutten-van Mölken MP, Beeh KM, et al. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med. 2011;364:1093–103. doi: 10.1056/NEJMoa1008378. [DOI] [PubMed] [Google Scholar]
- 37.Welte T, Miravitlles M, Hernandez P, Eriksson G, Peterson S, Polanowski T, et al. Efficacy and tolerability of budesonide/formoterol added to tiotropium in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;180:741–50. doi: 10.1164/rccm.200904-0492OC. [DOI] [PubMed] [Google Scholar]
- 38.Aaron SD, Vandemheen KL, Fergusson D, Maltais F, Bourbeau J, Goldstein R, et al. Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease: A randomized trial. Ann Intern Med. 2007;146:545–55. doi: 10.7326/0003-4819-146-8-200704170-00152. [DOI] [PubMed] [Google Scholar]
- 39.Karner C, Cates CJ. Combination inhaled steroid and long-acting beta(2)-agonist in addition to tiotropium versus tiotropium or combination alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011;3:CD008532. doi: 10.1002/14651858.CD008532.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maltais F, Bourbeau J, Shapiro S, Lacasse Y, Perrault H, Baltzan M, et al. Effects of home-based pulmonary rehabilitation in patients with chronic obstructive pulmonary disease: A randomized trial. Ann Intern Med. 2008;149:869–78. doi: 10.7326/0003-4819-149-12-200812160-00006. [DOI] [PubMed] [Google Scholar]
- 41.Lacasse Y, Goldstein R, Lasserson TJ, Martin S. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;4:CD003793. doi: 10.1002/14651858.CD003793.pub2. [DOI] [PubMed] [Google Scholar]
- 42.Cote CG, Celli BR. Pulmonary rehabilitation and the BODE index in COPD. Eur Respir J. 2005;26:630–6. doi: 10.1183/09031936.05.00045505. [DOI] [PubMed] [Google Scholar]
- 43.Carone M, Patessio A, Ambrosino N, Baiardi P, Balbi B, Balzano G, et al. Efficacy of pulmonary rehabilitation in chronic respiratory failure (CRF) due to chronic obstructive pulmonary disease (COPD): The Maugeri Study. Respir Med. 2007;101:2447–53. doi: 10.1016/j.rmed.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 44.Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: A clinical trial. Nocturnal Oxygen Therapy Trial Group. Ann Intern Med. 1980;93:391–8. doi: 10.7326/0003-4819-93-3-391. [DOI] [PubMed] [Google Scholar]
- 45.Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Report of the Medical Research Council Working Party. Lancet. 1981;1:681–6. [PubMed] [Google Scholar]
- 46.McEvoy RD, Pierce RJ, Hillman D, Esterman A, Ellis EE, Catcheside PG, et al. Nocturnal non-invasive nasal ventilation in stable hypercapnic COPD: A randomised controlled trial. Thorax. 2009;64:561–6. doi: 10.1136/thx.2008.108274. [DOI] [PubMed] [Google Scholar]
- 47.Light RW, Muro JR, Sato RI, Stansbury DW, Fischer CE, Brown SE. Effects of oral morphine on breathlessness and exercise tolerance in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1989;139:126–33. doi: 10.1164/ajrccm/139.1.126. [DOI] [PubMed] [Google Scholar]
- 48.Jennings AL, Davies AN, Higgins JP, Gibbs JS, Broadley KE. A systematic review of the use of opioids in the management of dyspnoea. Thorax. 2002;57:939–44. doi: 10.1136/thorax.57.11.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Foral PA, Malesker MA, Huerta G, Hilleman DE. Nebulized opioids use in COPD. Chest. 2004;125:691–4. doi: 10.1378/chest.125.2.691. [DOI] [PubMed] [Google Scholar]
- 50.Currow D, Johnson M, White P, Abernethy A. Evidence-based intervention for chronic refractory breathlessness: Practical therapies that make a difference. Br J Gen Pract. 2013;63:609–10. doi: 10.3399/bjgp13X674611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lanken PN, Terry PB, Delisser HM, Fahy BF, Hansen-Flaschen J, Heffner JE, et al. An official American Thoracic Society clinical policy statement: Palliative care for patients with respiratory diseases and critical illnesses. Am J Respir Crit Care Med. 2008;177:912–27. doi: 10.1164/rccm.200605-587ST. [DOI] [PubMed] [Google Scholar]
- 52.Mahler DA, Selecky PA, Harrod CG, Benditt JO, Carrieri-Kohlman V, Curtis JR, et al. American College of Chest Physicians consensus statement on the management of dyspnea in patients with advanced lung or heart disease. Chest. 2010;137:674–91. doi: 10.1378/chest.09-1543. [DOI] [PubMed] [Google Scholar]
- 53.Parshall MB, Schwartzstein RM, Adams L, Banzett RB, Manning HL, Bourbeau J, et al. An official American Thoracic Society statement: Update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185:435–52. doi: 10.1164/rccm.201111-2042ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marciniuk DD, Goodridge D, Hernandez P, Rocker G, Balter M, Bailey P, et al. Managing dyspnea in patients with advanced chronic obstructive pulmonary disease: A Canadian Thoracic Society clinical practice guideline. Can Respir J. 2011;18:69–78. doi: 10.1155/2011/745047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rocker GM, Sinuff T, Horton R, Hernandez P. Advanced chronic obstructive pulmonary disease: Innovative approaches to palliation. J Palliat Med. 2007;10:783–97. doi: 10.1089/jpm.2007.9951. [DOI] [PubMed] [Google Scholar]
- 56.Ekström MP, Bornefalk-Hermansson A, Abernethy AP, Currow DC. Safety of benzodiazepines and opioids in very severe respiratory disease: National prospective study. BMJ. 2014;348:g445. doi: 10.1136/bmj.g445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Murray SA, Kendall M, Boyd K, Sheikh A. Illness trajectories and palliative care. BMJ. 2005;330:1007–11. doi: 10.1136/bmj.330.7498.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.American Academy of Hospice and Palliative Medicine; Center to Advance Palliative Care; Hospice and Palliative Nurses Association; Last Acts Partnership; National Hospice and Palliative Care Organization. National Consensus Project for Quality Palliative Care: Clinical Practice Guidelines for quality palliative care, executive summary. J Palliat Med. 2004;7:611–27. doi: 10.1089/jpm.2004.7.611. [DOI] [PubMed] [Google Scholar]
- 59.Levy MH, Adolph MD, Back A, Block S, Codada SN, Dalal S, et al. Palliative care. J Natl Compr Canc Netw. 2012;10:1284–309. doi: 10.6004/jnccn.2012.0132. [DOI] [PubMed] [Google Scholar]
- 60.Foreman MG, Zhang L, Murphy J, Hansel NN, Make B, Hokanson JE, et al. Early-onset chronic obstructive pulmonary disease is associated with female sex, maternal factors, and African American race in the COPDGene Study. Am J Respir Crit Care Med. 2011;184:414–20. doi: 10.1164/rccm.201011-1928OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Curtis JR. Palliative and end-of-life care for patients with severe COPD. Eur Respir J. 2008;32:796–803. doi: 10.1183/09031936.00126107. [DOI] [PubMed] [Google Scholar]
- 62.Rocker GM, Cook D. ‘INSPIRED’ approaches to better care for patients with advanced COPD. Clin Invest Med. 2013;36:E114–20. doi: 10.25011/cim.v36i3.19721. [DOI] [PubMed] [Google Scholar]
- 63.Spathis A, Booth S. End of life care in chronic obstructive pulmonary disease: In search of a good death. Int J Chron Obstruct Pulmon Dis. 2008;3:11–29. doi: 10.2147/copd.s698. [DOI] [PMC free article] [PubMed] [Google Scholar]


