Abstract
Spinal muscular atrophy (SMA) is a genetic neurological disease that causes infant mortality; no effective therapies are currently available. SMA is due to homozygous mutations and/or deletions in the survival motor neuron 1 gene and subsequent reduction of the SMN protein, leading to the death of motor neurons. However, there is increasing evidence that in addition to motor neurons, other cell types are contributing to SMA pathology. In this review, we will discuss the involvement of non-motor neuronal cells, located both inside and outside the central nervous system, in disease onset and progression. Even if SMN restoration in motor neurons is needed, it has been shown that optimal phenotypic amelioration in animal models of SMA requires a more widespread SMN correction. It has been demonstrated that non-motor neuronal cells are also involved in disease pathogenesis and could have important therapeutic implications. For these reasons it will be crucial to take this evidence into account for the clinical translation of the novel therapeutic approaches.
Keywords: Spinal muscular atrophy, Pathogenesis, Therapy, Central nervous system
Introduction
Spinal muscular atrophy (SMA) is the most common genetic neurological disease leading to infant mortality. It is characterized predominantly by spinal motor neuron loss, muscle atrophy, and motor impairment [1, 2]. There is currently no effective treatment for this disease, although several promising therapeutic strategies are under development [3, 4]. SMA is caused by mutations in the survival motor neuron 1 (SMN1) gene, which result in functional SMN protein deficiency, leading to motor neuron degeneration.
SMN is ubiquitously expressed and its well-characterized function is linked to a critical pathway of RNA metabolism: the small nuclear ribonucleic proteins’ (snRNP) biogenesis [5]. Briefly SMN, in collaboration with partner proteins, catalyzes the assembly of snRNPs, which are the building blocks for pre-mRNA splicing [5].
From a clinical point of view, SMA is classified into three types based on age of onset and clinical course. Type I SMA is the most severe infant form, type II is an intermediate form affecting toddlers, and type III is a mild juvenile form. Type 1 SMA patients can present some systemic signs and symptoms such as autonomic dysfunction, cardiac impairment, and, rarely, skin necrosis [6, 7]. Indeed, these systemic features are even more prominent in the most widely used severe transgenic mouse models of SMA [8–10]. When SMN levels are dramatically diminished, this more widespread pathology is probably linked to the general RNA metabolism pathways described above [10]. On the other hand, in the mildest forms of SMA, in which SMN levels are relatively higher, only motor neurons are affected, suggesting that this cell type is more sensitive to SMN reduction. Thus, it is legitimate to hypothesize that there is a variable threshold of susceptibility to SMN reduction in different cell types. The level of SMN in other tissues could be important to better understand the role of SMN protein, and the effects of its depletion could be useful to develop new therapeutic strategies against SMA.
Humans and bonobos are the only species in which two copies of paralogous inverted SMN genes in chromosome 5 are present [11]. The paralogous gene SMN2 differs from SMN1 by a single C-to-T transition in exon 7 that modifies a splicing modulator and causes deletion of exon 7 in 90 % of SMN2 mRNA transcripts [12]. The SMN protein lacking exon 7 does not oligomerize efficiently and is quickly degraded, leading to the reduction of total SMN levels. The SMN2 gene produces approximately 10 % of full-length SMN2 protein [12], which is a major modulator of SMA clinical phenotype. As a result, only a small percentage of full-length, functional, and stable SMN protein is produced from SMN2. Due to this partial SMN production, the number of SMN2 copies is inversely correlated with disease severity in patients [13]. In fact, it is known that there are asymptomatic subjects carrying homozygous SMN1 gene mutations and multiple copies of SMN2; while at the other extreme, the total absence of SMN products is invariably fatal [5].
Highlighting the importance of SMN function, which is essential for every cell, the complete loss of SMN protein results in embryonic lethality in knockout animals [14]. In contrast, SMA is caused by various degrees of reduction in SMN levels. In line with this in Cre-Lox transgenic rodent models, in which full-length SMN is completely ablated in specific tissues, the absence of SMN protein leads to dramatic tissue-specific defects that are more severe than the typical SMA phenotype, where residual SMN protein levels are still present [15, 16]. Although some researchers consider that the motor neuron is the primary site of pathology in SMA and SMN restoration in these cells is sufficient for therapy [15, 17, 18]; other work suggests that multiple organs contribute to the phenotype, in particular in the most severe forms of SMA [19, 20].
In this review, we will discuss the involvement of non-motor neuronal cells, located both inside and outside the central nervous system (CNS), in SMA pathogenesis. We will discuss the different mechanisms that could play a role in disease initiation and progression, as well as the important implications for the optimal design of novel effective therapeutic strategies. Motor neurons are the predominant target in the majority of therapeutic approaches. However, the consideration of other cell types affected by SMN deficiency, at different time points of the disease, could be crucial for therapeutic success in SMA patients. Taking into consideration all these aspects, it is crucial to focus on targeting both motor neurons and other cell types and to define the therapeutic window when interventions can still be meaningful, when developing new therapeutic strategies.
Therapeutic implications in non-motor neuron cell types
Rodents, in contrast to humans, carry only the SMN gene. For the generation of mouse models of SMA, scientists have combined the genetic deletion of endogenous mouse SMN with the insertion of several copies of human SMN2 [21]. The genetic insertion of two copies of SMN2 and of a transgene missing the exon 7 sequence (SMNΔ7) in SMN knockout murine embryonic cells has led to the generation of a mouse strain known as SMNΔ7 [22]. SMNΔ7 mice are widely employed in preclinical studies of SMA since they recapitulate many key aspects of the disease, including severe progressive muscle weakness and an average lifespan of about 2 weeks [22]. Although there is limited muscle denervation and overall motor neuron loss, specific muscle groups and motor neuron subsets in these mice show greater vulnerability compared to others [23–25].
Several groups have shown that the restoration of SMN1 in different SMA mice model, using a motor neuron specific promoter [homeobox gene 9 (HB9) or choline acetyltransferase (ChAT)], resulted only in a modest extension of survival [17, 26, 27]. Conversely, SMN1 expression in SMA mice using a promoter highly expressed both in neurons and astrocytes (prion promoter), significantly extended their survival [15]. These crucial findings, together with others, suggest that astrocytes, sensory neurons, Schwann cells, and skeletal muscle may all contribute to the expression of the disease and its associated motor neuron loss [25, 27–32]. Additional evidence of the potential key role of non-motor neuronal cells in SMA pathogenesis was recently provided by an effort to up-regulate SMN protein, introducing the wild-type SMN1 gene [33–36], or by modulating SMN2 splicing with oligonucleotides or small molecules in mice (for review see [4, 37, 38]). Several recent studies have demonstrated that these strategies can significantly increase survival of SMA mice [38–44]. In particular, Foust and his group obtained the most profound phenotypic correction in terms of rescue of motor function, neuromuscular physiology, and life span [40]. Here, vascular delivery of scAAV9 encoding SMN at post-natal day 1 in SMA pups was employed to increase levels of SMN protein. In contrast, Hua used a different strategy based on antisense oligonucleotides that effectively corrected SMN2 splicing and restored SMN expression in motor neurons. In agreement with the first study, the systemic administration of gene-correcting agents to neonates robustly rescued the severe SMA mice phenotype [9]. Also, in a recent paper Hua and collaborators demonstrated that increasing SMN exclusively in peripheral tissues completely rescued necrosis in mild SMA mice, and significantly extended survival of severe SMA mice, with noticeable improvements in motor neuron survival, neuromuscular junction integrity, and motor function. Accordingly, they conclude that the SMA phenotype in murine models is not the result of a cell-autonomous defect of motor neurons [45].
The findings of the various studies investigating the role of SMN in cell types other than motor neurons are summarized in Table 1.
Table 1.
Summary of the participation different non-motor neuron cell types, intrinsic and extrinsic to the CNS, in SMA pathogenesis
| Cell type | Citation | Disease model | Observation |
|---|---|---|---|
| Involvement of non-motor neuronal cells located inside the CNS | |||
| Astrocytes | McGivern et al. [30] | SMAΔ7 mouse (Smn−/−, SMN2+/+; SMNΔ7+/+) | Early PD: no differences between wt and SMA mice |
| PD9 in SMA mice: augmented cell bodies; ↑ GFAP; thin and retracted processes | |||
| Production of pro-inflammatory cytokines through ERK1/2 activation | |||
| SMA iPSC-derived astrocytes | Mis-regulation of basal calcium | ||
| Decreased response to adenosine triphosphate stimulation | |||
| Tarabal et al. [67] | SMAΔ7 mouse | GFAP-positive profiles from PD4 | |
| ↑ Components of Notch signaling pathway, in particular Jagged 1 | |||
| Microglia | Tarabal et al. [67] | SMAΔ7 mouse | ↑ Anti-Iba1 positive microglial cells from PD7 |
| CD68 positive microglial cells | |||
| Microglial clearance of cellular debris during synapse degeneration/elimination | |||
| Interneurons and sensory neurons | Jablonka et al. [29] | SMA sensory neuron (E14 embryos derived) | Smaller growth cones |
| Shorter neurites | |||
| Reduced β actin levels of mRNA and protein | |||
| Schwab and Ebert [51] | Co-colture with SMA iPS-derived sensory neurons and wt iPS-derived MNs | SMA sensory neurons does notcontribute to MNs loss in this human stem cell system | |
| Lotti et al. [48] | SMA Drosophila | aberrant splicing of Stasimon gene | |
| isolated sensory neurons from Smn−/−; SMN2 mouse embryos | 1. Smaller terminal in the skin and | ||
| 2. Defects both in neurite growth and growth cone morphology | |||
| 3. Abnormal sensory conduction velocity | |||
| 4. Axonal degeneration | |||
| SMAΔ7 mouse | Alteration of monosynaptic connections between sensory neurons and motor neurons | ||
| Other cell types outside the CNS | |||
| Heart involvement | Elkohen et al. [119], Roos et al. [120], Tanaka et al. [122], Kimura et al. [127], Takahashi et al. [128], Distefano et al. [129] | SMA I patients | Atrial and ventricular septal defects |
| Dilated right ventricle | |||
| Anomalous development of the heart | |||
| Shababi et al. [133] | Severe model of SMA (Smn−/−, SMN2+/+) and the SMAΔ7 mouse | Cardiac fibrosis due to oxidative stress | |
| Heier et al. [8] | SMAΔ7 mouse | Bradyarrhythmia from PD2 (heart block and reduced ventricular depolarization efficiency) | |
| Sympathetic innervation defects and dilated cardiomyopathy at late stages of disease | |||
| Bevan et al. [131] | SMAΔ7 mouse | Bradycardia | |
| Dilated cardiomyopathy | |||
| Biondi et al. [132] | type 2 SMA-like mice | Cardiac muscle maturation delay | |
| Fibrosis | |||
| Alteration of cardiac electrical conduction velocity | |||
| Bradycardia and arrhythmia | |||
| Schwann cells involvement | Hunter et al. [84] | Schwann cells derived from SMA mice | ↓ Levels of SMN protein |
| Failed to respond normally to differentiation signals | |||
| SMA mice | Alterations in the expression of key myelin proteins (MPZ, PMP22, MBP) | ||
| ↓ Level of LAMA2 | |||
| Aghamaleky Sarvestany et al. [89] | Schwann cells isolated from SMA mice | SMN-dependent disruption changes in growth/proliferation, cell death/survival, and molecular transport pathways, changes in ubiquitination pathways proteins such as Uba1 | |
|
Neuromuscular junction and muscle cells involvement Neuromuscular junction and muscle cells involvement |
Kong et al. [23], Ling et al. [24], Mentis et al. [25], Kariya et al. [65], Dachs et al. [75], Lee et al. [90], Murray et al. [91] | SMAΔ7 mouse | Morphological and functional abnormalities of synapses |
| Bowerman et al. [80] | SMAΔ7 mouse | SMN levels in muscle fibers has no effects on SMA phenotype or on the survival | |
| Gavrilina et al. [15] | SMAΔ7 mouse | Defect in cytoskeleton organization | |
| Arlnold et al. [103], Boyer et al. [104], Cifuentes-Diaz et al. [105], Nicole et al. [106], Shafey et al. [107] | SMAΔ7 mouse | Defect in myoblast fusion, proliferation and in the correct formation of myotubes | |
| Dachs et al. [75] | SMAΔ7 mouse | ↓ Macrophage density | |
PD post-natal day, MNs motor neurons
Role of non-motor neuronal cells located inside the CNS
Interneurons and sensory neurons
Numerous in vitro and in vivo studies have shed light on discrete alterations in sensory neurons and interneurons in SMA. For instance, Jablonka et al. [29] have demonstrated that in Smn-deficient sensory neurons isolated from the severely affected SMA mouse model (Smn−/−; SMN2), growth cones are smaller, neurites are shorter, and levels of both β-actin mRNA and protein are reduced in comparison to neurons from control animals; without affecting the survival of these cells in culture [29]. In vivo, the sensory neurons of embryos from the same SMA mouse model did not develop normally. In particular, they exhibited smaller terminals in the skin and altered neurite growth and growth cone morphology compared to controls [29]. In the relatively less severe SMA mouse model, SMNΔ7, early alterations of monosynaptic connections between proprioceptive (sensory) neurons and motor neurons, such as reduction in the number of VGluT1+ synaptic terminals on motor neuron soma were shown to precede motor neuron loss [25]. Furthermore, a recent study performed by Zhang et al. [46], has found that motor neuron loss is preceded by a dysregulation of mRNAs critical for synaptic formation and sensory-motor circuitry in SMA mice spinal cord. Importantly, abnormal conduction velocity and axonal degeneration of sensory neurons are also reported in severe SMA type I patients [47]. In a Drosophila model of SMA, the groups of McCabe and Pellizzoni, have shown that when SMN is deleted in sensory neurons, their excitability is reduced causing secondary motor neuron dysfunction [28, 48]. In 2012, Pellizzoni’s group identified a novel gene called Stasimon, which is involved in U12 related splicing defects in the context of reduced SMN levels [48]. They demonstrated that aberrant splicing of Stasimon in cholinergic sensory neurons and interneurons causes SMN-related phenotypes, while its re-expression leads to the complete restoration of neuromuscular junction activity and muscle size.
In light of these studies suggesting that SMN levels and function in sensory neurons and interneurons contribute indirectly to motor neuron function, it has been hypothesized that some primary defects appearing in afferent sensory neurons may contribute to, or may exacerbate a secondary motor neuron dysfunction and loss [17, 24, 27]. In contrast, other studies point to motor neuron dysfunction triggering secondary sensory and interneuron deficits in SMA [17, 27, 49, 50]. In a cellular model employing iPSC-derived neurons, Schwab and Ebert [51] have used a direct co-culture approach to assess whether sensory neurons established from SMA-derived iPS cell lines can lead to WT motor neuron loss [51]. They found that SMA iPSC-derived sensory neurons have an negligible impact on motor neuron survival, or expression of glutamate transporters (VGLUT1, VGLUT2) in neurites and cell bodies of motor neuron; concluding that SMN-deficient sensory neurons are not the primary trigger of motor neuron loss [51]. As described above, it has been shown that motor neuron loss is preceded by a small reduction in VGLUT1+ bouton numbers on sensory neurons [24]. From a neuropathological point of view, at the end stage of the disease, the number of VGLUT1+ synapses decreases in motor neurons but not in sensory neurons, while VGLUT2 excitatory boutons reduce in L3–L5 lateral motor neurons, raising the possibility that synaptic defects might appear in interneuronal circuits [24]. This evidence lead Thirumalai and collaborators to conclude that the loss of proprioceptive synapses on motor neurons may be secondary to motor neuron pathology [50]. Similarly, amelioration of electrophysiological deficits and loss of sensory-motor synapses could be achieved through increasing SMN levels in motor neurons in SMN∆7 mice, and even in the severe murine model, suggesting that the reduced levels of SMN in motor neurons contributes to the motor circuit dysfunction [17, 27, 52].
In SMA, the interruption of sensory nerve impulses caused by an injury of the sensory nerve fibers is an early event leading to motor impairment [25]. Moreover, Renault et al. [53] showed reduced H-reflexes in type I human SMA patients that are responsible for the muscle weakness caused not only by proprioceptive abnormalities but also complicated by neuromuscular denervation and motor neuron loss. In addition to abnormal sensory nerve conduction velocity [47, 54, 55], nerve biopsies have revealed sensory axonal degeneration in SMA [47]. Overall, it remains unclear whether the loss of sensory-motor synapses is the cause, or the consequence of motor neuron loss; further studies are clearly needed to decipher the sequence of events in the pathophysiology of SMA.
Astrocytes
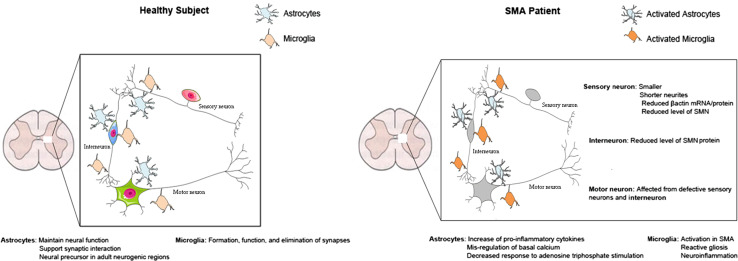
Astrocytes are the most abundant cell type in the CNS and are present both in white and grey matter. These cells are characterized by a star-shaped cytoplasm, comprised of intermediate filaments (glial fibers), extending numerous processes that surround adjacent blood vessels and neurons. Astrocytes have different roles, such as maintaining neuronal functions, supporting synaptic interactions, and serving as neural precursors in adult neurogenic regions (Fig. 1). Moreover, through the reuptake of neurotransmitters, they can contribute to overall synaptic activity; and are a critical component of the blood–brain barrier (BBB), regulating the transport of different molecules across this barrier [56]. Multiple cellular studies have demonstrated that astrocytes are able to release proteins involved in the control of neuronal maturation and differentiation. Through the release of neurotrophic factors, including neurotrophin-3 (NT-3) [57], brain-derived neurotrophic factor (BDNF), and nerve growth factor (NGF), astrocytes can enhance neural survival and control both neuronal differentiation and growth [58]. Furthermore, other molecules, such as S100B which is a glial-specific protein expressed primarily by astrocytes, can be released and exert neuroprotective effects against glutamate excitotoxicity [59].
Fig. 1.
Role of astrocytes and microglia in healthy subjects and SMA patients
In a neurodegenerative disorder such as SMA, there is growing evidence that astrocytic functions are disrupted, giving rise to the hypothesis that astrocytes could contribute to the disease process. In fact, during disease progression, astrocytes can become reactive, exhibiting both morphological and functional changes. McGivern et al. [30] recently demonstrated that astrocytes in the SMAΔ7 mouse spinal cord and even astrocytes derived from SMA-induced pluripotent stem cells show morphological and cellular alteration indicative of activation before the motor neuron loss. This indicates that there may be a direct consequence of SMN deficiency intrinsic to astrocytes, and that disruption of their function may be due to this loss rather than because of altered neuronal-glial signaling. No significant differences were reported between wild-type (WT) and SMA mouse astrocytes in early post-natal days, but at post-natal day 9 SMA astrocytes exhibited augmented cell bodies, increased expression of GFAP in the cytoplasm, and thin retracted processes. Moreover, reactive astrocytes produce pro-inflammatory cytokines, which can trigger an apoptotic cascade and motor neuron loss, in particular through ERK1 and ERK2, which are members of the MAPK signaling pathway [30]. McGivern et al. [30] have hypothesized that increased phosphorylation of ERK1/2 observed in SMA iPSC-derived astrocytes, can have a direct role in apoptosis’ induction through an increased expression of pro-inflammatory and pro-apoptotic cytokines such as TNFα, IL-1, and IL-6 [60–62]. In the context of growth factor deprivation, the same group also showed reduced secretion of GDNF in SMA iPSC-derived astrocyte cultures, which can be an indirect signal for the activation of the apoptotic pathway involving ERK. Astrocyte communication with other astrocytes and neurons can also take place through gap junctions, adenosine triphosphate (ATP) release, and calcium-dependent glio-transmission [63]. In a cellular model, McGivern et al. [30] found that there is a dysregulation of basal calcium and decreased response to ATP stimulation, confirming abnormal astrocyte function (Fig. 1). Rindt and colleagues examined the involvement of SMN deficiency specifically in astrocytes in post-mortem human tissue and in vivo models of SMA. They reported prominent astrogliosis in end stage SMA mice as well as post-mortem patient spinal cords. Importantly, restoration of SMN protein levels in astrocytes, using a viral vector-based approach, resulted in increased survival in both severe and intermediate models of SMA. In addition to an improvement of neuromuscular circuitry, the increased expression of pro-inflammatory cytokines was partially normalized in treated mice, suggesting that astrocytes directly contribute to the pathogenesis of SMA [64].
It is important to note that some groups have demonstrated that motor neuron loss is detectable only at the end stage of SMA [22, 23]. As is commonly observed in other neurodegenerative diseases, the earliest structural defects appear distally, involving the neuromuscular synapse in the case of SMA. Prior to death of the motor neuron, there are presynaptic defects that include loss of terminal arborization as well as intermediate filament aggregation, which causes intermittent neurotransmission failures [65]. For this reason, it may be inferred that MN death is a late event in SMA pathogenesis, preceded by the activation of astrocytes leading to secondary motor neuron degeneration [56]. As discussed above, cellular studies have demonstrated that astrocytes participate in the regulation of synaptic function by providing an optimal presynaptic environment, triggering synaptic maturation, and maintaining synaptic activity and stability [66]. In neurodegenerative diseases, their function becomes deregulated, leading to the loss of central synapses, as showed by Tarabal et al. [67] (Table 1).
Overall, there is growing evidence suggesting that although the expression of SMN in motor neurons is essential, its restoration is not sufficient to significantly enhance survival in mouse models of SMA. When SMN1 is expressed highly in both astrocytes and MNs, survival is maximized [26, 30]. These data implicate astrocytes in the pathogenesis of SMA and indicate that astrocytes could be an additional target for therapeutic intervention.
Microglia
Microglia represent the endogenous immune cells in spinal cord and in brain, and mediate the innate immune response in the CNS to various types of pathogenic insults. Microglia are derived from hematopoietic stem cells, particularly precursors of the monocyte and mesodermal lineages. Micro and macroglial cells have an important role in the formation, function, and elimination of synapses under normal and pathologic conditions (Fig. 1) [68]. Neuroinflammation plays a key role in the pathology of several neurodegenerative diseases, such as Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, and amyotrophic lateral sclerosis (ALS) [69–74]. In the case of SMA, microglial activation is observed early in the patient’s spinal cord [67]; however the pathological role of microglial cells in SMA has not yet been fully characterized. Recently, Tarabal et al. [67] (Table 1) detected activated microglia in lumbar spinal cord at different stages of a murine model of SMA [early post-natal, presymptomatic (P4–5), early symptomatic (P7–8), and end stage (P14–15)]. Immunoreactivity for the specific microglial marker, IbaI, was markedly increased, surrounding motor neurons in the ventral horn, in a time-dependent fashion in the lumbar spinal cord of SMN∆7 in comparison to WT animals. This increase was significant at P7–8, and persisted until the late stages of the disease. Moreover, in agreement with previous studies, they demonstrated an increased loss of glutamatergic terminals in SMN∆7 spinal cord during post-natal development [18, 24, 25, 75]. Importantly, in agreement with the findings of Mentis [25], but in contrast to Ling and colleagues, the study of Tarabal et al. [67] showed that the damage of central synapses in SMA precedes MN death, as discussed above. In contrast, Ling et al. [24] showed that while glutamateric terminals on motor neurons are reduced in SMA mice, this occurs in the absence of microgliosis in the spinal cord; leading these authors to conclude that neuroinflammation is not a major contributing factor to the disease mechanism in models of severe SMA [18].
The phagocytic function of microglia cells for the clearance of degenerating neurons has not been fully considered in the past, but it is an increasingly recognized phenomenon in many neurodegenerative diseases [76]. This activity of microglia is also involved in synaptic elimination. In a mouse model of SMA, active microglial cells were detected engulfing structural complexes including damaged presynaptic terminals and postsynaptic dendrites [67]. These findings do not exclude the relevance of this cell type to the elimination of cellular fragments during synapse degeneration [67]. Examination of SMA post-mortem spinal cord reveals a severe loss of myelinated fibers with numerous glial bundles in the anterior horns [77]. These glial bundles are most abundant in the lumbar regions and are observed only in the anterior spinal roots and not in the posterior roots. Glial bundles are characteristic, but not specific, to SMA and it is hypothesized that they are secondary to neuronal degeneration, rather than causing the degeneration of anterior horn cells [77]. Another interesting finding raised in this study is that the loss of GABAergic and glutamatergic synapses on MNs could result from the induction of neuronal nitric oxide synthase (nNOS), which is upregulated in SMN∆7 MNs [67]. It is known that nitric oxide (NO) can induce synaptic degeneration following a physical injury to motor nerves; and is also found in ALS [78]. Moreover, NO produced via up-regulation of nNOS could lead to activation of the RhoA/Rho kinase (ROCK) pathway [79]. This pathway is abnormally activated in the SMA mice, and ROCK inhibitor treatment improves maturation of the NMJ, and increases survival in treated mice [80].
Other cell types outside the central nervous system involved in SMA
Peripheral nervous system involvement: Schwann cells
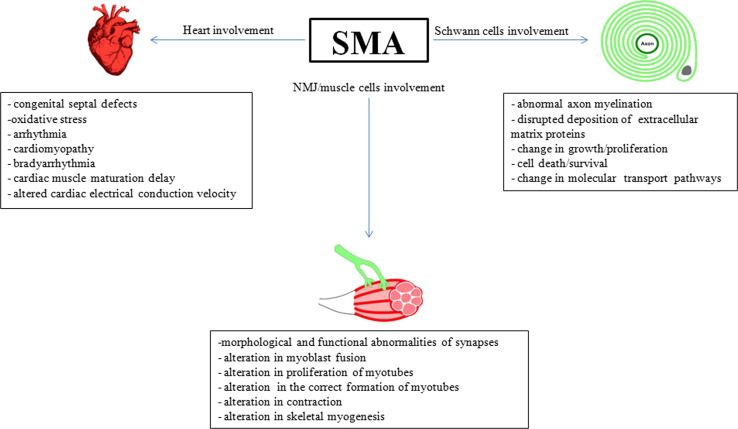
Schwann cells are the main glial cells of the peripheral nervous system (PNS). There are two types of Schwann cells, myelinating and non-myelinating, that are essential both to axon stability and neuronal survival, notably through the production of proteins involved in the formation and maintenance of the extracellular matrix (ECM) [81, 82]. In addition to this, myelinating Schwann cells also form the protective myelin sheath wrapping around axons of motor and sensory neurons, which allows a substantial increase in nerve conduction velocity.
Myelinating Schwann cell dysfunction is known to play a crucial role in hereditary peripheral neuropathies that are usually associated with axonal atrophy and degeneration of lower motor and sensory neurons [83]. More recently, low levels of SMN were reported to cause changes in Schwann cells that lead to abnormal axon myelination and interrupted the deposition of extracellular matrix proteins in peripheral nerves (Fig. 2) [84]. In this study, Hunter et al. demonstrated that Schwann cells, isolated from SMA mice, had reduced levels of SMN as expected, but did not respond normally to differentiation signals in culture. Concurrently, they observed morphological imperfections in myelination in SMA mice that were associated with alterations in the expression of myelin-associated proteins, such as peripheral myelin protein 22 (PMP22), myelin protein zero (MPZ), and myelin basic protein (MBP) [85]. They further demonstrated that primary Schwann cells derived from SMA transgenic mice cannot successfully myelinate healthy neurons in co-culture, and that they exert a detrimental influence on neuritic stability. One possible explanation for these changes could be that low levels of SMN impair the regulation of expression of myelin proteins, which normally occurs during post-natal development [84]. By genetically correcting SMN levels in Schwann cells, Hunter et al. showed that these defects were SMN-dependent and reversible. Alternatively, these myelination defects could also be explained by the reduced capacity of SMA-derived Schwann cells to produce key proteins composing the ECM of peripheral nerves. This is supported by other studies showing that the expression levels of several ECM proteins, including laminin alpha 2 (LAMA2), are severely altered in the context of SMN deficiency [86, 87]. Consistent with this, it was shown that impairment of extracellular laminin-mediated control of beta-actin translation is contributing to SMA motor neuron axonal defects [88].
Fig. 2.
The effects of SMA on non-motor neuronal tissue
To understand how SMN deficiency can disrupt Schwann cell physiological functions, Aghamaleky Sarvestany et al. [89] performed label-free proteomics analyses of Schwann cells obtained from SMA mouse peripheral nerve. Through this approach, they found that several pathways were altered in SMA Schwann cells including growth, proliferation, cell death, survival, and molecular transport. Of particular interest, functional cluster analysis revealed that ubiquitination pathways are profoundly disrupted in SMN-deficient Schwann cells. For example, reduced levels of ubiquitin-like modifier activating enzyme 1 (uba1) were observed [89]. In fact, suppression of Uba1 through pharmacological treatment in WT Schwann cells is sufficient to recapitulate the defective myelination phenotype presented by SMA Schwann cells [89]. Microarray analyses of Schwann cell myelination-related genes revealed disrupted gene expression in tissue from SMA mice in comparison to WT [86, 87].
Altogether, these data highlight the important role of the SMN protein in regulating the ubiquitination pathway and maintaining the homeostasis of Schwann cells.
Neuromuscular junction and muscle cell involvement
Longitudinal studies in a severe mouse model of SMA have revealed that early symptomatic stages of the disease are characterized by morphological and functional anomalies at the synapse; affecting both NMJs [23, 24, 65, 75, 90, 91] and synaptic inputs to motor neurons in the spinal cord [24, 25]. To understand the underlying mechanisms of this early synaptic pathology in SMA, Martinez and colleagues have recently generated three lines of SMA mice that express increased levels of SMN in motor neurons and/or muscle [27]. In this study, they showed that the increase of SMN expression in motor neurons improved synaptic function both at the NMJ and motor neuron somas, whereas selective over-expression of SMN in muscle restores muscle mass without affecting synaptic integrity [27]. They concluded that SMN could have a role in the growth of muscle that is independent of its effect on motor neuron synaptic function; suggesting that SMN has multiple roles in the motor neuron unit (Fig. 2).
Some studies have reported that high levels of SMN expression in muscle can affect the SMA phenotype in Drosophila melanogaster [5, 92]. To investigate whether this is also the case in mammals, Gavrilina et al. [15] have used transgenic SMA mouse models in which SMN expression is restored in skeletal muscle fibers under the human skeletal actin (HSA) promoter [93–95] and in neurons under the prion promoter (PrP) [96], which is also known to lead to high expression in astrocytes [15, 96]. In this study, both lines were null for the murine SMN gene, and homozygous for the human SMN2 gene. They found that full-length SMN expressed under the PrP promoter had a strong beneficial impact on survival and phenotype in the severe SMA mouse [15]. In contrast, restoration of SMN expression in muscle alone was unable to correct the phenotype of the disease. This confirms that re-establishment of SMN expression in neurons, and possibly astrocytes, is required for maximal disease-modifying effect in SMA [15].
Many studies have reported a direct or indirect interaction of SMN with molecular modulators of actin dynamics such as plastin 3, profilin, small Rho GTPases Cdc42, and RhoA, as well as ROCK, a direct downstream effector of RhoA [97–100]. If activity of the RhoA/ROCK pathway is increased in SMN-depleted neuronal cells and tissue, inhibition of actin-mediated neuronal outgrowth and differentiation is observed. Conversely, when SMA mice are treated with ROCK inhibitors, they show an improvement of skeletal muscle mass and NMJ morphological maturation and an accompanying increase in life span [80, 101] (Table 1), with no change in the number of surviving motor neurons or in the expression of SMN protein [102].
SMN has also been implicated in myoblast fusion, proliferation, and in the correct formation of myotubes [103–107] (Fig. 2; Table 1). Among its many functions, the RhoA/ROCK pathway is also involved at multiple points, regulating also muscle contraction [108] and skeletal myogenesis [109]. It is possible that modulation of myogenic regulatory factors as well as actin-dependent myogenesis via regulation of ROCK signaling is sufficient to improve survival in mouse models of SMA. This raises the possibility that, to ameliorate disease progression, a modulation of myogenic regulatory factors and an actin-dependent regulation of myogenesis might be considered as a therapeutic target [102].
Altogether, these data demonstrate that the expression of high levels of SMN in neurons and astrocytes contribute to myofiber growth and development. Regardless for the full restoration of muscle fibers in severe form of SMA it is necessary to target other muscle cells, such as the satellite cells, which are crucial in the maintenance and regeneration of the muscle mass.
This possibility is supported by the significant effects of ROCK inhibitors in SMA mice, which bypass the replacement of SMN full-length protein by targeting downstream pathogenic events.
Other targets, independent of efforts to enhance SMN protein levels in muscle tissue, have been explored as possible disease-modifying approaches. Rose and collaborators [110] investigated the role of follistatin, a cystine-rich glycoprotein that binds to and inhibits several TGF-β family members [23, 24], including myostatin [5, 17, 25], in SMA progression. They demonstrated that recombinant human follistatin enhances muscle mass in WT and SMA mice. This finding suggests that the administration of follistatin and the subsequent inhibition of the myostatin pathway are beneficial in SMA models as they preserve the expression of muscle-derived neurotrophic factors in SMA mice.
Recently, our group [111] examined quadriceps muscle biopsies from 24 patients with genetically documented SMA, and paraspinal muscle biopsies from three patients with SMA-II. We found that cytochrome-c oxidase (COX) deficiency was more evident in muscle from patients <with SMA-I and SMA-II compared to healthy controls, and that muscle mtDNA content and citrate synthase activity were also reduced in all two SMA types. Our results strongly support the conclusion that an altered regulation of myogenesis and downregulated mitochondrial biogenesis contribute to pathologic changes in the muscle of patients with SMA [111].
Motor neuron survival and neuroprotection
A substance beneficial for motor neuron survival is insulin-like growth factor I (IGF-1). Shababi and colleagues also reported that the addition of the neurotrophic factor IGF-1 determine neuroprotection and SMN induction providing greater protection in an SMA animal model. They demonstrated that an intracerebroventricular injection of the trans-splicing/IGF vector significantly increased SMN protein in brain and spinal cord of SMAΔ7 mice and lessened the severity of disease as evidenced by an extension of life span and increased body mass [112].
Tsai and colleagues evaluated the efficacy of intravenous administration of a recombinant AAV1 vector encoding human insulin-like growth factor-1 (IGF-1) in a severe mouse model of SMA. Affected mice treated with AAV1-IGF-1 on post-natal day 1 exhibited reduced motor neuron degeneration, cardiac, and muscle atrophy as well as a greater extent of innervation at the neuromuscular junctions compared to untreated transgenic mice. Moreover, they demonstrated that treated animals had prolonged lifespan, increased body weight, and improved motor coordination. Importantly, IGF-1 over-expression led to an increase in SMN protein levels in multiple tissues including spinal cord and muscle [113]; although the relevant site of action of IGF-1 for boosting SMN levels was not determined.
Vascular development and hypoxia of motor neurons
Somers et al. [114] investigated whether vascular defects are involved in severe forms of SMA. They found that, in both mouse models and patients, abnormalities in vascular development lead to functional hypoxia of motor neurons. In particular, mouse models showed an aberrant vasculature in post-natal SMA proximal skeletal muscle and similar vascular changes were present in SMA patients. Next, they moved their attention to the spinal cord, trying to find a correlation between the vascular changes and the loss of motor neuron cell bodies, which was probably caused by a defect in angiogenesis, leading to hypovascularity and thus increasing the diffusion distances between capillaries and motor neurons in the ventral grey horn.
The study also showed that, in mice, capillaries at birth are normal, but fail to develop properly either in spinal cord or in proximal skeletal muscles and these results are mirrored by the human phenotype. Capillaries seem to be not only reduced in number, but it is also shown that Claudin-5, a component of endothelial cell tight junctions, is not completely localized at blood vessels, particularly in small caliber vessels, thus contributing to incomplete post-natal development and maturation of the blood–spinal cord barrier (BSCB) that in turn do not acquire its full normal function.
All these evidences point out the hypothesis that vascular defect-induced hypoxia could generate further damage to motor neuron, not only directly but also by damaging BSCB [115].
These events create a link between vascular pathology and the particular vulnerability of the neuromuscular system in SMA, suggesting that the vascular system could be a novel therapeutic target in order to ameliorate functional hypoxia and BSCB defects.
Immune system dysfunction in SMA
The extent of involvement of the immune system in SMA has not been studied in depth to this point. An original study from Dachs et al. [116] investigated the stimulation of apoptosis and macrophage recruitment in SMA muscles. Surprisingly, macrophage infiltration in SMA muscle was approximately 25–50 % lower in comparison to healthy controls. This observation was tissue-specific, as no differences were observed in the liver and spleen between WT and SMA mice [116]. The authors concluded that the muscle-specific reduction in macrophage infiltration results from a primary defect in the immune system of severe SMA mice, due to a specific atrophy of immune organs, such as spleen and thymus. Similar alterations, such as spleen atrophy and systemic immune aberrations were previously reported in murine models of ALS [117]. Considering the pathologic alteration identified in the immune system organs of SMA animals, this field of study will require further attention in SMA patients.
Cardiac involvement
The presence of heart alterations have been reported in the most severe forms of SMA; caused either by congenital anomalies manifesting during cardiogenesis [118–122], or secondary to autonomic nervous system defects [123–128]. Many authors suggest that in juvenile cases of SMA, in particular for SMA types I and II, the presence of cardiac involvement is likely secondary to chronic respiratory insufficiency, which is a common feature of the disease. Investigators hypothesized that ventricular arrhythmia, bundle-branch, and atrioventricular blocks are provoked by pulmonary and respiratory defects, highlighting the importance of respiratory assistance in preventing the onset of cardiological alterations [119, 120, 122, 127–129]. While previous clinical reports of SMA I patients did not explicitly determine if the cardiac defects were secondary to respiratory distress; recently, however, congenital heart anomalies are being described more frequently upon autopsy and include: dilated right ventricle, atrial and ventricular septal defects. The most common congenital heart defect is an anomalous development of the heart, referred to as hypoplastic left heart syndrome [121, 124, 130]. Analysis performed by Rudnik-Schoneborn and collaborators support a crucial role for SMN protein in cardiac development. In fact, they found that among patients with a single copy of SMN2, the incidence of congenital septal defects is approaching 75 %; whereas among people without SMA, its frequency is closer to 1 in 50 million [121].
Preclinical studies in animal models of SMA have confirmed the presence of cardiac dysfunction [8, 10, 131–133]. Shababi et al. [133] compared the very severe model of SMA (Smn−/−, SMN2+/+) and the well-established severe model for SMA, SMAΔ7 model (Smn−/−, SMN2+/+; SMNΔ7+/+), to examine the role of SMN protein in cardiogenesis and the contribution of heart anomalies to the SMA pathological phenotype. In the very severe model, as early as the embryonic stage, there was evidence of cardiac remodeling, well in advance of motor neuron loss. In the SMAΔ7 mouse model, similar structural heart defects have been described in the early post-natal period. The authors suggested that oxidative stress could lead to cardiac fibrosis, which starts at the time of symptom onset and increases significantly in the following days (Fig. 2) [10, 134].
In addition to structural defects, there is increasing evidence that arrhythmia and cardiomyopathy are widespread in SMA patients. In light of this, Heier et al. [8] investigated whether such phenotypes are also present in SMA mouse models. Transgenic mice developed a severe bradyarrhythmia characterized by heart block and reduced ventricular depolarization as early as 2 days of age, before the onset of neuromuscular symptoms (Fig. 2). However, at later stages of the disease, dilated cardiomyopathy as well as sympathetic innervation defects are involved in heart failure. Moreover, mice treated with trichostatin A, a histone deacetylase inhibitor, showed improved heart rate and cardiac size. An improvement in neuromuscular function was also noted; however, affected mice died as a result of persistent bradyarrhythmia. This work further strengthens the notion that the involvement of cardiac arrhythmia is an early and progressive feature of SMA [8].
Previously, Foust et al. [135] demonstrated that early post-natal delivery of SMN1 in SMNΔ7 mice using self-complementary AAV type 9 (scAAV9) improves both life span and neuromuscular function. Based on these findings, Bevan et al. [131] studied the effects of scAAV9 treatment on heart defects in the SMNΔ7 mouse model. They reported that treatment with scAAV9-SMN, also at the early post-natal stage, prevented bradycardia and the early development of dilated cardiomyopathy. They hypothesized that the success of this treatment was not only due to the targeting of spinal motor neurons, for which this subtype of AAV has a high tropism, but also to the transduction of autonomic nervous system neurons. However, as treated animals still exhibited contractility impairment, additional mechanisms, potentially initiated prior to the administration of the virus, they are also involved in SMA-related heart defects. In fact, because the delivery of SMN1 was performed post-natally, it is difficult to conclude whether the remaining heart defects are due to embryonic autonomic nervous system dysfunction, or other cardiogenesis deficits [131]. Addressing this question, in 2012, Biondi et al. [132] showed that cardiac alterations in type 2 SMA transgenic mice are largely caused by intrinsic heart defects and not by autonomic impairment. They examined the effects of physical exercise on cardiorespiratory function in this SMA mouse model and observed many benefits, including a reduction in the delay of cardiac muscle maturation, a lessening of fibrosis, enhancement of cardiac electrical conduction velocity, and a decrease in arrhythmias and bradycardia; resulting in a partial restoration of cardiac function. In addition, their data show that the sympathetic system contributes to the amelioration of arrhythmia and bradycardia in this model [10, 132].
Also Shababi treated SMN∆7 model using scAAV9 expressing the full-length SMN cDNA. They showed that fibrosis, oxidative stress activation, vascular remodeling, and a significant decrease in the number of capillaries exist in the SMA heart. The cardiac structural defects were improved in the treated animals, however, the level of impairment was still significant compared to the age-matched wild-type littermates. Functional analysis by in vivo cardiac magnetic resonance imaging revealed that the heart of the treated SMA mice still exhibits functional defects. They conclude that cardiac abnormalities are only partially rescued in post-birth treated SMA animals and these abnormalities may contribute to the premature death of vector-treated SMA animals with seemingly rescued motor function but an average life span of less than 70 days as reported in several studies [134].
In addition to direct effects on the structure and function of the heart itself, investigators have observed abnormalities in the vascular system of infants affected by SMA. Autonomic dysfunction and, particularly sympathetic dysfunction, has been hypothesized to be a primary source of the vascular perfusion abnormalities in SMA [136]. Vascular defects include coolness and poor perfusion of distal extremities; and occasional digital necrosis has been reported in SMA type I children [123]. Because vascular and innervation patterning are closely related, it is intuitively evident that there is an integral and codependent relationship between innervation and vascularization in motor neuron disease [137]. This occurs in particular in congenital or early infantile onset SMA when the growth and development of the vascular network is critical.
Taken together, the findings from multiple preclinical in vivo studies and the emerging clinical findings in SMA patients, suggest a key role of SMN protein in cardiogenesis, providing a new area of investigation that could be essential for optimal clinical success in the treatment of SMA.
Bone, pancreas, liver system and gastro-intestinal tract involvement
The impact of SMN deficiency in bone, intestine, pancreas, and liver has also been documented. It is known that for patients affected by SMA as well as in the various SMA animal models, there is a high incidence of fractures and hypercalcemia, indicative of a role of SMN protein in bone function. In particular, children affected by SMA often suffer from severe osteopenia and fractures after a minimal trauma. In the most severe cases, congenital bone fractures and thinner ribs have been reported [138]. Moreover, a decrease of bone mineral density with increasing age in SMA patients has also been described [139]. Recently, a study revealed a key role of SMN protein in skeletal development critically dependent upon an interaction between SMN protein and osteoclast stimulatory factor. It was hypothesized that osteoclast stimulatory factor and SMN play a joint role in the cellular signal transduction cascade regulating the release of factors that stimulate skeletal development [140].
Although the pathological impact of SMN deficiency in digestive system is not entirely recognized as cause of death in SMA, alterations in the intestine, pancreas, and liver have been reported in SMA patients and animal models.
Patients with SMA type II and type IV show severe constipation [141, 142] and reduction in term of neural tissue in small intestine and colon [143].
Gombash et al. [144] employed two mouse models of SMA to determine whether functional gastro-intestinal complications are a direct consequence of SMN deficiency or a secondary event. With this study they demonstrated that SMN deficiency in enteric nervous system (ENS) neurons caused constipation, delayed gastric emptying, slow intestinal transit, and reduced colonic motility (similar to what is reported in SMA patients), in the absence of gross anatomical or histopathological abnormalities. In particular, SMN deficiency led to disrupted ENS signaling to the smooth muscle of the colon but did not cause enteric neuron loss. These findings indicate that the alterations in the autonomic nervous system contribute to intestinal dysfunctions [20].
Regarding pancreas, occasional cases of pathological defects associated with SMA have been reported; in particular, diabetes and alterations in glucose metabolism in SMA Type II and III patients [145], and acute pancreatitis in SMA Type I patients [146]. The SMN ± mouse model displayed abnormal localization of glucagon-producing α-cells within the pancreatic islets and increased number of insulin-producing β cells, which caused hyperinsulinemia and increased hepatic glucagon sensitivity. Since these dysfunctions appeared after the onset of symptoms, the pathological pancreatic phenotype seems to be the direct result of SMN deficiency in the pancreas and independent of the neuronal phenotype SMA [147].
The importance of SMN protein in the development and function of the liver was demonstrated by Vitte et al. [148] using the Δ7 SMA in vivo model. In this study, a mutation in exon 7 of murine Smn induced liver failure and late embryonic lethality [148]. Moreover, subcutaneous injection of therapeutic oligonucleotide (ASO), which restores SMN protein levels, increased IGF-1 levels in the liver and rescued the pathological phenotype [9], highlighting the involvement of liver in SMA. However, is still not fully clear whether the lack of SMN has a direct or indirect effect on metabolic abnormalities [20].
Among the metabolic defects, abnormal fatty acids metabolism is the most common defect reported in patients SMA type II [149–151]. In particular, SMA patients suffer of mild or moderate dicarboxylic aciduria [149, 150] and increased level of carnitine esterified [149].
Normal levels of ketone bodies, which are secondary products of fatty acids mitochondrial β-oxidation in the liver, indicate a specific defect in fatty acid metabolism of muscles rather than in the liver [20].
In order to investigate whether a fatty acid metabolism defect is a consequence of muscle denervation and atrophy, fatty acid levels in the plasma of severe SMA infants were measured and compared with control patients. The investigators found an increased ratio of dodecanoic acid to tetradecanoic acid in all SMA patients. These data suggest that the fatty acid metabolism dysfunction in SMA is not the consequence of SMA-related immobility, systemic illness, muscle denervation, or muscle atrophy. Rather these abnormalities may be directly related to the loss of SMN1 or neighboring genes [150]. For this reason, the evaluation of lipid content in the liver will be informative with respect to the effects of SMN deficiency on metabolic abnormalities [20].
Conclusions
Currently, there are no effective treatments available for SMA. There are, however, multiple ongoing clinical trials showing promising efficacy in ameliorating certain disease phenotypes. Historically, for SMA as in other neurodegenerative diseases, neuronal degeneration was widely considered to be strictly cell autonomous and the sole contributor to the clinical expression of the disease. However, the past few years have seen the accumulation of an overwhelming body of evidence that other cell types in the CNS, such as astrocytes, microglia, and sensory neurons; and even outside the CNS, such as Schwann cells, muscle cells or heart, can be key components in the disease initiation and/or progression, as well as in the emergence of clinical symptoms. Here, we have reviewed and discussed the increasingly recognized involvement of these different systems in SMA which can have great consequences for the development of optimal therapeutic strategies for this aggressive disease.
Until recently, astrocytes and microglia were thought to be solely involved in the secondary response to a primary neuronal injury, via a process known as “reactive gliosis”. While the pathologic roles of glial cells in SMA have not been characterized as extensively as in other motor neuron diseases such as ALS, numerous alterations in SMA astrocytes and microglia could potentially initiate neurodegeneration as well as propagate injury. For example, restoration of SMN expression both in motor neurons and in astrocytes is necessary to greatly extend animal survival in a mouse model of SMA. In keeping with this, astrocytes and microglia should be thought as prime additional targets for the development of therapeutic interventions. Similarly, the roles of sensory neurons and Schwann cells have also recently gained prominence in the pathogenesis of SMA. In fact, several clinical observations made in type I SMA patients have indicated that these cells are also affected. In addition, other tissues outside the CNS contribute to the overall phenotype of SMA pathology. For example, several preclinical studies and clinical findings in SMA patients suggest that SMN protein plays an important role in cardiogenesis; these findings provide a new field of investigation that could be helpful to improve clinical success in the treatment of SMA.
Thus, while it is likely that motor neurons have an increased vulnerability to SMN deficiency, the involvement of different tissue in SMA pathology, inside and outside the CNS, warrants further investigation. Currently, the accumulating evidence concerning the involvement of cell types other than motor neurons is creating a paradigm shift in SMA and many other neurodegenerative diseases; what should we target, when, how and for how long? As discussed in this review, a first important point regarding the future development of genetic therapy is to consider that restoration of SMN expression in both central compartments (neurons/astrocytes) and peripheral compartments (muscle/Schwann cells) will be essential for an ideal disease-modifying treatment for SMA. Thus, while it is now largely recognized that each cell type may require different levels of SMN to exert its proper biological role, the regulation of SMN levels within different tissues and systems during development in animal models and humans remains to be investigated. The elucidation of levels of SMN that are required, regionally and temporally, is central to the development of successful therapies (genetic or otherwise), not only for the most severe SMA patients but also for the milder type 2 and 3 SMA patients who appear to be much less responsive to SMN-restorative strategies than Type 1 patients. In keeping with this, unraveling the molecular pathways in SMA-relevant cell types that are defective after the SMN requirement window has passed, may open new therapeutic strategies downstream of SMN, which could be more beneficial to milder SMA patients with later onset. These are among the critical questions that future translational research must address to finally make a cure for SMA a reality.
Acknowledgments
Is gratefully acknowledged the financial support from the following research Grants: FP7-IRSES: “No-MND”.
Footnotes
D. Papadimitriou, D.B. Re and S. Corti are co-senior authors.
References
- 1.Monani UR. Spinal muscular atrophy: a deficiency in a ubiquitous protein; a motor neuron-specific disease. Neuron. 2005;48(6):885–896. doi: 10.1016/j.neuron.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Sumner CJ. Molecular mechanisms of spinal muscular atrophy. J Child Neurol. 2007;22(8):979–989. doi: 10.1177/0883073807305787. [DOI] [PubMed] [Google Scholar]
- 3.Zanetta C, Nizzardo M, Simone C, Monguzzi E, Bresolin N, Comi GP, Corti S. Molecular therapeutic strategies for spinal muscular atrophies: current and future clinical trials. Clin Ther. 2014;36(1):128–140. doi: 10.1016/j.clinthera.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Zanetta C, Riboldi G, Nizzardo M, Simone C, Faravelli I, Bresolin N, Comi GP, Corti S. Molecular, genetic and stem cell-mediated therapeutic strategies for spinal muscular atrophy (SMA) J Cell Mol Med. 2014;18(2):187–196. doi: 10.1111/jcmm.12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burghes AH, Beattie CE. Spinal muscular atrophy: why do low levels of survival motor neuron protein make motor neurons sick? Nat Rev Neurosci. 2009;10(8):597–609. doi: 10.1038/nrn2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harding BN, Kariya S, Monani UR, Chung WK, Benton M, Yum SW, Tennekoon G, Finkel RS. Spectrum of neuropathophysiology in spinal muscular atrophy type I. J Neuropathol Exp Neurol. 2015;74(1):15–24. doi: 10.1097/NEN.0000000000000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khera S, Ghuliani R. Type 0 spinal muscular atrophy with multisystem involvement. Indian Pediatr. 2014;51(11):923–924. doi: 10.1007/s13312-015-0557-6. [DOI] [PubMed] [Google Scholar]
- 8.Heier CR, Satta R, Lutz C, DiDonato CJ. Arrhythmia and cardiac defects are a feature of spinal muscular atrophy model mice. Hum Mol Genet. 2010;19(20):3906–3918. doi: 10.1093/hmg/ddq330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hua Y, Sahashi K, Rigo F, Hung G, Horev G, Bennett CF, Krainer AR. Peripheral SMN restoration is essential for long-term rescue of a severe spinal muscular atrophy mouse model. Nature. 2011;478(7367):123–126. doi: 10.1038/nature10485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shababi M, Habibi J, Yang HT, Vale SM, Sewell WA, Lorson CL. Cardiac defects contribute to the pathology of spinal muscular atrophy models. Hum Mol Genet. 2010;19(20):4059–4071. doi: 10.1093/hmg/ddq329. [DOI] [PubMed] [Google Scholar]
- 11.Swoboda KJ. SMN-targeted therapeutics for spinal muscular atrophy: are we SMArt enough yet? J Clin Investig. 2014;124(2):487–490. doi: 10.1172/JCI74142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lorson CL, Strasswimmer J, Yao JM, Baleja JD, Hahnen E, Wirth B, Le T, Burghes AH, Androphy EJ. SMN oligomerization defect correlates with spinal muscular atrophy severity. Nat Genet. 1998;19(1):63–66. doi: 10.1038/ng0598-63. [DOI] [PubMed] [Google Scholar]
- 13.D’Amico A, Mercuri E, Tiziano FD, Bertini E. Spinal muscular atrophy. Orphanet J Rare Dis. 2011;6:71. doi: 10.1186/1750-1172-6-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schrank B, Gotz R, Gunnersen JM, Ure JM, Toyka KV, Smith AG, Sendtner M. Inactivation of the survival motor neuron gene, a candidate gene for human spinal muscular atrophy, leads to massive cell death in early mouse embryos. Proc Natl Acad Sci USA. 1997;94(18):9920–9925. doi: 10.1073/pnas.94.18.9920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gavrilina TO, McGovern VL, Workman E, Crawford TO, Gogliotti RG, DiDonato CJ, Monani UR, Morris GE, Burghes AH. Neuronal SMN expression corrects spinal muscular atrophy in severe SMA mice while muscle-specific SMN expression has no phenotypic effect. Hum Mol Genet. 2008;17(8):1063–1075. doi: 10.1093/hmg/ddm379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sohal DS, Nghiem M, Crackower MA, Witt SA, Kimball TR, Tymitz KM, Penninger JM, Molkentin JD. Temporally regulated and tissue-specific gene manipulations in the adult and embryonic heart using a tamoxifen-inducible Cre protein. Circ Res. 2001;89(1):20–25. doi: 10.1161/hh1301.092687. [DOI] [PubMed] [Google Scholar]
- 17.Gogliotti RG, Quinlan KA, Barlow CB, Heier CR, Heckman CJ, Didonato CJ. Motor neuron rescue in spinal muscular atrophy mice demonstrates that sensory-motor defects are a consequence, not a cause, of motor neuron dysfunction. J Neurosci. 2012;32(11):3818–3829. doi: 10.1523/JNEUROSCI.5775-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park GH, Maeno-Hikichi Y, Awano T, Landmesser LT, Monani UR. Reduced survival of motor neuron (SMN) protein in motor neuronal progenitors functions cell autonomously to cause spinal muscular atrophy in model mice expressing the human centromeric (SMN2) gene. J Neurosci. 2010;30(36):12005–12019. doi: 10.1523/JNEUROSCI.2208-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamilton G, Gillingwater TH. Spinal muscular atrophy: going beyond the motor neuron. Trends Mol Med. 2013;19(1):40–50. doi: 10.1016/j.molmed.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Shababi M, Lorson CL, Rudnik-Schoneborn SS. Spinal muscular atrophy: a motor neuron disorder or a multi-organ disease? J Anat. 2014;224(1):15–28. doi: 10.1111/joa.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGovern VL, Gavrilina TO, Beattie CE, Burghes AHM. Embryonic motor axon development in the severe SMA mouse. Hum Mol Genet. 2008;17(18):2900–2909. doi: 10.1093/hmg/ddn189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le TT, Pham LT, Butchbach ME, Zhang HL, Monani UR, Coovert DD, Gavrilina TO, Xing L, Bassell GJ, Burghes AH. SMNDelta7, the major product of the centromeric survival motor neuron (SMN2) gene, extends survival in mice with spinal muscular atrophy and associates with full-length SMN. Hum Mol Genet. 2005;14(6):845–857. doi: 10.1093/hmg/ddi078. [DOI] [PubMed] [Google Scholar]
- 23.Kong L, Wang X, Choe DW, Polley M, Burnett BG, Bosch-Marce M, Griffin JW, Rich MM, Sumner CJ. Impaired synaptic vesicle release and immaturity of neuromuscular junctions in spinal muscular atrophy mice. J Neurosci. 2009;29(3):842–851. doi: 10.1523/JNEUROSCI.4434-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ling KK, Lin MY, Zingg B, Feng Z, Ko CP. Synaptic defects in the spinal and neuromuscular circuitry in a mouse model of spinal muscular atrophy. PLoS One. 2010;5(11):e15457. doi: 10.1371/journal.pone.0015457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mentis GZ, Blivis D, Liu W, Drobac E, Crowder ME, Kong L, Alvarez FJ, Sumner CJ, O’Donovan MJ. Early functional impairment of sensory-motor connectivity in a mouse model of spinal muscular atrophy. Neuron. 2011;69(3):453–467. doi: 10.1016/j.neuron.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee AJ, Awano T, Park GH, Monani UR. Limited phenotypic effects of selectively augmenting the SMN protein in the neurons of a mouse model of severe spinal muscular atrophy. PLoS One. 2012;7(9):e46353. doi: 10.1371/journal.pone.0046353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martinez TL, Kong L, Wang X, Osborne MA, Crowder ME, Van Meerbeke JP, Xu X, Davis C, Wooley J, Goldhamer DJ, Lutz CM, Rich MM, Sumner CJ. Survival motor neuron protein in motor neurons determines synaptic integrity in spinal muscular atrophy. J Neurosci. 2012;32(25):8703–8715. doi: 10.1523/JNEUROSCI.0204-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imlach WL, Beck ES, Choi BJ, Lotti F, Pellizzoni L, McCabe BD. SMN is required for sensory-motor circuit function in Drosophila. Cell. 2012;151(2):427–439. doi: 10.1016/j.cell.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jablonka S, Karle K, Sandner B, Andreassi C, von Au K, Sendtner M. Distinct and overlapping alterations in motor and sensory neurons in a mouse model of spinal muscular atrophy. Hum Mol Genet. 2006;15(3):511–518. doi: 10.1093/hmg/ddi467. [DOI] [PubMed] [Google Scholar]
- 30.McGivern JV, Patitucci TN, Nord JA, Barabas ME, Stucky CL, Ebert AD. Spinal muscular atrophy astrocytes exhibit abnormal calcium regulation and reduced growth factor production. Glia. 2013;61(9):1418–1428. doi: 10.1002/glia.22522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murray LM, Beauvais A, Bhanot K, Kothary R. Defects in neuromuscular junction remodelling in the Smn(2B/−) mouse model of spinal muscular atrophy. Neurobiol Dis. 2013;49:57–67. doi: 10.1016/j.nbd.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 32.Voigt T. Early effects of carbachol on the morphology of motor endplates of mammalian skeletal muscle fibers. Muscle Nerve. 2010;41(3):399–405. doi: 10.1002/mus.21508. [DOI] [PubMed] [Google Scholar]
- 33.Benkhelifa-Ziyyat S, Besse A, Roda M, Duque S, Astord S, Carcenac R, Marais T, Barkats M. Intramuscular scAAV9-SMN injection mediates widespread gene delivery to the spinal cord and decreases disease severity in SMA mice. Mol Ther. 2013;21(2):282–290. doi: 10.1038/mt.2012.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dominguez E, Marais T, Chatauret N, Benkhelifa-Ziyyat S, Duque S, Ravassard P, Carcenac R, Astord S, Pereira de Moura A, Voit T, Barkats M. Intravenous scAAV9 delivery of a codon-optimized SMN1 sequence rescues SMA mice. Hum Mol Genet. 2011;20(4):681–693. doi: 10.1093/hmg/ddq514. [DOI] [PubMed] [Google Scholar]
- 35.Glascock JJ, Osman EY, Wetz MJ, Krogman MM, Shababi M, Lorson CL. Decreasing disease severity in symptomatic, Smn(−/−); SMN2(+/+), spinal muscular atrophy mice following scAAV9-SMN delivery. Hum Gene Ther. 2012;23(3):330–335. doi: 10.1089/hum.2011.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glascock JJ, Shababi M, Wetz MJ, Krogman MM, Lorson CL. Direct central nervous system delivery provides enhanced protection following vector mediated gene replacement in a severe model of spinal muscular atrophy. Biochem Biophys Res Commun. 2012;417(1):376–381. doi: 10.1016/j.bbrc.2011.11.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Howell MD, Singh NN, Singh RN. Advances in therapeutic development for spinal muscular atrophy. Future Med Chem. 2014;6(9):1081–1099. doi: 10.4155/fmc.14.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Porensky PN, Mitrpant C, McGovern VL, Bevan AK, Foust KD, Kaspar BK, Wilton SD, Burghes AH. A single administration of morpholino antisense oligomer rescues spinal muscular atrophy in mouse. Hum Mol Genet. 2012;21(7):1625–1638. doi: 10.1093/hmg/ddr600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duque SI, Arnold WD, Odermatt P, Li X, Porensky PN, Schmelzer L, Meyer K, Kolb SJ, Schumperli D, Kaspar BK, Burghes AH. A large animal model of spinal muscular atrophy and correction of phenotype. Ann Neurol. 2014 doi: 10.1002/ana.24332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Foust KD, Wang X, McGovern VL, Braun L, Bevan AK, Haidet AM, Le TT, Morales PR, Rich MM, Burghes AH, Kaspar BK. Rescue of the spinal muscular atrophy phenotype in a mouse model by early postnatal delivery of SMN. Nat Biotechnol. 2010;28(3):271–274. doi: 10.1038/nbt.1610. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Hua Y, Sahashi K, Hung G, Rigo F, Passini MA, Bennett CF, Krainer AR. Antisense correction of SMN2 splicing in the CNS rescues necrosis in a type III SMA mouse model. Genes Dev. 2010;24(15):1634–1644. doi: 10.1101/gad.1941310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meyer K, Ferraiuolo L, Schmelzer L, Braun L, McGovern V, Likhite S, Michels O, Govoni A, Fitzgerald J, Morales P, Foust KD, Mendell JR, Burghes AH, Kaspar BK. Improving single injection CSF delivery of AAV9-mediated gene therapy for SMA: a dose-response study in mice and nonhuman primates. Mol Ther. 2014 doi: 10.1038/mt.2014.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mitrpant C, Porensky P, Zhou H, Price L, Muntoni F, Fletcher S, Wilton SD, Burghes AH. Improved antisense oligonucleotide design to suppress aberrant SMN2 gene transcript processing: towards a treatment for spinal muscular atrophy. PLoS One. 2013;8(4):e62114. doi: 10.1371/journal.pone.0062114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Passini MA, Bu J, Roskelley EM, Richards AM, Sardi SP, O’Riordan CR, Klinger KW, Shihabuddin LS, Cheng SH. CNS-targeted gene therapy improves survival and motor function in a mouse model of spinal muscular atrophy. J Clin Investig. 2010;120(4):1253–1264. doi: 10.1172/JCI41615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hua Y, Liu YH, Sahashi K, Rigo F, Bennett CF, Krainer AR. Motor neuron cell-nonautonomous rescue of spinal muscular atrophy phenotypes in mild and severe transgenic mouse models. Genes Dev. 2015;29(3):288–297. doi: 10.1101/gad.256644.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Z, Pinto AM, Wan L, Wang W, Berg MG, Oliva I, Singh LN, Dengler C, Wei Z, Dreyfuss G. Dysregulation of synaptogenesis genes antecedes motor neuron pathology in spinal muscular atrophy. Proc Natl Acad Sci USA. 2013;110(48):19348–19353. doi: 10.1073/pnas.1319280110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rudnik-Schoneborn S, Goebel HH, Schlote W, Molaian S, Omran H, Ketelsen U, Korinthenberg R, Wenzel D, Lauffer H, Kreiss-Nachtsheim M, Wirth B, Zerres K. Classical infantile spinal muscular atrophy with SMN deficiency causes sensory neuronopathy. Neurology. 2003;60(6):983–987. doi: 10.1212/01.WNL.0000052788.39340.45. [DOI] [PubMed] [Google Scholar]
- 48.Lotti F, Imlach WL, Saieva L, Beck ES, le Hao T, Li DK, Jiao W, Mentis GZ, Beattie CE, McCabe BD, Pellizzoni L. An SMN-dependent U12 splicing event essential for motor circuit function. Cell. 2012;151(2):440–454. doi: 10.1016/j.cell.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park KA, Fehrenbacher JC, Thompson EL, Duarte DB, Hingtgen CM, Vasko MR. Signaling pathways that mediate nerve growth factor-induced increase in expression and release of calcitonin gene-related peptide from sensory neurons. Neuroscience. 2010;171(3):910–923. doi: 10.1016/j.neuroscience.2010.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thirumalai V, Behrend RM, Birineni S, Liu W, Blivis D, O’Donovan MJ. Preservation of VGLUT1 synapses on ventral calbindin-immunoreactive interneurons and normal locomotor function in a mouse model of spinal muscular atrophy. J Neurophysiol. 2013;109(3):702–710. doi: 10.1152/jn.00601.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwab AJ, Ebert AD. Sensory neurons do not induce motor neuron loss in a human stem cell model of spinal muscular atrophy. PLoS One. 2014;9(7):e103112. doi: 10.1371/journal.pone.0103112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iascone DM, Henderson CE, Lee JC. Spinal muscular atrophy: from tissue specificity to therapeutic strategies. F1000prime Rep. 2015;7:04. doi: 10.12703/P7-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Renault F, Raimbault J, Praud JP, Laget P. Electromyographic study of 50 cases of Werdnig-Hoffmann disease. Revue d’electroencephalographie et de neurophysiologie clinique. 1983;13(3):301–305. doi: 10.1016/S0370-4475(83)80042-2. [DOI] [PubMed] [Google Scholar]
- 54.Anagnostou E, Miller SP, Guiot MC, Karpati G, Simard L, Dilenge ME, Shevell MI. Type I spinal muscular atrophy can mimic sensory-motor axonal neuropathy. J Child Neurol. 2005;20(2):147–150. doi: 10.1177/08830738050200022101. [DOI] [PubMed] [Google Scholar]
- 55.Omran H, Ketelsen UP, Heinen F, Sauer M, Rudnik-Schoneborn S, Wirth B, Zerres K, Kratzer W, Korinthenberg R. Axonal neuropathy and predominance of type II myofibers in infantile spinal muscular atrophy. J Child Neurol. 1998;13(7):327–331. doi: 10.1177/088307389801300704. [DOI] [PubMed] [Google Scholar]
- 56.Wang DD, Bordey A. The astrocyte odyssey. Prog Neurobiol. 2008;86(4):342–367. doi: 10.1016/j.pneurobio.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rudge JS, Alderson RF, Pasnikowski E, McClain J, Ip NY, Lindsay RM. Expression of ciliary neurotrophic factor and the neurotrophins-nerve growth factor, brain-derived neurotrophic factor and neurotrophin 3-in cultured rat hippocampal astrocytes. Euro J Neurosci. 1992;4(6):459–471. doi: 10.1111/j.1460-9568.1992.tb00896.x. [DOI] [PubMed] [Google Scholar]
- 58.Ojeda SR, Ma YJ, Lee BJ, Prevot V. Glia-to-neuron signaling and the neuroendocrine control of female puberty. Recent Prog Horm Res. 2000;55:197–223. [PubMed] [Google Scholar]
- 59.Donato R. S100: a multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int J Biochem Cell Biol. 2001;33(7):637–668. doi: 10.1016/S1357-2725(01)00046-2. [DOI] [PubMed] [Google Scholar]
- 60.Jo SK, Cho WY, Sung SA, Kim HK, Won NH. MEK inhibitor, U0126, attenuates cisplatin-induced renal injury by decreasing inflammation and apoptosis. Kidney Int. 2005;67(2):458–466. doi: 10.1111/j.1523-1755.2005.67102.x. [DOI] [PubMed] [Google Scholar]
- 61.Ridet JL, Malhotra SK, Privat A, Gage FH. Reactive astrocytes: cellular and molecular cues to biological function. Trends Neurosci. 1997;20(12):570–577. doi: 10.1016/S0166-2236(97)01139-9. [DOI] [PubMed] [Google Scholar]
- 62.Wang W, Shi L, Xie Y, Ma C, Li W, Su X, Huang S, Chen R, Zhu Z, Mao Z, Han Y, Li M. SP600125, a new JNK inhibitor, protects dopaminergic neurons in the MPTP model of Parkinson’s disease. Neurosci Res. 2004;48(2):195–202. doi: 10.1016/j.neures.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 63.Parpura V, Zorec R. Gliotransmission: exocytotic release from astrocytes. Brain Res Rev. 2010;63(1–2):83–92. doi: 10.1016/j.brainresrev.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rindt H, Feng Z, Mazzasette C, Glascock JJ, Valdivia D, Pyles N, Crawford TO, Swoboda KJ, Patitucci TN, Ebert AD, Sumner CJ, Ko CP, Lorson CL. Astrocytes influence the severity of spinal muscular atrophy. Hum Mol Genet. 2015;24(14):4094–4102. doi: 10.1093/hmg/ddv148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kariya S, Park GH, Maeno-Hikichi Y, Leykekhman O, Lutz C, Arkovitz MS, Landmesser LT, Monani UR. Reduced SMN protein impairs maturation of the neuromuscular junctions in mouse models of spinal muscular atrophy. Hum Mol Genet. 2008;17(16):2552–2569. doi: 10.1093/hmg/ddn156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ullian EM, Sapperstein SK, Christopherson KS, Barres BA. Control of synapse number by glia. Science. 2001;291(5504):657–661. doi: 10.1126/science.291.5504.657. [DOI] [PubMed] [Google Scholar]
- 67.Tarabal O, Caraballo-Miralles V, Cardona-Rossinyol A, Correa FJ, Olmos G, Llado J, Esquerda JE, Caldero J. Mechanisms involved in spinal cord central synapse loss in a mouse model of spinal muscular atrophy. J Neuropathol Exp Neurol. 2014;73(6):519–535. doi: 10.1097/NEN.0000000000000074. [DOI] [PubMed] [Google Scholar]
- 68.Eroglu C, Barres BA. Regulation of synaptic connectivity by glia. Nature. 2010;468(7321):223–231. doi: 10.1038/nature09612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Eikelenboom P, Bate C, Van Gool WA, Hoozemans JJ, Rozemuller JM, Veerhuis R, Williams A. Neuroinflammation in Alzheimer’s disease and prion disease. Glia. 2002;40(2):232–239. doi: 10.1002/glia.10146. [DOI] [PubMed] [Google Scholar]
- 70.Evans CF, Davtyan H, Petrushina I, Hovakimyan A, Davtyan A, Hannaman D, Cribbs DH, Agadjanyan MG, Ghochikyan A. Epitope-based DNA vaccine for Alzheimer’s disease: translational study in macaques. Alzheimers Dement. 2014;10(3):284–295. doi: 10.1016/j.jalz.2013.04.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Frank-Cannon TC, Alto LT, McAlpine FE, Tansey MG. Does neuroinflammation fan the flame in neurodegenerative diseases? Mol Neurodegener. 2009;4:47. doi: 10.1186/1750-1326-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McGeer PL, McGeer EG. Inflammatory processes in amyotrophic lateral sclerosis. Muscle Nerve. 2002;26(4):459–470. doi: 10.1002/mus.10191. [DOI] [PubMed] [Google Scholar]
- 73.Moisse K, Strong MJ. Innate immunity in amyotrophic lateral sclerosis. Biochim Biophys Acta. 2006;1762(11–12):1083–1093. doi: 10.1016/j.bbadis.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 74.Sargsyan SA, Monk PN, Shaw PJ. Microglia as potential contributors to motor neuron injury in amyotrophic lateral sclerosis. Glia. 2005;51(4):241–253. doi: 10.1002/glia.20210. [DOI] [PubMed] [Google Scholar]
- 75.Dachs E, Piedrafita L, Hereu M, Esquerda JE, Caldero J. Chronic treatment with lithium does not improve neuromuscular phenotype in a mouse model of severe spinal muscular atrophy. Neuroscience. 2013;250:417–433. doi: 10.1016/j.neuroscience.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 76.Bowen S, Ateh DD, Deinhardt K, Bird MM, Price KM, Baker CS, Robson JC, Swash M, Shamsuddin W, Kawar S, El-Tawil T, Roos J, Hoyle A, Nickols CD, Knowles CH, Pullen AH, Luthert PJ, Weller RO, Hafezparast M, Franklin RJ, Revesz T, King RH, Berninghausen O, Fisher EM, Schiavo G, Martin JE. The phagocytic capacity of neurones. Euro J Neurosci. 2007;25(10):2947–2955. doi: 10.1111/j.1460-9568.2007.05554.x. [DOI] [PubMed] [Google Scholar]
- 77.Yamamoto T, Iwasaki Y, Konno H, Kudo H. Glial bundle formation in spinal roots following experimental neuronopathy. Ann Neurol. 1986;20(2):267–271. doi: 10.1002/ana.410200215. [DOI] [PubMed] [Google Scholar]
- 78.Moreno-Lopez A, Holttum S, Oddy M. A grounded theory investigation of life experience and the role of social support for adolescent offspring after parental brain injury. Brain Inj. 2011;25(12):1221–1233. doi: 10.3109/02699052.2011.608205. [DOI] [PubMed] [Google Scholar]
- 79.Sunico CR, Gonzalez-Forero D, Dominguez G, Garcia-Verdugo JM, Moreno-Lopez B. Nitric oxide induces pathological synapse loss by a protein kinase G-, Rho kinase-dependent mechanism preceded by myosin light chain phosphorylation. J Neurosci. 2010;30(3):973–984. doi: 10.1523/JNEUROSCI.3911-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bowerman M, Beauvais A, Anderson CL, Kothary R. Rho-kinase inactivation prolongs survival of an intermediate SMA mouse model. Hum Mol Genet. 2010;19(8):1468–1478. doi: 10.1093/hmg/ddq021. [DOI] [PubMed] [Google Scholar]
- 81.Chernousov MA, Yu WM, Chen ZL, Carey DJ, Strickland S. Regulation of Schwann cell function by the extracellular matrix. Glia. 2008;56(14):1498–1507. doi: 10.1002/glia.20740. [DOI] [PubMed] [Google Scholar]
- 82.McKee KK, Yang DH, Patel R, Chen ZL, Strickland S, Takagi J, Sekiguchi K, Yurchenco PD. Schwann cell myelination requires integration of laminin activities. J Cell Sci. 2012;125(Pt 19):4609–4619. doi: 10.1242/jcs.107995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Simons M, Trotter J. Wrapping it up: the cell biology of myelination. Curr Opin Neurobiol. 2007;17(5):533–540. doi: 10.1016/j.conb.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 84.Hunter G, Aghamaleky Sarvestany A, Roche SL, Symes RC, Gillingwater TH. SMN-dependent intrinsic defects in Schwann cells in mouse models of spinal muscular atrophy. Hum Mol Genet. 2014;23(9):2235–2250. doi: 10.1093/hmg/ddt612. [DOI] [PubMed] [Google Scholar]
- 85.Desarnaud F, Do Thi AN, Brown AM, Lemke G, Suter U, Baulieu EE, Schumacher M. Progesterone stimulates the activity of the promoters of peripheral myelin protein-22 and protein zero genes in Schwann cells. J Neurochem. 1998;71(4):1765–1768. doi: 10.1046/j.1471-4159.1998.71041765.x. [DOI] [PubMed] [Google Scholar]
- 86.Baumer D, Lee S, Nicholson G, Davies JL, Parkinson NJ, Murray LM, Gillingwater TH, Ansorge O, Davies KE, Talbot K. Alternative splicing events are a late feature of pathology in a mouse model of spinal muscular atrophy. PLoS Genet. 2009;5(12):e1000773. doi: 10.1371/journal.pgen.1000773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Murray LM, Lee S, Baumer D, Parson SH, Talbot K, Gillingwater TH. Pre-symptomatic development of lower motor neuron connectivity in a mouse model of severe spinal muscular atrophy. Hum Mol Genet. 2010;19(3):420–433. doi: 10.1093/hmg/ddp506. [DOI] [PubMed] [Google Scholar]
- 88.Rathod R, Havlicek S, Frank N, Blum R, Sendtner M. Laminin induced local axonal translation of beta-actin mRNA is impaired in SMN-deficient motoneurons. Histochem Cell Biol. 2012;138(5):737–748. doi: 10.1007/s00418-012-0989-1. [DOI] [PubMed] [Google Scholar]
- 89.Aghamaleky Sarvestany A, Hunter G, Tavendale A, Lamont DJ, Llavero Hurtado M, Graham LC, Wishart TM, Gillingwater TH. Label-free quantitative proteomic profiling identifies disruption of ubiquitin homeostasis as a key driver of Schwann cell defects in spinal muscular atrophy. J Proteome Res. 2014;13(11):4546–4557. doi: 10.1021/pr500492j. [DOI] [PubMed] [Google Scholar]
- 90.Lee YI, Mikesh M, Smith I, Rimer M, Thompson W. Muscles in a mouse model of spinal muscular atrophy show profound defects in neuromuscular development even in the absence of failure in neuromuscular transmission or loss of motor neurons. Dev Biol. 2011;356(2):432–444. doi: 10.1016/j.ydbio.2011.05.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Murray LM, Comley LH, Thomson D, Parkinson N, Talbot K, Gillingwater TH. Selective vulnerability of motor neurons and dissociation of pre- and post-synaptic pathology at the neuromuscular junction in mouse models of spinal muscular atrophy. Hum Mol Genet. 2008;17(7):949–962. doi: 10.1093/hmg/ddm367. [DOI] [PubMed] [Google Scholar]
- 92.Rajendra TK, Gonsalvez GB, Walker MP, Shpargel KB, Salz HK, Matera AG. A Drosophila melanogaster model of spinal muscular atrophy reveals a function for SMN in striated muscle. J Cell Biol. 2007;176(6):831–841. doi: 10.1083/jcb.200610053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brennan KJ, Hardeman EC. Quantitative analysis of the human alpha-skeletal actin gene in transgenic mice. J Biol Chem. 1993;268(1):719–725. [PubMed] [Google Scholar]
- 94.Miniou P, Tiziano D, Frugier T, Roblot N, Le Meur M, Melki J. Gene targeting restricted to mouse striated muscle lineage. Nucleic Acids Res. 1999;27(19):e27. doi: 10.1093/nar/27.19.e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Leu M, Bellmunt E, Schwander M, Farinas I, Brenner HR, Muller U. Erbb2 regulates neuromuscular synapse formation and is essential for muscle spindle development. Development. 2003;130(11):2291–2301. doi: 10.1242/dev.00447. [DOI] [PubMed] [Google Scholar]
- 96.Wang J, Xu G, Slunt HH, Gonzales V, Coonfield M, Fromholt D, Copeland NG, Jenkins NA, Borchelt DR. Coincident thresholds of mutant protein for paralytic disease and protein aggregation caused by restrictively expressed superoxide dismutase cDNA. Neurobiol Dis. 2005;20(3):943–952. doi: 10.1016/j.nbd.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 97.Bowerman M, Shafey D, Kothary R. Smn depletion alters profilin II expression and leads to upregulation of the RhoA/ROCK pathway and defects in neuronal integrity. J Mol Neurosci (MN) 2007;32(2):120–131. doi: 10.1007/s12031-007-0024-5. [DOI] [PubMed] [Google Scholar]
- 98.Giesemann T, Rathke-Hartlieb S, Rothkegel M, Bartsch JW, Buchmeier S, Jockusch BM, Jockusch H. A role for polyproline motifs in the spinal muscular atrophy protein SMN. Profilins bind to and colocalize with SMN in nuclear gems. J Biol Chem. 1999;274(53):37908–37914. doi: 10.1074/jbc.274.53.37908. [DOI] [PubMed] [Google Scholar]
- 99.Nolle A, Zeug A, van Bergeijk J, Tonges L, Gerhard R, Brinkmann H, Al Rayes S, Hensel N, Schill Y, Apkhazava D, Jablonka S, O’Mer J, Srivastav RK, Baasner A, Lingor P, Wirth B, Ponimaskin E, Niedenthal R, Grothe C, Claus P. The spinal muscular atrophy disease protein SMN is linked to the Rho-kinase pathway via profilin. Hum Mol Genet. 2011;20(24):4865–4878. doi: 10.1093/hmg/ddr425. [DOI] [PubMed] [Google Scholar]
- 100.Oprea GE, Krober S, McWhorter ML, Rossoll W, Muller S, Krawczak M, Bassell GJ, Beattie CE, Wirth B. Plastin 3 is a protective modifier of autosomal recessive spinal muscular atrophy. Science. 2008;320(5875):524–527. doi: 10.1126/science.1155085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bowerman M, Murray LM, Beauvais A, Pinheiro B, Kothary R. A critical SMN threshold in mice dictates onset of an intermediate spinal muscular atrophy phenotype associated with a distinct neuromuscular junction pathology. Neuromuscul Disord (NMD) 2012;22(3):263–276. doi: 10.1016/j.nmd.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 102.Coque E, Raoul C, Bowerman M. ROCK inhibition as a therapy for spinal muscular atrophy: understanding the repercussions on multiple cellular targets. Front Neurosci. 2014;8:271. doi: 10.3389/fnins.2014.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Arnold AS, Gueye M, Guettier-Sigrist S, Courdier-Fruh I, Coupin G, Poindron P, Gies JP. Reduced expression of nicotinic AChRs in myotubes from spinal muscular atrophy I patients. Lab Investig. 2004;84(10):1271–1278. doi: 10.1038/labinvest.3700163. [DOI] [PubMed] [Google Scholar]
- 104.Boyer JG, Deguise MO, Murray LM, Yazdani A, De Repentigny Y, Boudreau-Lariviere C, Kothary R. Myogenic program dysregulation is contributory to disease pathogenesis in spinal muscular atrophy. Hum Mol Genet. 2014;23(16):4249–4259. doi: 10.1093/hmg/ddu142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cifuentes-Diaz C, Frugier T, Tiziano FD, Lacene E, Roblot N, Joshi V, Moreau MH, Melki J. Deletion of murine SMN exon 7 directed to skeletal muscle leads to severe muscular dystrophy. J Cell Biol. 2001;152(5):1107–1114. doi: 10.1083/jcb.152.5.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nicole S, Desforges B, Millet G, Lesbordes J, Cifuentes-Diaz C, Vertes D, Cao ML, De Backer F, Languille L, Roblot N, Joshi V, Gillis JM, Melki J. Intact satellite cells lead to remarkable protection against SMN gene defect in differentiated skeletal muscle. J Cell Biol. 2003;161(3):571–582. doi: 10.1083/jcb.200210117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shafey D, Cote PD, Kothary R. Hypomorphic SMN knockdown C2C12 myoblasts reveal intrinsic defects in myoblast fusion and myotube morphology. Exp Cell Res. 2005;311(1):49–61. doi: 10.1016/j.yexcr.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 108.Formigli L, Meacci E, Vassalli M, Nosi D, Quercioli F, Tiribilli B, Tani A, Squecco R, Francini F, Bruni P, Zecchi Orlandini S. Sphingosine 1-phosphate induces cell contraction via calcium-independent/Rho-dependent pathways in undifferentiated skeletal muscle cells. J Cell Physiol. 2004;198(1):1–11. doi: 10.1002/jcp.10366. [DOI] [PubMed] [Google Scholar]
- 109.Bryan BA, Li D, Wu X, Liu M. The Rho family of small GTPases: crucial regulators of skeletal myogenesis. Cell Mol Life Sci (CMLS) 2005;62(14):1547–1555. doi: 10.1007/s00018-005-5029-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rose FF, Jr, Mattis VB, Rindt H, Lorson CL. Delivery of recombinant follistatin lessens disease severity in a mouse model of spinal muscular atrophy. Hum Mol Genet. 2009;18(6):997–1005. doi: 10.1093/hmg/ddn426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ripolone M, Ronchi D, Violano R, Vallejo D, Fagiolari G, Barca E, Lucchini V, Colombo I, Villa L, Berardinelli A, Balottin U, Morandi L, Mora M, Bordoni A, Fortunato F, Corti S, Parisi D, Toscano A, Sciacco M, DiMauro S, Comi GP, Moggio M. Impaired muscle mitochondrial biogenesis and myogenesis in spinal muscular atrophy. JAMA Neurol. 2015 doi: 10.1001/jamaneurol.2015.0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shababi M, Glascock J, Lorson CL. Combination of SMN trans-splicing and a neurotrophic factor increases the life span and body mass in a severe model of spinal muscular atrophy. Hum Gene Ther. 2011;22(2):135–144. doi: 10.1089/hum.2010.114. [DOI] [PubMed] [Google Scholar]
- 113.Tsai LK, Chen CL, Ting CH, Lin-Chao S, Hwu WL, Dodge JC, Passini MA, Cheng SH. Systemic administration of a recombinant AAV1 vector encoding IGF-1 improves disease manifestations in SMA mice. Mol Ther. 2014;22(8):1450–1459. doi: 10.1038/mt.2014.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Somers E, Lees RD, Hoban K, Sleigh JN, Zhou H, Muntoni F, Talbot K, Gillingwater TH, Parson SH. Vascular defects and spinal cord hypoxia in spinal muscular atrophy. Ann Neurol. 2015 doi: 10.1002/ana.24549. [DOI] [PubMed] [Google Scholar]
- 115.Garbuzova-Davis S, Hernandez-Ontiveros DG, Rodrigues MC, Haller E, Frisina-Deyo A, Mirtyl S, Sallot S, Saporta S, Borlongan CV, Sanberg PR. Impaired blood–brain/spinal cord barrier in ALS patients. Brain Res. 2012;1469:114–128. doi: 10.1016/j.brainres.2012.05.056. [DOI] [PubMed] [Google Scholar]
- 116.Dachs E, Hereu M, Piedrafita L, Casanovas A, Caldero J, Esquerda JE. Defective neuromuscular junction organization and postnatal myogenesis in mice with severe spinal muscular atrophy. J Neuropathol Exp Neurol. 2011;70(6):444–461. doi: 10.1097/NEN.0b013e31821cbd8b. [DOI] [PubMed] [Google Scholar]
- 117.Banerjee R, Mosley RL, Reynolds AD, Dhar A, Jackson-Lewis V, Gordon PH, Przedborski S, Gendelman HE. Adaptive immune neuroprotection in G93A-SOD1 amyotrophic lateral sclerosis mice. PLoS One. 2008;3(7):e2740. doi: 10.1371/journal.pone.0002740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bach JR. Medical considerations of long-term survival of Werdnig-Hoffmann disease. Am J Phys Med Rehabil Assoc Acad Physiatr. 2007;86(5):349–355. doi: 10.1097/PHM.0b013e31804b1d66. [DOI] [PubMed] [Google Scholar]
- 119.Elkohen M, Vaksmann G, Elkohen MR, Francart C, Foucher C, Rey C. Cardiac involvement in Kugelberg–Welander disease. A prospective study of 8 cases. Arch Mal Coeur Vaiss. 1996;89(5):611–617. [PubMed] [Google Scholar]
- 120.Roos M, Sarkozy A, Chierchia GB, De Wilde P, Schmedding E, Brugada P. Malignant ventricular arrhythmia in a case of adult onset of spinal muscular atrophy (Kugelberg–Welander disease) J Cardiovasc Electrophysiol. 2009;20(3):342–344. doi: 10.1111/j.1540-8167.2008.01327.x. [DOI] [PubMed] [Google Scholar]
- 121.Rudnik-Schoneborn S, Heller R, Berg C, Betzler C, Grimm T, Eggermann T, Eggermann K, Wirth R, Wirth B, Zerres K. Congenital heart disease is a feature of severe infantile spinal muscular atrophy. J Med Genet. 2008;45(10):635–638. doi: 10.1136/jmg.2008.057950. [DOI] [PubMed] [Google Scholar]
- 122.Tanaka H, Uemura N, Toyama Y, Kudo A, Ohkatsu Y. Cardiac involvement in the Kugelbert–Welander syndrome. Am J Cardiol. 1976;38(4):528–532. doi: 10.1016/0002-9149(76)90473-2. [DOI] [PubMed] [Google Scholar]
- 123.Araujo A, Araujo M, Swoboda KJ. Vascular perfusion abnormalities in infants with spinal muscular atrophy. J Pediatr. 2009;155(2):292–294. doi: 10.1016/j.jpeds.2009.01.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Finsterer J, Stollberger C. Cardiac involvement in Werdnig–Hoffmann’s spinal muscular atrophy. Cardiology. 1999;92(3):178–182. doi: 10.1159/000006968. [DOI] [PubMed] [Google Scholar]
- 125.Hachiya Y, Arai H, Hayashi M, Kumada S, Furushima W, Ohtsuka E, Ito Y, Uchiyama A, Kurata K. Autonomic dysfunction in cases of spinal muscular atrophy type 1 with long survival. Brain Dev. 2005;27(8):574–578. doi: 10.1016/j.braindev.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 126.Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- 127.Kimura S, Yokota H, Tateda K, Miyamoto K, Yamamoto K, Shibata J. A case of the Kugelberg–Welander syndrome complicated with cardiac lesions. Jpn Heart J. 1980;21(3):417–422. doi: 10.1536/ihj.21.417. [DOI] [PubMed] [Google Scholar]
- 128.Takahashi N, Shimada T, Ishibashi Y, Sugamori T, Hirano Y, Oyake N, Murakami Y. Cardiac involvement in Kugelberg–Welander disease: a case report and review. Am J Med Sci. 2006;332(6):354–356. doi: 10.1097/00000441-200612000-00009. [DOI] [PubMed] [Google Scholar]
- 129.Distefano G, Sciacca P, Parisi MG, Parano E, Smilari P, Marletta M, Fiumara A (1994) [Heart involvement in progressive spinal muscular atrophy. A review of the literature and case histories in childhood]. La Pediatria medica e chirurgica: Medical and surgical pediatrics 16(2):125–128 [PubMed]
- 130.Menke LA, Poll-The BT, Clur SA, Bilardo CM, van der Wal AC, Lemmink HH, Cobben JM. Congenital heart defects in spinal muscular atrophy type I: a clinical report of two siblings and a review of the literature. Am J Med Genet A. 2008;146A(6):740–744. doi: 10.1002/ajmg.a.32233. [DOI] [PubMed] [Google Scholar]
- 131.Bevan AK, Hutchinson KR, Foust KD, Braun L, McGovern VL, Schmelzer L, Ward JG, Petruska JC, Lucchesi PA, Burghes AH, Kaspar BK. Early heart failure in the SMNDelta7 model of spinal muscular atrophy and correction by postnatal scAAV9-SMN delivery. Hum Mol Genet. 2010;19(20):3895–3905. doi: 10.1093/hmg/ddq300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Biondi O, Lopes P, Desseille C, Branchu J, Chali F, Salah AB, Pariset C, Chanoine C, Charbonnier F. Physical exercise reduces cardiac defects in type 2 spinal muscular atrophy-like mice. J Physiol. 2012;590(Pt 22):5907–5925. doi: 10.1113/jphysiol.2012.238196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shababi M, Habibi J, Yang HT, Vale SM, Sewell WA, Lorson CL. Cardiac defects contribute to the pathology of spinal muscular atrophy models. Hum Mol Genet. 2010;19(20):4059–4071. doi: 10.1093/hmg/ddq329. [DOI] [PubMed] [Google Scholar]
- 134.Shababi M, Habibi J, Ma L, Glascock JJ, Sowers JR, Lorson CL. Partial restoration of cardio-vascular defects in a rescued severe model of spinal muscular atrophy. J Mol Cell Cardiol. 2012;52(5):1074–1082. doi: 10.1016/j.yjmcc.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Foust KD, Wang XY, McGovern VL, Braun L, Bevan AK, Haidet AM, Le TT, Morales PR, Rich MM, Burghes AHM, Kaspar BK. Rescue of the spinal muscular atrophy phenotype in a mouse model by early postnatal delivery of SMN. Nat Biotechnol. 2010;28(3):271–274. doi: 10.1038/nbt.1610. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 136.Arai H, Tanabe Y, Hachiya Y, Otsuka E, Kumada S, Furushima W, Kohyama J, Yamashita S, Takanashi J, Kohno Y. Finger cold-induced vasodilatation, sympathetic skin response, and R–R interval variation in patients with progressive spinal muscular atrophy. J Child Neurol. 2005;20(11):871–875. doi: 10.1177/08830738050200110301. [DOI] [PubMed] [Google Scholar]
- 137.Lambrechts D, Carmeliet P. VEGF at the neurovascular interface: therapeutic implications for motor neuron disease. Biochim Biophys Acta. 2006;1762(11–12):1109–1121. doi: 10.1016/j.bbadis.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 138.Felderhoff-Mueser U, Grohmann K, Harder A, Stadelmann C, Zerres K, Buhrer C, Obladen M. Severe spinal muscular atrophy variant associated with congenital bone fractures. J Child Neurol. 2002;17(9):718–721. doi: 10.1177/088307380201700915. [DOI] [PubMed] [Google Scholar]
- 139.Swoboda KJ, Prior TW, Scott CB, McNaught TP, Wride MC, Reyna SP, Bromberg MB. Natural history of denervation in SMA: relation to age, SMN2 copy number, and function. Ann Neurol. 2005;57(5):704–712. doi: 10.1002/ana.20473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Shanmugarajan S, Swoboda KJ, Iannaccone ST, Ries WL, Maria BL, Reddy SV. Congenital bone fractures in spinal muscular atrophy: functional role for SMN protein in bone remodeling. J Child Neurol. 2007;22(8):967–973. doi: 10.1177/0883073807305664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ionasescu V, Christensen J, Hart M. Intestinal pseudo-obstruction in adult spinal muscular atrophy. Muscle Nerve. 1994;17(8):946–948. doi: 10.1002/mus.880170816. [DOI] [PubMed] [Google Scholar]
- 142.Khawaja K, Houlsby WT, Watson S, Bushby K, Cheetham T. Hypercalcaemia in infancy; a presenting feature of spinal muscular atrophy. Arch Dis Child. 2004;89(4):384–385. doi: 10.1136/adc.2003.028225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Galvis DA, Ang SM, Wells TR, Landing BH, Romansky SG. Microdissection study of the myenteric plexus in acardia, ataxia-telangiectasia, cystic fibrosis, extrahepatic biliary atresia, pediatric AIDS and Werdnig–Hoffmann disease. Pediatr Pathol. 1992;12(3):385–395. doi: 10.3109/15513819209023317. [DOI] [PubMed] [Google Scholar]
- 144.Gombash SE, Cowley CJ, Fitzgerald JA, Iyer CC, Fried D, McGovern VL, Williams KC, Burghes AH, Christofi FL, Gulbransen BD, Foust KD. SMN deficiency disrupts gastrointestinal and enteric nervous system function in mice. Hum Mol Genet. 2015 doi: 10.1093/hmg/ddv292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bowerman M, Swoboda KJ, Michalski JP, Wang GS, Reeks C, Beauvais A, Murphy K, Woulfe J, Screaton RA, Scott FW, Kothary R. Glucose metabolism and pancreatic defects in spinal muscular atrophy. Ann Neurol. 2012;72(2):256–268. doi: 10.1002/ana.23582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bach JR. The use of mechanical ventilation is appropriate in children with genetically proven spinal muscular atrophy type 1: the motion for. Paediatr Respir Rev. 2008;9(1):45–50. doi: 10.1016/j.prrv.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 147.Bowerman M, Michalski JP, Beauvais A, Murray LM, DeRepentigny Y, Kothary R. Defects in pancreatic development and glucose metabolism in SMN-depleted mice independent of canonical spinal muscular atrophy neuromuscular pathology. Hum Mol Genet. 2014;23(13):3432–3444. doi: 10.1093/hmg/ddu052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Vitte JM, Davoult B, Roblot N, Mayer M, Joshi V, Courageot S, Tronche F, Vadrot J, Moreau MH, Kemeny F, Melki J. Deletion of murine Smn exon 7 directed to liver leads to severe defect of liver development associated with iron overload. Am J Pathol. 2004;165(5):1731–1741. doi: 10.1016/S0002-9440(10)63428-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tein I, Sloane AE, Donner EJ, Lehotay DC, Millington DS, Kelley RI. Fatty acid oxidation abnormalities in childhood-onset spinal muscular atrophy: primary or secondary defect(s)? Pediatr Neurol. 1995;12(1):21–30. doi: 10.1016/0887-8994(94)00100-G. [DOI] [PubMed] [Google Scholar]
- 150.Crawford TO, Sladky JT, Hurko O, Besner-Johnston A, Kelley RI. Abnormal fatty acid metabolism in childhood spinal muscular atrophy. Ann Neurol. 1999;45(3):337–343. doi: 10.1002/1531-8249(199903)45:3<337::AID-ANA9>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 151.Zolkipli Z, Sherlock M, Biggar WD, Taylor G, Hutchison JS, Peliowski A, Alman BA, Ling SC, Tein I. Abnormal fatty acid metabolism in spinal muscular atrophy may predispose to perioperative risks. Euro J Paediatr Neurol (EJPN) 2012;16(5):549–553. doi: 10.1016/j.ejpn.2012.01.004. [DOI] [PubMed] [Google Scholar]