Abstract
Background
Intolerance of the esophageal manometry catheter may prolong high-resolution manometry (HRM) studies and increase patient distress. We assessed the impact of obtaining the landmark phase at the end of the study when the patient has acclimatized to the HRM catheter.
Methods
366 patients (mean age 55.4 ± 0.8 years, 62.0% female) undergoing esophageal HRM over a 1-year period were studied. The standard protocol consisted of the landmark phase, 10 5 mL water swallows 20–30 s apart, and multiple rapid swallows where 4–6 2 mL swallows were administered in rapid succession. The modified protocol consisted of the landmark phase at the end of the study after test swallows. Study duration, technical characteristics, indications, and motor findings were compared between standard and modified protocols.
Key Results
Of the 366 patients, 89.6% underwent the standard protocol (study duration 12.9 ± 0.3 min). In 10.4% with poor catheter tolerance undergoing the modified protocol, study duration was significantly longer (15.6 ± 1.0 min, p = 0.004) despite similar duration of study maneuvers. Only elevated upper esophageal sphincter basal pressures at the beginning of the study segregated modified protocol patients. The 95th percentile time to landmark phase in the standard protocol patients was 6.1 min; as many as 31.4% of modified protocol patients could not obtain their first study maneuver within this period (p = 0.0003). Interpretation was not impacted by shifting the landmark phase to the end of the study.
Conclusions & Inferences
Modification of the HRM study protocol with the landmark phase obtained at the end of the study optimizes study duration without compromising quality.
Keywords: basal UES pressure, esophageal high-resolution manometry, landmark phase
INTRODUCTION
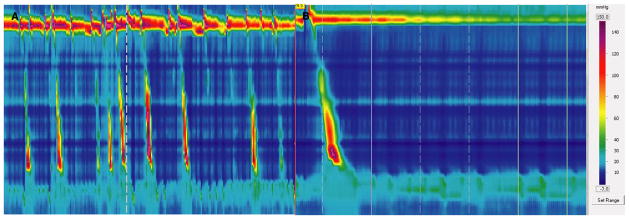
Esophageal high-resolution manometry (HRM) studies are quicker and easier to perform than conventional manometry, as appropriate catheter placement is visually evident and re-positioning is unnecessary during the study.1,2 Landmark phase measurements, during which baseline sphincter location and pressures are established, are typically performed during a quiet phase without swallows at the start of the HRM study protocol.2,3 Repetitive swallowing, gagging, retching, and intolerance of the catheter compromise landmark phase measurements and prolong HRM studies.3 It is our experience that the patient is most tolerant of the catheter at the end of the study (Fig. 1).
Figure 1.
Repetitive swallowing precluding recording of the landmark phase at the beginning of the study (A). However, at the end of the study, the same patient is calm and able to withhold swallowing for a landmark phase to be recorded (B).
In this study, we aimed to assess the impact of acquiring the landmark phase at the end of the HRM study on study duration and quality.
METHODS
Patients
All adults (≥18 years) undergoing HRM studies at our institution during 2011 were eligible for inclusion. In this uncontrolled study, HRM operators were instructed to proceed with test swallows if a swallow-free phase could not be immediately obtained, and obtain a quiet period for landmark phase measurements at the end of the study instead. No specific time criteria were dictated to the operators. Patients undergoing high-resolution impedance manometry (HRIM), which requires a distinct longer protocol with provocative swallows, and aborted HRM studies were excluded. This study protocol was approved by the Human Research Protection Office at Washington University in St. Louis.
Data collection
All subjects were studied after an overnight fast, using methodology described elsewhere4,5 The standard protocol consisted of the landmark phase (20 s or at least 3 respiratory cycles of swallow-free baseline recording), 10 5 mL water swallows 20–30 s apart, and one set of multiple rapid swallows. The modified protocol started with the 10 water swallows, followed by one set of multiple rapid swallows, and the landmark phase at the end of the study.5 Data acquisition, display, and analysis were performed using a dedicated computerized system (ManoView, Given Imaging, Los Angeles, CA, USA).
Subject demographics, study indications, motor findings, and technical characteristics of the study were recorded. High-resolution manometry time intervals collected included total study duration, time to first swallow, time to landmark phase (standard swallow protocol), and duration of swallow phase. Major motor disorders were characterized according to Chicago Classification criteria.6
Data analysis
Data are reported as the mean ± SEM unless stated otherwise. Categorical data were compared using the chi-squared test; grouped data were compared using the two-tailed Student’s t-test. Predictors of catheter intolerance were assessed using univariate and multivariate linear regression analyses. In all cases, p < 0.05 was required for statistical significance. All statistical analyses were performed using IBM SPSS Statistics V.21.0 (Armonk, NY, USA).
RESULTS
Of 488 esophageal HRM studies performed during the study period, 13 (2.7%) were aborted because of extreme intolerance or refusal to continue the study, and 109 (22.3%) were HRIM studies; these were excluded. The remaining 366 unique studies consisted of 328 (89.6%) in the standard protocol group, and 38 (10.4%) in the modified protocol group. The modified protocol group was younger than the standard protocol group (p = 0.002, Table 1), but other demographic parameters and presenting symptoms were similar.
Table 1.
Demographics and clinical characteristics
| All subjects (n = 366) | Standard protocol (n = 328) | Modified protocol (n = 38) | p-value | |
|---|---|---|---|---|
| Mean age (year) ± SEM | 55.4 ± 0.8 | 56.2 ± 0.8 | 48.0 ± 2.8 | 0.002 |
| Gender (F) | 227, 62.0% | 202, 61.6% | 25, 65.8% | 0.613 |
| Study durations | ||||
| Total study duration (min) | 13.2 ± 0.02 | 12.9 ± 0.3 | 15.6 ± 9.6 | 0.004 |
| Time to first swallow (min) | 3.6 ± 0.2 | 3.3 ± 0.2 | 5.8 ± 0.8 | 0.006 |
| Time to landmark phase (min) | 3.2 ± 0.3 | 1.9 ± 0.1 | 14.6 ± 1.0 | <0.001 |
| Swallow phase duration (min) | 9.7 ± 0.2 | 9.6 ± 0.2 | 9.9 ± 0.5 | 0.706 |
| Motor parameters | ||||
| UES basal pressure (mmHg) | 42.7 ± 1.6 | 42.5 ± 1.7 | 44.4 ± 5.5 | 0.725 |
| 81.5 ± 8.1* | <0.0001 | |||
| LES basal pressure (mmHg) | 13.0 ± 0.7 | 13.1 ± 0.7 | 12.0 ± 1.7 | 0.629 |
| 12.3 ± 2.0† | 0.930 | |||
| Mean DCI (mmHg.cm.s) | 1810.7 ± 108.0 | 1834.3 ± 117.7 | 1622.3 ± 244.4 | 0.538 |
| Highest DCI (mmHg.cm.s) | 3177.0 ± 205.2 | 3254.0 ± 226.1 | 2561.7 ± 359.5 | 0.290 |
| Distal latency (s) | 10.5 ± 3.6 | 10.9 ± 4.1 | 7.0 ± 0.3 | 0.733 |
| Motor diagnoses | ||||
| Chicago Classification diagnosis | 214, 58.5% | 194, 59.1% | 20, 52.6% | 0.440 |
| Major motor disorders | 120, 32.8% | 113, 34.5% | 7, 18.4% | 0.05 |
| Achalasia | 53, 14.5% | 50, 15.2% | 3, 7.9% | 0.223 |
| Hypercontractile disorder | 23, 6.3% | 22, 6.7% | 1, 2.6% | 0.491 |
| Distal esophageal spasm | 21, 5.7% | 20, 6.1% | 1, 2.6% | 0.710 |
| Aperistalsis | 23, 6.3% | 21, 6.4% | 2, 5.2% | 1.0 |
UES basal pressures at the beginning of the study;
LES basal pressures at beginning of the study.
UES, upper esophageal sphincter; LES, lower esophageal sphincter; DCI, distal contractile integral.
Mean study duration was longer by 20.6% in the modified protocol group (p = 0.004), primarily because the first point of protocol data acquisition was later in the modified protocol group (p < 0.0001, Table 1). Despite this delay, the duration of the swallow phases of the study were similar in both groups (p = 0.71), suggesting that the modified protocol did not affect data acquisition. Furthermore, all subjects had a median of 10 wet swallows in either group. Reasons for inability to obtain a swallow-free interval included repetitive swallowing in 27 patients (71.1%) and gagging in 21 (55.3%); 10 patients (26.3%) had both repetitive swallowing and gagging.
Demographics, presenting symptoms, and major motor diagnoses did not predict need for a modified protocol. Esophageal body contraction vigor (DCI), timing of smooth muscle contraction (distal latency), and LES metrics were also not different between the two groups (Table 1). However, upper esophageal sphincter (UES) basal pressure at the start of the study was significantly higher in patients who underwent the modified protocol (81.5 ± 8.1 mmHg, p < 0.0001 compared to mean recorded UES basal pressures for the entire cohort), although UES basal pressures at the end of the study (43.2 ± 4.8 mmHg) were similar to that recorded in the standard protocol patients at the beginning of the study (41.3 ± 1.5 mmHg).
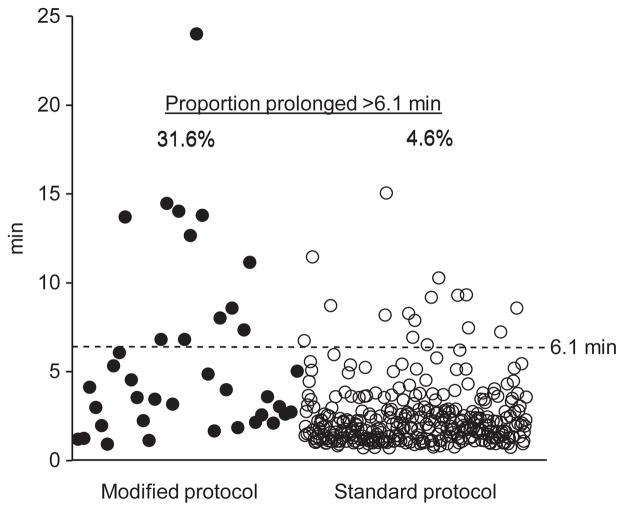
Using the 95th percentile of time to landmark phase in the standard protocol group (6.1 min) as the threshold, 95.4% in the standard group had landmark phase and 89.6% had their first test swallow recorded within this time frame (Fig. 2), with a study duration of 11.9 ± 0.2 min. In the 4.6% of standard protocol patients with landmark phase recording beyond the 6.1 min threshold, the study duration was 21.4 ± 1.3 min, 79.8% longer compared to those within the 6.1 min threshold (p < 0.0001). In the modified protocol, 26 patients (68.4%) had their first test swallow within the 6.1 min threshold, with a study duration of 12.7 ± 0.3 min; when the first swallow was past this threshold, study duration was 73.2% longer, 22.0 ± 1.5 min (p < 0.0001).
Figure 2.
When compared to the 95th percentile time duration to first study maneuver (landmark phase assessment) in standard protocol patients (6.1 min), as many as 31.6% of modified protocol subjects could not obtain their first swallow within this time frame (p = 0.0003). We propose that if the patient cannot refrain from swallowing past 5–6 min, the study should proceed with test swallows rather than wait for a quiet period for the landmark phase recording, which can be subsequently obtained at the end of the study.
DISCUSSION
In this study, we demonstrate that obtaining the landmark phase at the end of the HRM study (modified swallow protocol) shortens study duration without compromising study quality. A high UES basal pressure at the start of the study may identify patients unable to refrain from repetitive swallowing or gagging at the start of the study. We propose moving the landmark phase to the end of the HRM study if a swallow- or artifact-free phase cannot be acquired within the first 5–6 min of the HRM study.
Assessment of esophageal motor function may be hindered by technically imperfect studies,2,7,8 defined as aberrant pressure signal acquisition, failure of the catheter to traverse the esophagogastric junction, or fewer than 7 evaluable swallows, and encountered 21% of the time at a tertiary motility laboratory.7 Some of these technical flaws can be avoided if the operator is experienced and patient.7–9 We report that 2.7% of studies are aborted because of factors beyond the operator’s control. Existing data suggests that another 1.4–2.0% of HRM studies may have critical imperfections curtailing their clinical value.7,9 In our hands, in over 85% of patients, the study can be initiated quickly following catheter positioning. However, initial intolerance of the catheter can prolong HRM studies, leading to patient distress, and can contribute to an insufficient number of swallows. We report that 12.8% of patients undergoing esophageal HRM cannot begin their first protocol maneuver within the first 6 min of the study; in these patients, the study duration is almost 80% longer. This 6-min mark could represent a critical point, in that if the patient does not sufficiently settle down for a swallow-free landmark phase within this period, it is more efficient for the patient to focus on test swallows than to refrain from swallowing, reserving the landmark phase for the end of the study. This modification of protocol does not compromise the HRM study, and study duration from the start to the end of the protocol maneuvers is the same regardless of whether the landmark phase is obtained first or last.
While clinical characteristics did not segregate the two study groups, UES basal pressure was significantly higher at the start of the study in the modified protocol group. As the UES represents volitional skeletal muscle, we speculate that anxiety and tensing up of oropharyngeal musculature results in increased UES basal pressure. The UES pressure has been noted to be hyperdynamic, with high postswallow residual pressures in globus, for instance, which could have a superimposed anxiety component.10,11 We propose that a high resting UES basal pressure could prompt moving the landmark phase to the end of the study.
Our study also highlights the role of the operator or experienced technician, who needs to recognize artifacts and imperfections as they occur, and implement corrective measures to ensure high-quality studies.2,3,8 Our study has a few limitations. Patients were identified retrospectively, and all limitations of a retrospective study apply. The decision to obtain the landmark phase at the end of the study was not randomized, but left open in an exploratory fashion to the discretion of the operator. The landmark phase was not repeated at the end of the study in standard protocol patients. Further, non-oropharyngeal or non-esophageal factors contributing to catheter intolerance and patient discomfort were not addressed. Finally, our results may not apply to HRM systems designed by manufacturers other than the Given system. Nevertheless, we feel our results contribute to optimizing the HRM study protocol.
In summary, starting with a test swallow protocol and obtaining the landmark phase at the end of the HRM study will shorten the procedure without compromising quality. High UES basal pressures at the start of the study or catheter intolerance lasting 5–6 min are factors that could prompt obtaining the landmark phase at the end of the study.
Key Messages.
In patients who are intolerant of the esophageal HRM catheter at the beginning of the study, obtaining the landmark phase at the end of the study when the patient has acclimatized to the catheter can be successfully performed without compromising study quality or interpretation.
Upon retrospectively comparing HRM study metrics and motor findings between standard protocol and this modification of the study protocol, duration of study maneuvers, study interpretation and motor diagnoses were not impacted; elevated UES pressures at the start of the study identified patients needing the modified protocol. We propose that catheter intolerance or inability to obtain a landmark phase in the first 5–6 min should prompt modification of the study protocol where test swallows are obtained first, followed by the landmark phase.
Acknowledgments
Guarantor of the article: C. Prakash Gyawali, MD.
FUNDING
Partially funded by a grant from Mentors in Medicine, Department of Internal Medicine, Washington University in St. Louis (AP and AD) for providing time for data collection.
Footnotes
Presented in Preliminary Form at the Annual Meeting of the American Gastroenterological Association, Orlando, May 2013.
DISCLOSURE
No conflicts of interest exist for any of the authors.
AUTHOR CONTRIBUTION
AP study design, data collection and analysis, manuscript preparation and review; AD data collection and analysis, manuscript review; FM data collection and analysis, manuscript review; CPG study concept and design, study supervision, data analysis and interpretation, manuscript preparation and review. All authors approved the final version of the manuscript. No writing assistance was obtained.
References
- 1.Kahrilas PJ. Esophageal motor disorders in terms of high-resolution esophageal pressure topography: what has changed? Am J Gastroenterol. 2010;105:981–7. doi: 10.1038/ajg.2010.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gyawali CP, Bredenoord AJ, Conklin JL, Fox M, Pandolfino JE, Peters JH, Roman S, Staiano A, et al. Evaluation of esophageal motor function in clinical practice. Neurogastroenterol Motil. 2013;25:99–133. doi: 10.1111/nmo.12071. [DOI] [PubMed] [Google Scholar]
- 3.Gyawali CP, Patel A. Esophageal motor function: technical aspects of manometry. Gastrointest Endosc Clin N Am. 2014;24:527–43. doi: 10.1016/j.giec.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Porter RF, Kumar N, Drapekin JE, Gyawali CP. Fragmented esophageal smooth muscle contraction segments on high resolution manometry: a marker of esophageal hypomotility. Neurogastroenterol Motil. 2012;24:763–8. e353. doi: 10.1111/j.1365-2982.2012.01930.x. [DOI] [PubMed] [Google Scholar]
- 5.Shaker A, Stoikes N, Drapekin J, Kushnir V, Brunt LM, Gyawali CP. Multiple rapid swallow responses during esophageal high-resolution manometry reflect esophageal body peristaltic reserve. Am J Gastroenterol. 2013;108:1706–12. doi: 10.1038/ajg.2013.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bredenoord AJ, Fox M, Kahrilas PJ, Pandolfino JE, Schwizer W, Smout AJ International High Resolution Manometry Working Group. Chicago classification criteria of esophageal motility disorders defined in high resolution esophageal pressure topography. Neurogastroenterol Motil. 2012;24(Suppl 1):57–65. doi: 10.1111/j.1365-2982.2011.01834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roman S, Kahrilas PJ, Boris L, Bidari K, Luger D, Pandolfino JE. High-resolution manometry studies are frequently imperfect but usually still interpretable. Clin Gastroenterol Hepatol. 2011;9:1050–5. doi: 10.1016/j.cgh.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gyawali CP. Making the most of imperfect high-resolution manometry studies. Clin Gastroenterol Hepatol. 2011;9:1015–6. doi: 10.1016/j.cgh.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 9.Chan WW, Haroian LR, Gyawali CP. Value of preoperative esophageal function studies before laparoscopic antireflux surgery. Surg Endosc. 2011;25:2943–9. doi: 10.1007/s00464-011-1646-9. [DOI] [PubMed] [Google Scholar]
- 10.Kwiatek MA, Mirza F, Kahrilas PJ, Pandolfino JE. Hyperdynamic upper esophageal sphincter pressure: a manometric observation in patients reporting globus sensation. Am J Gastroenterol. 2009;104:289–98. doi: 10.1038/ajg.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peng L, Patel A, Kushnir V, Gyawali CP. Assessment of upper esophageal sphincter function on high-resolution manometry: identification of predictors of globus symptoms. J Clin Gastroenterol. 2014 doi: 10.1097/MCG.0000000000000078. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]