Abstract
Factors associated with maintaining good cognition into old age are unclear. Decreased brain-derived neurotrophic factor (BDNF) contributes to memory loss in Alzheimer's disease (AD), and soluble assemblies of amyloid-beta (Aβ) and tau contribute to neurodegeneration. However, it is unknown whether AD-type neuropathology, soluble Aβ and tau, or levels of BDNF and its receptor TrkB correlate with dementia in the oldest-old. We examined these targets in post-mortem Brodmann's areas 7 and 9 (BA7, BA9) in 4 groups of subjects >90 years old: 1) No Dementia/No AD Pathology, 2) No Dementia/AD Pathology, 3) Dementia/No AD Pathology, 4) Dementia/AD Pathology. In BA7, BDNF mRNA correlated with MMSE scores and was decreased in demented vs. non-demented subjects, regardless of pathology. Soluble Aβ42 was increased in both groups with AD pathology, demented or not, compared to No dementia/No AD Pathology subjects. Groups did not differ in TrkB isoform levels or in levels of total soluble tau, individual tau isoforms, threonine-181 tau phosphorylation or ratio of phosphorylated 3R to 4R isoforms. In BA9, soluble Aβ42 correlated with MMSE scores and with BDNF mRNA expression. Thus, soluble Aβ42 and BDNF, but not TrkB or soluble tau, correlate with dementia in the oldest-old.
Introduction
The rapidly growing population of oldest-old (nonagenerians and centenarians) brings with it an urgent need for a better understanding of age-related neurodegenerative diseases like Alzheimer's disease (AD) in this age group. There is substantial evidence suggesting that neurotrophic factors, mainly BDNF, play an important role in the etiology of AD (Fahnestock, 2011; Siegel et al., 2000; Murer et al., 2001). Most studies on younger-old subjects (mainly in their seventies and eighties) have demonstrated that areas of the brain predominantly affected by AD-type pathology, hippocampus, cortex and basal forebrain, exhibit down-regulated levels of BDNF (Connor et al., 1997; Ferrer et al., 1999; Garzon et al., 2002; Hock et al., 2000; Holsinger et al., 2000; Peng et al., 2005; Phillips et al., 1991). Reduction of BDNF protein in the parietal cortex has been shown to correlate with cognitive decline (Peng et al., 2005), suggesting that this decrease could be associated with the pathogenesis of AD. In vitro studies revealed that soluble oligomeric Aβ42 and not fibrillar (plaque) Aβ42 is the species responsible for decreased BDNF mRNA expression (Garzon & Fahnestock, 2007).
BDNF is vital for learning and memory (Lu et al., 2014; Yamada & Nabeshima, 2003). Restoring BDNF levels in animal models of AD by delivery of protein, viral vectors or stem cells (Nagahara et al., 2009; Blurton-Jones et al., 2009) or by lifestyle changes such as antioxidant diet and environmental enrichment (physical and cognitive exercise and social interaction; Fahnestock et al., 2012) counteracts learning and memory deficits. BDNF exerts its biological actions through its receptor, TrkB. In the human brain, there are 3 alternatively-spliced transcripts of TrkB translated into 3 isoforms: TrkB-FL, the full-length signaling receptor with a catalytic tyrosine kinase domain, and two truncated forms, TrkB-T1 and TrkB-Shc, lacking the kinase domain (Stoilov et al., 2002; Luberg et. al., 2010). Reports of TrkB expression in brain tissue of AD subjects are mixed: increased, no change and decreased levels have been reported (Connor et al., 1996; Allen et al., 1999; Savaskan et al., 2000; Ginsberg, 2006b; Ferrer, 1999; Wong et al., 2012).
The neuropathology of AD includes extracellular amyloid plaques containing fibrillar, insoluble Aβ, composed mainly of the most fibrillogenic form of Aβ, Aβ42, and intracellular neurofibrillary tangles containing hyperphosphorylated tau. Despite a relatively well-documented association between the amount of AD-type neuropathology and the severity of cognitive impairment (Arriagada et al., 1992; Bennett et al., 2004; Berg et al., 1998; Braak et al., 1993; Gold et al., 2000; Nagy et al., 1995), there is a lack of agreement regarding the contribution of amyloid plaques and neurofibrillary tangles towards a decline in cognition (Castellani et al., 2006; Lee et al., 2005; Nelson et al., 2007). This disagreement led to the emerging idea that AD-type dementia might be caused not by plaques, but rather by different pools and assemblies of soluble Aβ (Kuo et al., 1996; Gong et al., 2003; Lesné et al., 2006; McDonald et al., 2010; Steinerman et al., 2008).
Tau is a natively unfolded, highly soluble, microtubule-associated protein that exists in brain in 6 different isoforms (Goedert et al., 1988; Lewis et al., 1988). The isoforms differ by absence (0N form) or presence of N-terminal inserts of either 29 amino acids (encoded by exon 2; 1N form) or 58 amino acids (encoded by exons 2 and 3; 2N form) and inclusion of 3 or 4 repeats in the C-terminal microtubule binding domain (3R or 4R) (Goedert et al., 1989; Goedert & Jakes, 1990). The 4th 31-amino-acid repeat is encoded by exon 10, such that isoforms are also designated by the presence (4R) or absence (3R) of exon 10. The isoforms are differentially expressed during development and are differentially distributed (Kosik et al., 1989; Goedert & Jakes, 1990), which suggests they have different physiological roles. Structural differences between tau isoforms could also play a role in pathology. For example, 3R and 4R isoforms have distinct microtubule binding structures, which differentially promote the assembly of microtubules (Goode et al., 2000). Whether the ratio of 4R/3R isoforms is altered in AD is controversial (Hong et al., 1998; Ginsberg et al., 2006a). Tau protein contains multiple putative serine (Ser), threonine (Thr) and tyrosine phosphorylation sites which are phosphorylated in both normal and fibrillar tau (reviewed in Lee et al., 2001; Buée et al., 2000). During postmortem delay the sites undergo rapid dephosphorylation at different rates. The site Thr181 (AT270) is one of the more stable phosphorylation sites in tau (Matsuo et al., 1994). Tau phosphorylated at Thr181 is considered as a biomarker of AD: its levels are increased in cerebrospinal fluid of subjects with AD (Vanmechelen et al., 2000; Kapaki et al., 2007).
In the oldest-old population, it is unclear whether Aβ and tau pathologies correlate with cognitive status. There are reports of a lack of association between dementia and pathology in this population (Haroutunian et al., 2008; Savva et al., 2009) as well as studies reporting strong correlations of Aβ and tau pathologies with dementia (Gold et al., 2000; Nelson et al., 2007; Dolan et al., 2010). Robinson et al. (2011, 2014) showed that dementia in subjects of the 90+ Study is strongly associated with plaque and tangle measures. In our cohort there are demented subgroups with and without pathology, affording an opportunity to study the correlation of dementia with the less traditional measures of soluble Aβ and tau. Although the majority of nonagenarians and centenarians are demented, there is a subgroup without dementia, and studying those subjects may help us to determine the factors associated with maintaining good cognition even to very old age. Furthermore, there is no published data on BDNF and TrkB transcript/isoform expression in the oldest-old or their association with cognitive status and AD pathology in this group. In this study, we examined mRNA and protein expression of BDNF and TrkB isoforms in Brodmann Area 7 (BA7) of subjects over 90 years old from the 90+ Study, we measured the levels of soluble Aβ42 and tau and we investigated the association of the measured variables with cognitive status. We chose BA7 because it is an area of parietal cortex that we have previously studied intensively for its BDNF expression and regulation (Holsinger et al., 2000; Garzon et al., 2002; Michalski & Fahnestock, 2003; Peng et al., 2005), and it is an area known to be severely affected in AD (Bruner & Jacobs, 2013). We also examined some of these targets and correlations in prefrontal cortex (Brodmann Area 9), another area severely affected in AD (DeKosky & Scheff, 1990).
Materials and Methods
Subject Characteristics
Fifty-one subjects from the 90+ Study, a longitudinal study of aging and dementia in southern California (Kawas, 2006) were divided into 4 clinicopathologic groups based on the presence of AD-type pathology (extracellular Aβ plaques and intracellular tau pathology) and AD-type clinical dementia. Pathological diagnosis was determined by a neuropathologist who was blinded to clinical status. Aβ pathology was rated using Consortium to Establish a Registry for Alzheimer's disease (CERAD) plaque scores (score 0-3, Mirra et al., 1991). Tau pathology was assessed using Braak staging (stages I-VI, Braak & Braak, 1991). Dementia diagnoses were determined applying the Diagnostic and Statistical Manual of Mental Disorders 4th edition criteria (American Psychiatric Association, 1994). Cognitive status was determined during a multidisciplinary consensus conference after death, using all available information and blinded to pathological evaluations. Information included longitudinal neuropsychological testing (including the Mini-Mental State Examination [MMSE]), neurological examinations, informant questionnaires (Kawas et al., 1994), medical records and clinical neuroimaging when available. For a more complete description of 90+ Study procedures, see Corrada et al. (2012) and Kawas et al. (2015). The first group of subjects, considered controls, consisted of 13 subjects, not demented and without AD pathology (ND/NP). About half of the controls did have minor plaque and tangle pathology. The second group contained 14 non-demented subjects, 6 of whom were classified as Cognitive Impairment, not demented (CIND), and 8 with normal cognition, all of them with AD pathology (ND/P). While CIND included people who would be considered MCI, it was defined as people with cognitive impairment involving memory or other domains such as executive function, language, etc., but not meeting criteria for dementia, typically because they did not have functional loss in activities of daily living, or did not have more than one cognitive domain affected. One of the subjects in this group with normal cognition was identified in a boxplot of BDNF mRNA analysis as an outlier (BDNF mRNA copies were greater than 1.5 times the interquartile range) and therefore was excluded from this part of the study (mRNA analysis). The third group included 9 demented subjects without AD pathology (D/NP). All 9 subjects from this latter group (D/NP) exhibited a low number of tangles, identified as Braak stages II or III. Seven of them had some Aβ pathology, classified as CERAD plaque score 1, and two subjects’ plaque scores were 0. With this minor amount of pathology, subjects did not reach the criteria for AD diagnosis. In addition to minor tau and amyloid beta pathology, some subjects exhibited a low level of other pathologies: 4 subjects had microinfarcts, one had white matter disease (subcortical arteriolosclerotic leukoencephalopathy) and one had both of these plus Lacunes or large infarcts. The fourth group contained 15 subjects with typical AD-type dementia and pathology (D/P). Western blot measurements were not obtained for one of the D/P samples. The MMSE score for one of the samples from the D/P group was not available. Characteristics of the 90+ Study samples are summarized in Table 1.
Table 1.
Characteristics of clinicopathologic groups.
| Characteristics | All subjects n=51 | Not Demented/No Pathology n=13 | Not Demented/Pathology n=14 | Demented/No Pathology n=9 | Demented/Pathology n=15 |
|---|---|---|---|---|---|
| Gender, % female | 71 | 46 | 86 | 67 | 80 |
| ApoE e4 (% e4 allele) | 12 (24) | 2 (15) | 2 (14) | 3 (33) | 5 (33) |
| MMSE total score, mean (range) | 19.86 (0-30) | 27.38 (24-29) | 26.57 (16-30) | 14.89 (3-22) | 9.36 (0-28) |
| Age in years, mean (range) | 97.43 (92-105) | 97.38 (93-101) | 97.07 (94-101) | 98.44 (93-103) | 97.2 (92-105) |
| Months between MMSE & death, mean (range) | 5.46 (0-44) | 5.15 (0-13) | 4.64 (1-14) | 3.22 (0-5) | 8.00 (0-44) |
| PMI in hours, Mean (range) | 5.15 (2.2-18.5) | 4.85 (2.2-8.8) | 4.78 (3.3-11.5) | 5.48 (3.0-14.4) | 5.55 (2.8-18.5) |
| Braak tangle stage, mean (range) | 3.78 (II-VI) | 2.62 (II-III) | 4.07 (III-VI) | 2.44 (II-III) | 5.33 (III-VI) |
| CERAD plaque score, mean (range) | 1.57 (0-3) | 0.23 (0-1) | 2.29 (2-3) | 0.78 (0-1) | 2.53 (2-3) |
| Cognitive diagnosis, N (%), Normal | 21 (41) | 13 (100) | 8 (57) | 0 (0) | 0 (0) |
| CIND | 6 (12) | 0 (0) | 6 (43) | 0 (0) | 0 (0) |
| Dementia | 24 (47) | 0 (0) | 0 (0) | 9 (100) | 15 (100) |
RNA Extraction
RNA was extracted from approximately 100 mg of frozen post-mortem tissue of BA7 or BA9. Each sample was homogenized in TRIzol® (Invitrogen, Burlington, ON, Canada) at a ratio of 1 ml of TRIzol® per 100 mg of tissue using a sonic dismembrator (Fisher Scientific, Toronto, ON, Canada). Invitrogen's protocol was followed through the collection of the RNA-containing aqueous phase. The RNA was further purified and DNase treated using an RNeasy® Mini Kit (Qiagen, Mississauga, ON, Canada) according to the manufacturer's instructions. Concentration and purity of RNA were determined by absorbance at 260, 280 and 230 nm using a Thermo Scientific NanoDrop 2000c (Fisher Scientific, Toronto, ON, Canada). RNA integrity was visualized by agarose gel electrophoresis.
Real-time RT-qPCR
One microgram of each RNA sample was reverse transcribed in a 20μl reaction using Superscript III® (Invitrogen) following the manufacturer's protocol. Negative controls lacking reverse transcriptase were included to confirm lack of genomic DNA contamination.
PCR primers were designed with PRIMER3 software (freeware program online, Rozen & Skaletsky, 2000) and were synthesized at the MOBIX facility at McMaster University. Primer sequences and PCR product sizes are shown in Table 2. For all PCR reactions, 300 nM of the forward and reverse primers were used. Each 20 μl real-time PCR reaction contained 10 μl of SYBR® Green qPCR SuperMix UDG (Invitrogen), 30 nM of reference dye ROX (Invitrogen) and cDNA derived from 50 ng RNA per sample (5 or 50 ng for β-actin) or standards. Standards for BDNF and TrkB isoforms were PCR products generated using target-specific primers. PCR products were gel purified using a Qiagen kit and quantified by spectrophotometry (Thermo Scientific NanoDrop 2000c; Fisher Scientific, Toronto, ON, Canada). Standards for β-actin were generated from a commercially available plasmid (Invitrogen). Real-time amplifications were carried out in triplicate (or duplicate for TrkB-T1) using the MX3000P PCR system (Stratagene, La Jolla, CA, USA) and the following thermal profile: 2 min at 50°C, 2 min at 95°C followed by 40 cycles of 95°C for 30 s, 58°C for 30 s, and 72°C for 45 s for BDNF and TrkB isoforms. The thermal profile for β-actin was: 95°C for 15 s, 58°C for 30 s, and 72°C for 30 s. Standard curve R2 values were > 0.995 and efficiencies were > 90%. Following 40 cycles of amplification, a dissociation curve was added to determine if any secondary products had formed. mRNA copy numbers of BDNF and TrkB mRNAs are presented as a ratio to copy numbers of the housekeeping gene β-actin.
Table 2.
Sequences of PCR primers.
| Target and accession number | Forward primer | Reverse primer | PCR product size (bp) |
|---|---|---|---|
| BDNF NM_001709 | 5′-AAA CAT CCG AGG ACA AGG TG -3′ | 5′- AGA AGA GGA GGC TCC AAA GG-3′ | 250 |
| TrkB-FL NM_001018064 | 5′-GGC CCA GAT GCT GTC ATT AT-3′ | 5′-TCC TGC TCA GGA CAG AGG TT-3′ | 206 |
| TrkB-Shc NM_001018066.2 | 5′-GGC CCA GAT GCT GTC ATT AT-3′ | 5′-AGG CAT GGA TTT AGC CTC CT-3′ | 191 |
| TrkB-T1 NM_001007097 | 5′- TGC CTT TTG GTA ATG CTG TTT-3′ | 5′-GGC TTC ATA TAG TAC AGC CTC CA-3′ | 265 |
| β-actin NM_001101 | 5′-CTC TTC CAG CCT TCC TTC-3′ | 5′-TGT TGG CGT ACA GGT CTT-3′ | 109 |
Protein extraction and measurements
Extraction of soluble Aβ was based on previously published methods with some modifications (Steinerman, 2008; Lesné et al., 2006; Kawarabayashi et al., 2001). Briefly, approximately 100 mg of post-mortem tissue from each subject was sonicated in Tris Buffered Saline (TBS), containing 25 mM Tris pH7.5, 150 mM NaCl, 10 mM EDTA, and protease and phosphatase inhibitor cocktails (Roche, Mississauga, ON, Canada) using a sonic dismembrator (Fisher Scientific) at a ratio of 1ml of buffer per 100mg of tissue. This procedure allowed us to extract an enriched pool of proteins containing extracellular, soluble Aβ (Lesné et al., 2006). The homogenates were kept on ice for 5-10min and then centrifuged at 14 000 × g for 15 min at 4°C to remove cellular debris and fibrillar Aβ. Preparation of homogenates for soluble tau analysis was based on published methods (Guillozet-Bongaarts et al., 2005; Tremblay et al., 2007) with some modification. Our tissue extract contained pooled TBS-soluble and detergent-soluble fractions of protein. Each sample (100 ±1.4 mg) was sonicated in Tris Buffered Saline containing detergent (TBS/detergent; 50 mM Tris pH 7.6, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100) with phosphatase and protease inhibitors as above. Homogenates were kept on ice for 5-10 minutes, then centrifuged at 14,000 × g for 10 min at 4°C. TBS /detergent-soluble protein preparations were used also for analysis of BDNF and TrkB.
The supernatants obtained from both methods were collected, aliquoted and kept frozen at −80°C until use. Concentrations of total TBS-so luble and total TBS/detergent-soluble proteins were established using the Dc™ protein assay (Bio-Rad Laboratories, Mississauga, ON, Canada). Levels of Aβ42 and Aβ40 in the TBS-soluble fraction were assayed using the Colorimetric BetaMark™ Beta-Amyloid x-42 or x-40 ELISA Kit (Covance, Montreal, Quebec, Canada or Biolegend, San Diego, CA, USA). Tau was assayed by Tau [total] Human ELISA kit (Invitrogen) and BDNF by Human BDNF DuoSet ELISA kit (R & D Systems, Minneapolis, MN, USA) in the TBS/detergent-soluble homogenate. Each sample and standard was assayed in duplicate, and the manufacturer's protocols were followed. Values obtained by ELISAs are presented as amount of measured protein per mg (or μg for tau) of total protein.
For analysis of phospho-tau, total tau and TrkB isoforms, 40 μg of each TBS/detergent homogenate was resolved on an 8% polyacrylamide gel. Gel electrophoresis and transfer were done as previously described (Fahnestock et al., 2001) with the exception that Immobilon-FL membrane was used (Millipore, Etobicoke, ON, Canada). An unphosphorylated human recombinant tau ladder (rPeptide, Bogart, GA, USA) was run on some blots. For tau detection, the antibodies 39E10, Tau5, Tau12 and Tau46 (Covance, Montreal, Quebec, Canada) were used; for TrkB isoform detection, a rabbit monoclonal antibody (Cell Signalling Technology, Danver, MA, USA) was used. To investigate phosphorylation of tau protein at different sites, antibodies directed against Thr181 and Ser203 (Cell Signalling Technology) and Thr231 (Covance) were tested. Membranes used for analysis of phospho-tau, total tau and TrkB isoforms were cut at 75kDa, and the upper half was incubated for 48 hr with TrkB antibody (1:1000 dilution). The lower half was incubated overnight with rabbit monoclonal phospho-tau Thr181 antibody (1:1000 dilution), with mouse monoclonal 39E10 antibody (1:500 dilution) and then with β-actin antibody (dilution 1:4000; Sigma, Oakville, ON, Canada). Primary and secondary antibodies (Goat Anti-rabbit IRDye® 680 and Goat Anti-Mouse IRDye® 800CW; LI-COR Biosciences, Lincoln, Nebraska, USA; dilutions 1:8000) were diluted in Odyssey Blocking Buffer (LI-COR Biosciences) mixed 1:1 with PBS and containing 0.05% Tween-20. Signals were detected and quantified using an Odyssey® Imaging System (LI-COR Biosciences).
Statistical Analysis
Statistical analyses were performed using SPSS 20.0 software (SPSS, Chicago, IL, USA) and GraphPad Prism software version 3.03 (GraphPad, La Jolla, CA, USA). A one-way ANOVA followed by post hoc Tukey's test was used to analyze differences between the four clinicopathologic groups, whereas a Student t-test was used to compare two groups. Differences were considered significant when the associated p value was less than 0.05. MMSE scores closest to death were used for the analyses. Correlation analyses were performed using the Pearson test. Correlations were considered significant when p<0.05 or p<0.01 (2-tailed).
Results
Sample and group characteristics
Mean post-mortem interval (PMI) of all the samples was 5.15 ± 0.39 hours, and PMIs were not different between clinicopathologic groups (one-way ANOVA, p=0.86). The groups did not differ in age, gender or presence of APOE e4 allele (one-way ANOVA, p=0.72, p=0.12 and p=0.51, respectively). The average time between the last MMSE and death was 5.5 months, and this interval was not different between groups (one-way ANOVA, p=0.45).
We examined tissues from the superior parietal cortex (BA7) and the prefrontal cortex (BA9). For two of the subjects from group ND/P, BA7 samples were precuneus sections. None of the measurements for these precuneus samples were different from the rest of the samples in the ND/P group.
BDNF
BDNF mRNA expression for each sample is presented as a ratio of BDNF mRNA copies to mRNA copies of the housekeeping gene β-actin. The clinicopathologic groups did not differ in levels of β-actin mRNA (data not shown, one-way ANOVA, p = 0.471 for BA7, p = 0.361 for BA9).
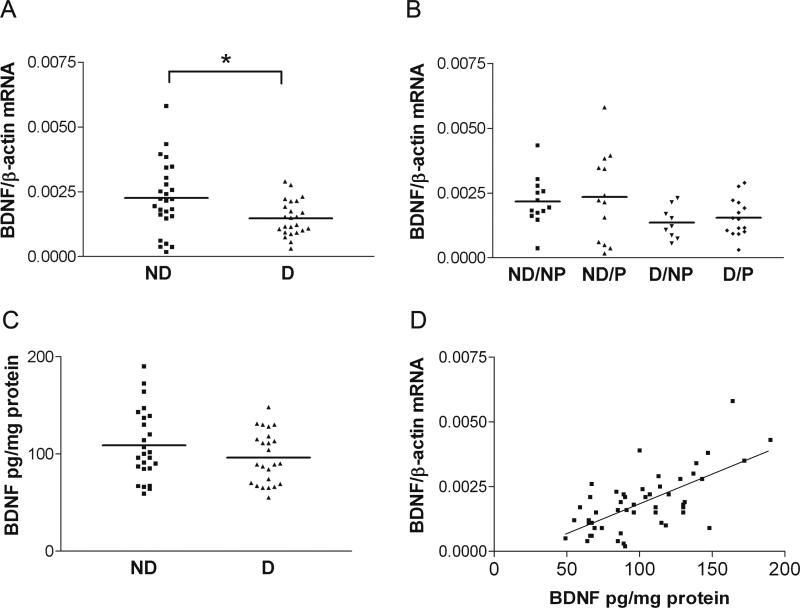
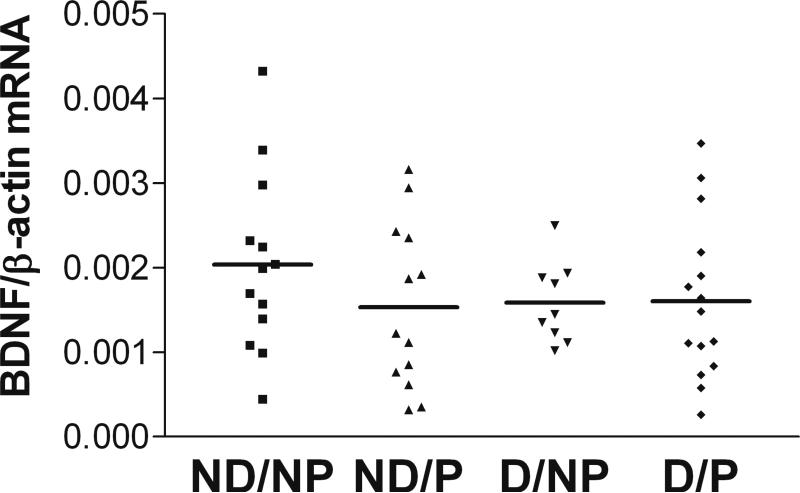
Normalized BDNF mRNA expression in BA7 of demented subjects (with or without pathology, D/NP+D/P, n=24) was significantly decreased compared to dementia-free subjects (with or without pathology, ND/NP+ND/P, n=26; Figure 1A, two-tailed Student t-test, p=0.014). Comparing levels of BDNF mRNA in the four separate clinicopathologic groups, subjects in both demented groups exhibited a trend towards lower levels of BDNF mRNA than subjects in both non-demented groups (Figure 1B, one-way ANOVA, p=0.1). Neither the 4 clinicopathologic groups (data not shown) nor the demented subjects compared to non-demented subjects (regardless of pathology) differed significantly in BDNF protein levels (Figure 1C, one-tailed Student t-test, p=0.11). However, there was a highly significant correlation between levels of BDNF mRNA and BDNF protein (Figure 1D, Pearson correlation, r=0.658, p<0.001). BDNF mRNA expression levels in BA9 were not different between clinicopathologic groups (Figure 2, one-way ANOVA, p=0.492). Normalized BDNF mRNA expression in BA9 of demented subjects (D/NP+D/P) was not significantly different from dementia-free subjects (data not shown, ND/NP+ND/P, two-tailed Student t-test, p=0.48).
Figure 1. BDNF mRNA and protein expression in BA7.
A: BDNF mRNA expression is decreased in demented subjects, regardless of pathology. Demented subjects (D/P+D/NP, n=24) compared to not demented (ND/NP+ND/P, n=26, two-tailed Student t-test, p=0.014). B: Trend towards significant differences between the four clinicopathologic groups broken down by cognition and pathology (one-way ANOVA, p=0.1). Clinicopathologic groups: (1) ND/NP, not demented with no pathology, control group, n=13, (2) ND/P, not demented with pathology, n=13 (protein n=14), (3) D/NP, demented with no pathology, n=9, (4) D/P, demented with pathology, typical AD, n=15. C: No difference between groups in level of BDNF protein (one-tailed Student t-test, p=0.11). Horizontal bars represent the group mean. D: Highly significant correlation of BDNF mRNA with BDNF protein (Pearson correlation, r=0.658, p<0.001).
Figure 2. No difference between the four clinicopathologic groups in BDNF mRNA expression in BA9.
Clinicopathologic groups: ND/NP, not demented with no pathology, control group, n=13; ND/P, not demented with pathology, n=13; D/NP, demented with no pathology, n=9; D/P, demented with pathology, typical AD, n=15. One-way ANOVA, p=0.492. Horizontal bars represent the group mean.
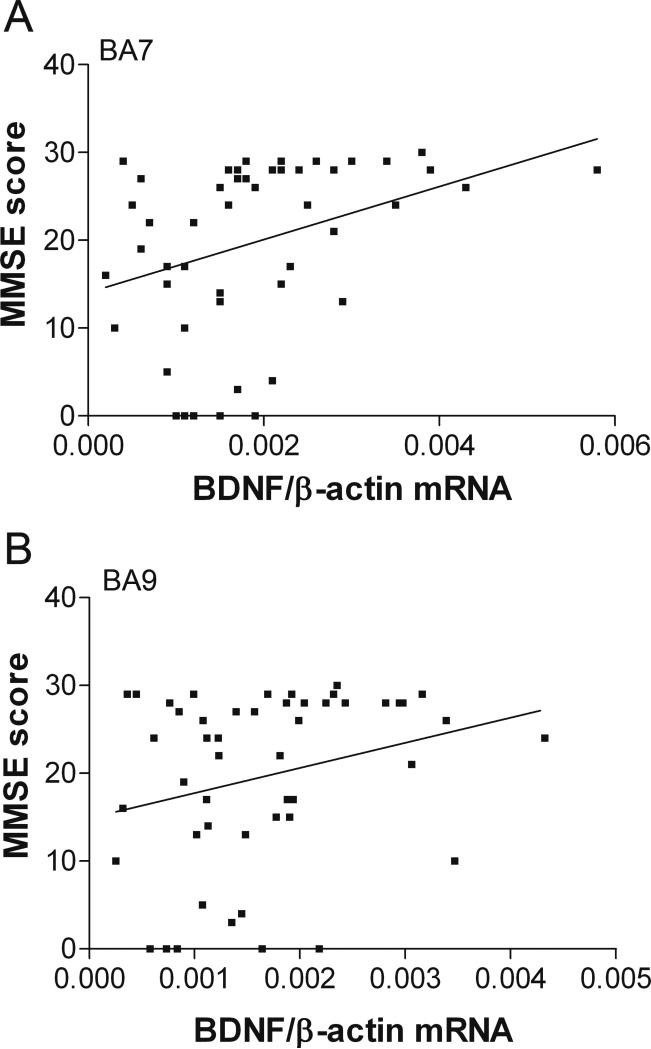
Further statistical analysis of BDNF mRNA expression in BA7 and BA9 revealed a significant positive correlation between BDNF mRNA expression of the subjects and their MMSE scores (BA7; Figure 3A, Pearson correlation r=0.350, p<0.05) or a strong trend (BA9; Figure 3B, Pearson correlation, r=0.263, p=0.068). There was a lack of correlation between BDNF mRNA and either CERAD plaque scores or Braak tangle stages (data not shown, BA7: Pearson correlation, r=−0.054 and r=−0.116, respectively, p>0.05; BA9: Pearson correlation r=−0.122 and r=−0.024, respectively, p>0.05).
Figure 3. Correlations of BDNF mRNA expression in BA7 and BA9 with MMSE scores.
A: BA7, Pearson correlation, r=0.350, p<0.05. B: BA9, Pearson correlation, r=0.263, p=0.068.
TrkB isoforms
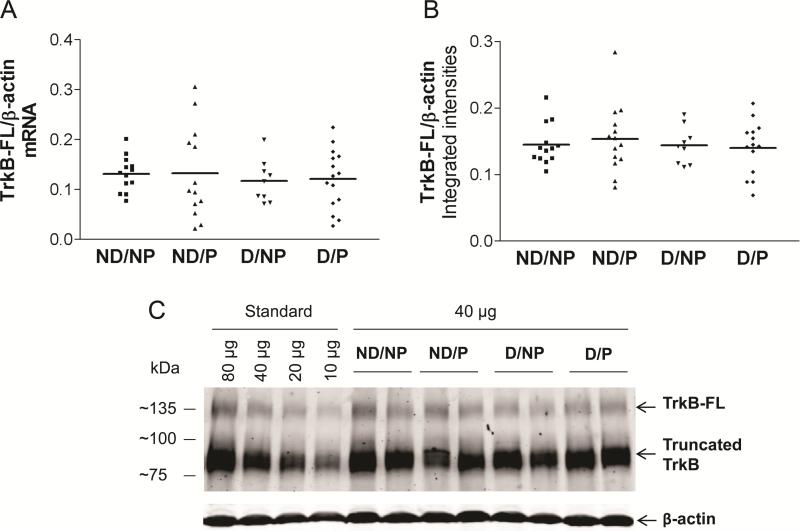
Real-time qRT-PCR analysis of mRNA expression for all 3 isoforms of TrkB (TrkB-FL, TrkB-Shc and TrkB-T1) in BA7 did not reveal any differences between groups (Figure 4A,TrkBFL; data not shown for truncated forms; one-way ANOVA, p>0.05 for each target). Western blotting also did not detect any differences between groups in the protein levels of TrkB-FL (140 kDa band) or truncated forms TrkB-Shc and TrkB-T1 (90kDa band, Figure 4B,C). Correlation analysis of TrkB mRNA isoform expression revealed that TrkB-FL and TrkB-Shc were positively associated with BDNF expression (Pearson correlation, r=0.720 and r=0.706, respectively, p<0.001) but TrkB-T1 was not (Pearson correlation, r=0.17, p>0.05). Similarly, TrkB-FL protein correlated with BDNF protein (Pearson correlation, r=0.358, p<0.05), and with TrkB-FL mRNA (Pearson correlation, r=0.331, p<0.05). None of the TrkB isoforms correlated with either CERAD plaque scores or Braak tangle stages (data not shown, p>0.05).
Figure 4. Full-length TrkB (TrkB-FL) mRNA and protein levels do not differ between groups.
(one-way ANOVA, p>0.05; Clinicopathologic groups: (1) ND/NP, not demented with no pathology, control group, n=13; (2) ND/P, not demented with pathology, n=13 (protein n=14); (3) D/NP, demented with no pathology, n=9; (4) D/P, demented with pathology, typical AD, n=15 (protein n=14). Horizontal bars represent the group mean. A: TrkB-FL mRNA, B: TrkB-FL protein C: Representative TrkB Western blot.
TBS-soluble Aβ42 and Aβ40
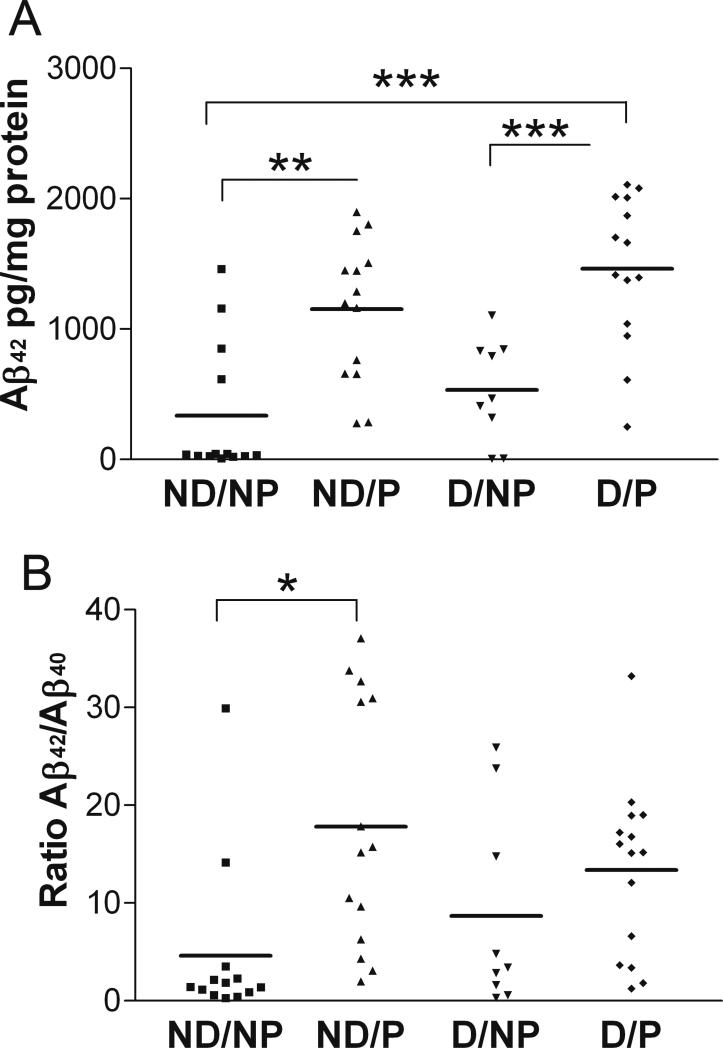
There were highly significant differences between clinicopathologic groups in the amount of TBS-soluble Aβ42 in BA7 (Figure 5A, one-way ANOVA, p<0.0001). The dementia- and pathology-free control group (ND/NP) exhibited the lowest level of soluble Aβ42, similar to the level of Aβ42 in the demented group without pathology (D/NP), whereas groups with AD-type pathology, demented (D/P) or not (ND/P), showed a 3-4-fold increase in the amount of soluble Aβ42 compared to the control group (post hoc Tukey's test, p<0.001 and p<0.01, respectively). There were no differences between clinicopathologic groups in the amount of TBS-soluble Aβ40 in BA7 (data not shown, one-way ANOVA, p=0.515), but there were significant differences between groups in the ratio of Aβ42/Aβ40 (Figure 5B, one-way ANOVA, p=0.010). The Aβ42/Aβ40 ratio distinguished between the non-demented groups with and without pathology, but not between the demented groups.
Figure 5. Significant differences between clinicopathologic groups in TBS-soluble Aβ42 (A) and Aβ42/Aβ40 ratio (B) in BA7.
Clinicopathologic groups: (1) ND/NP, not demented with no pathology, control group, n=13; (2) ND/P, not demented with pathology, n=14; (3) D/NP, demented with no pathology, n=9; (4) D/P, demented with pathology, typical AD, n=15. Horizontal bars represent the group mean. One-way ANOVA and post hoc Tukey's test, ***p<0.001, **p<0.01, *p<0.05.
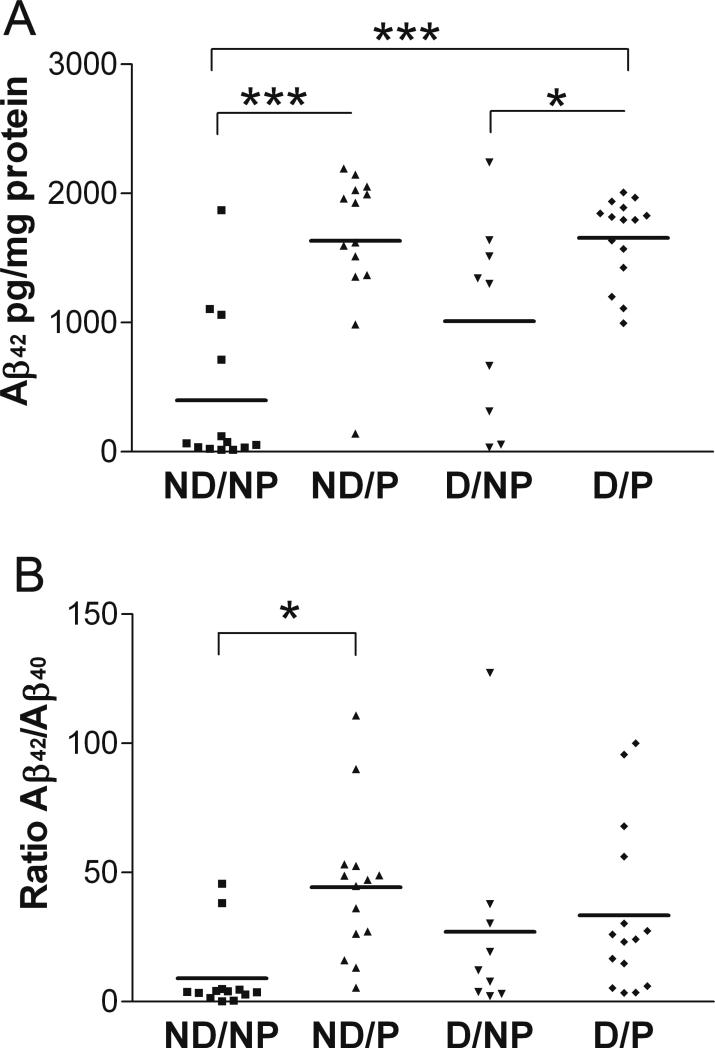
Levels of soluble Aβ42 in BA9 were also significantly different between clinicopathologic groups (Figure 6A, one-way ANOVA, p<0.0001). As in BA7, the ND/NP group had the lowest level of Aβ42, and both groups with AD-type pathology exhibited the highest levels of Aβ42. Similar to BA7, levels of TBS-soluble Aβ40 in BA9 were not different between groups (data not shown, one-way ANOVA, p=0.346), but there were significant between-group differences in the ratio of Aβ42/Aβ40 (Figure 6B, one-way ANOVA, p=0.024). As in BA7, whereas Aβ42 levels distinguished pathology from pathology-free groups for both demented and non-demented cases, the ratio of Aβ42/Aβ40 distinguished only the two non-demented groups from one another.
Figure 6. Significant differences between clinicopathologic groups in TBS-soluble Aβ42 (A) and Aβ42/Aβ40 ratio (B) in BA9.
Clinicopathologic groups: (1) ND/NP, not demented with no pathology, control group, n=13; (2) ND/P, not demented with pathology, n=14; (3) D/NP, demented with no pathology, n=9; (4) D/P, demented with pathology, typical AD, n=15. Horizontal bars represent the group mean. One-way ANOVA and post hoc Tukey's test ***p<0.001, *p<0.05.
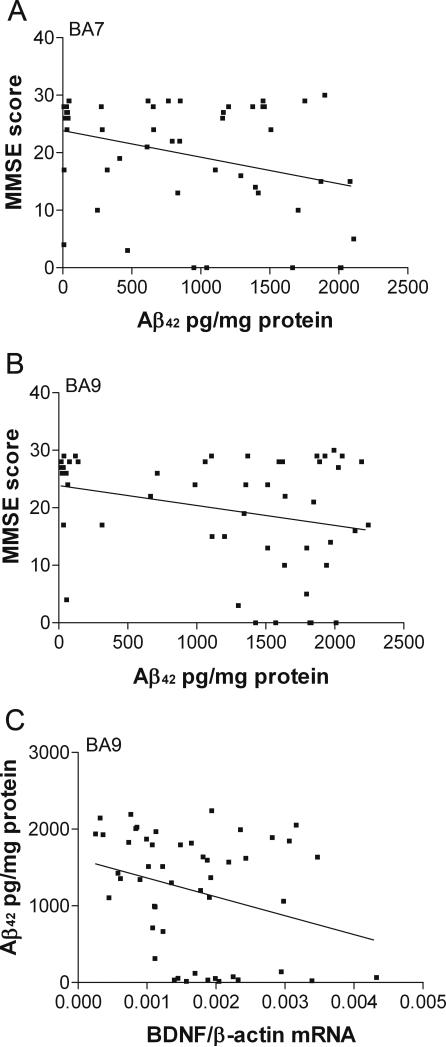
Correlation analysis revealed a significant negative association of soluble Aβ42 with MMSE scores in BA7 (Figure 7A, Pearson correlation, r=−0.318, p<0.05) and a highly significant positive correlation with Braak stages (Pearson correlation, r=0.709, p<0.001) and CERAD scores (Pearson correlation, r=0.725, p<0.001). There was no correlation in BA7 between soluble Aβ42 and levels of BDNF or TrkB isoforms (Pearson correlation, p>0.05, data not shown).
Figure 7. Correlations of soluble Aβ42 with MMSE scores and with BDNF mRNA.
Pearson correlations. A: BA7, Aβ42 and MMSE, r=−0.318, p<0.05; B: BA9, Aβ42 and MMSE, r=−0.248, p=0.082; C: BA9, Aβ42 and BDNF mRNA, r=−0.296, p=0.037.
In BA9 there was a strong trend towards a significant negative correlation of Aβ42 with MMSE scores (Figure 7B, Pearson correlation, r=−0.249, p=0.082). Similarly to BA7, there was also a highly significant correlation of soluble Aβ42 with Braak stages (Pearson correlation, r=0.505, p<0.001) and CERAD scores (Pearson correlation, r=0.709, p<0.001, data not shown). Unlike in BA7, BDNF mRNA expression of BA9 significantly inversely correlated with levels of soluble Aβ42 (Figure 7C, Pearson correlation, r=−0.289, p<0.05).
Ratios of soluble Aβ42/Aβ40 in BA7 and BA9 correlated with Braak stages (Pearson correlation, BA7: r=0.368, p<0.01; BA9: r=0.273, p=0.052) and CERAD scores (Pearson correlation, BA7: r=0.533, p<0.001; BA9: r=0.417, p<0.01, data not shown). There was a lack of correlation of Aβ42/Aβ40 in both areas with MMSE and BDNF mRNA levels and of Aβ42/Aβ40 in BA7 with TrkB or tau (Pearson correlation, p>0.05, data not shown).
TBS/detergent-soluble tau
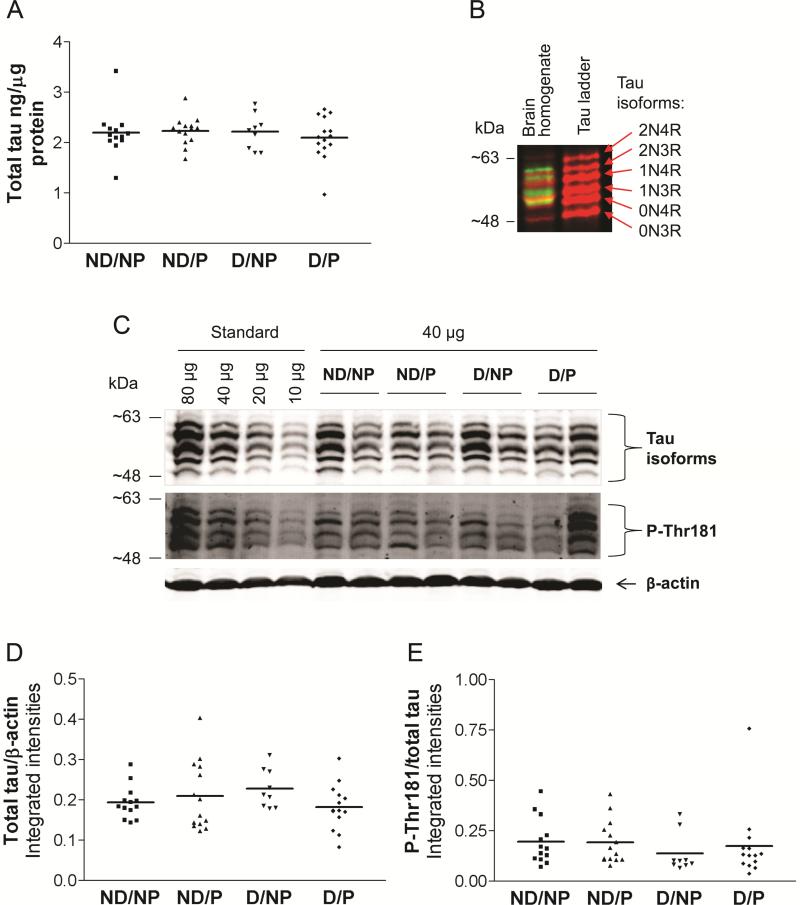
ELISA measurements did not detect any differences between clinicopathologic groups in total soluble tau levels in BA7 (Figure 8A). By Western blotting, bands at apparent molecular weights 48-65 kDa, representing different isoforms of tau (Goedert & Jakes, 1990; Deshpande et al., 2008), were detected by multiple anti-tau antibodies, 39E10, tau5, tau12, and tau46 (data not shown), directed against epitopes along the entire tau molecule (Table 3). Measurements of total tau (6 immunoreactive tau bands) for all samples were carried out on blots probed with 39E10 antibody directed against the proline-rich region of tau, which is present in all isoforms (Figure 8B,C). No differences were detected between clinicopathologic groups in total soluble tau as detected by Western blotting (Figure 8D). Furthermore, neither individual tau isoforms nor the ratio of 4R/3R tau differed between groups (one-way ANOVA, p>0.05, data not shown).
Figure 8. No difference between clinicopathologic groups in levels of soluble total tau or tau phosphorylated at Thr181 analysed by ELISA and Western blot.
Clinicopathologic groups: (1) ND/NP, not demented with no pathology, control group, n=13, (2) ND/P, not demented with pathology, n=14, (3) D/NP, demented with no pathology, n=9, (4) D/P, demented with pathology, typical AD, n=15 (Western blot n=14). Horizontal bars represent the group mean. A: total tau levels measured by ELISA. B: 39E10 antibody detects 6 isoforms of tau in a human recombinant tau ladder and in human brain homogenates (red color); antibody against tau phosphorylated at Threonine-181 detects 4 phosphorylated isoforms (green color) and does not cross-react with an unphosphorylated human recombinant tau ladder. C: Representative Western blot probed with mouse monoclonal 39E10 antibody (total tau) and with rabbit monoclonal phospho-tau antibody (Thr181) D: Total soluble tau analysed by Western blotting. E: Phosphorylation of tau at site Thr181 analysed by Western blotting. One-way ANOVA, p>0.05.
Table 3.
Tau antibodies used in this study and their epitopes.
| Antibody | Epitope* |
|---|---|
| 39E10 | 189-195, proline-rich domain |
| Tau5 | 210-230, proline-rich domain |
| Tau12 | 2-23, N-terminus |
| Tau46 | 404-441, C-terminus |
| p-Thr181 | Tau phosphorylated at Thr181 |
| p-Thr231 | Tau phosphorylated at Thr231 |
| p-Ser203 | Tau phosphorylated at Ser203 |
Epitope numbering is according to the longest CNS tau isoform 2N4R.
Antibodies 39E10 and p-Thr181 (BOLD) were used for measurements of immunoreactive tau bands in all samples.
Phospho-tau antibodies directed against Thr231 and Ser203 did not generate a quantifiable signal. Antibody against tau phosphorylated at site Thr181 detected 4 bands in human brain homogenates and did not react with an unphosphorylated human recombinant tau ladder (Figure 8B,C). Densitometry analysis did not reveal any differences between groups in tau phosphorylation at Thr181 (Figure 8E, one-way ANOVA, p=0.532) or in the ratio of phosphorylated 3R/4R isoforms (one-way ANOVA, p>0.05, data not shown)
Discussion
In this study, we analysed BDNF and TrkB isoform mRNA expression and their protein levels in the 90+ Study cohort. We show here that BDNF mRNA is down-regulated in the parietal cortex of subjects with AD (pathology and dementia) in the oldest-old cohort, in agreement with previous results in younger-old subjects (Garzon et al., 2002; Holsinger et al., 2000). We also show that subjects with dementia exhibit lower levels of BDNF mRNA than non-demented groups, regardless of pathology. We did not detect differences in BDNF protein levels between demented and non-demented subjects, which could be attributed to the greater sensitivity of qRT-PCR vs. ELISA. BDNF mRNA measured here by the highly sensitive quantitative RT-PCR assay detected a 34-fold change between the lowest and highest tested samples, whereas the ELISA detected only a 4-fold range in BDNF protein levels. Despite the difference in methods, samples which were high or low in qRT-PCR were high or low in the ELISA, which led to a highly significant correlation between BDNF mRNA and protein levels. Further strong involvement of BDNF in cognition is supported by a significant correlation of BDNF expression in BA7 and BA9 with MMSE scores of these subjects, as previously shown in younger-old samples in parietal cortex (Peng et al., 2005). Lastly, these findings are consistent with previous data in the oldest-old (Robinson et al., 2014) showing that hippocampal synaptic loss, which may be mediated by declining BDNF levels, correlates with cognitive impairment.
We also found that the four clinicopathologic groups do not differ in TrkB mRNA expression. Lack of alteration of TrkB mRNA or its isoforms in AD has previously been reported in human temporal and occipital cortices (Wong et al., 2012), and by our group in a canine model of age-related cognitive impairment (Fahnestock et al., 2012). Savaskan et al. (2000) reported no change in TrkB immunoreactivity in human AD parietal cortex compared to controls.
Soluble Aβ42 has emerged as a potentially toxic molecule, explaining why insoluble, fibrillar Aβ42 plaques often do not correlate well with cognitive decline in AD subjects (Benilova et al., 2012). However, for the segment of the oldest-old population in our study, AD pathology represented by CERAD scores and Braak stages correlated well with cognitive status of the subjects, as did soluble Aβ42 levels in both parietal and frontal cortex. Soluble Aβ42 assayed by ELISA in this study likely represented soluble extracellular Aβ (Lesné et al., 2006), containing a mixture of Aβ monomers, dimers, trimers, aggregates and some higher molecular weight species. However, this fraction was free from fibrillar Aβ which was removed by centrifugation (McDonald et al., 2010). Although we cannot determine which soluble Aβ42 species correlated with dementia in our study, TBS-soluble monomers and dimers have been implicated (McDonald et al., 2010). In both parietal and frontal cortex, soluble Aβ42, but not the ratio of Aβ42/Aβ40, distinguished groups with pathology from groups without pathology. Soluble Aβ42 was therefore a better indicator of Aβ42 toxicity than was the ratio of Aβ42/Aβ40.
There were no differences in total tau levels between clinicopathologic groups. Tau isoforms are differentially expressed during development and are differentially distributed (Goedert & Jakes, 1990), which suggests they may have different physiological roles. However, we did not find differences between clinicopathologic groups in levels of individual tau isoforms. The ratio of 3R/4R isoforms in normal brain is approximately 1, and this ratio is altered in some neurodegenerative diseases (Hong et al., 1998; Ginsberg et al., 2006a). In our study we did not find differences between clinicopathologic groups in 3R/4R ratio, in agreement with Hong et al. (1998). Regulation of tau isoform expression is region and cell-specific (Ginsberg et al., 2006a; McMillan et al., 2008) which might explain discrepancies between ours and Ginsberg et al. (2006a) results. Phosphorylation of tau at Thr181, considered a biomarker of AD, was detectable in post-mortem BA7 samples. However, clinicopathologic groups did not differ either in the level of phosphorylation at this site or in the ratio of phosphorylated 3R/4R isoforms. Despite short post mortem delays of the human samples, we cannot exclude that endogenous rapid dephosphorylation, heavily depending on postmortem interval and temperature of stored samples (Matsuo et al., 1994; Oka et al., 2011; Li et al., 2003), affected the results.
The findings reported here are from BA7 and BA9 and may not be representative of all regions of the brain. Nevertheless, in the 90+ study, the pathological diagnosis was not sufficient for cognitive decline. However, the significant inverse correlation of soluble Aβ42 with MMSE scores supports a growing consensus that it is the soluble, aggregated protein that is toxic, rather than the form precipitated as plaques or tangles.
In conclusion, both BDNF mRNA and soluble, extracellular-enriched Aβ42 correlate with cognitive status in the oldest old. This is consistent with our previous results showing that the reduction in BDNF correlates with the degree of dementia in younger subjects with MCI and AD (Peng et al., 2005) and that soluble, oligomeric Aβ down-regulates BDNF in vitro (Garzon & Fahnestock, 2007). In contrast, we found no changes in levels of tau, phospho-tau, TrkB or its truncated isoforms in the oldest old, regardless of degree of pathology or cognitive status. The presence of TrkB is significant because it suggests that neurons in the aged and/or demented brain may still have the capacity to respond to increases in BDNF affected by exogenous administration, pharmaceutical activation or lifestyle changes.
Highlights.
Goal: determine factors associated with maintaining good cognition into very old age
Study: cortex of subjects >90 yrs old, demented or not, with or without AD pathology
Soluble Aβ and BDNF levels correlated with the degree of dementia in the oldest old
No differences between groups in TrkB or tau isoforms or in tau phosphorylation
Acknowledgments
This work was supported by grant number 1430 from the Alzheimer Society of Canada and grant number MOP-102723 from the Canadian Institutes of Health Research to MF, and by NIH grant numbers R01 AG21055, R01 AG042444 and P50 AG16573 to MMC and CHK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen SJ, Wilcock GK, Dawbarn D. Profound and selective loss of catalytic TrkB immunoreactivity in Alzheimer's disease. Biochem Biophys Res Commun. 1999;264(3):648–651. doi: 10.1006/bbrc.1999.1561. [DOI] [PubMed] [Google Scholar]
- Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology. 1992;42:631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE. Neurofibrillary tangles mediate the association of amyloid load with clinical Alzheimer disease and level of cognitive function. Arch Neurol. 2004;61:378–384. doi: 10.1001/archneur.61.3.378. [DOI] [PubMed] [Google Scholar]
- Berg L, McKeel DW, Jr, Miller JP, Storandt M, Rubin EH, Morris JC, Baty J, Coats M, Norton J, Goate AM, Price JL, Gearing M, Mirra SS, Saunders AM, et al. Clinicopathologic studies in cognitively healthy aging and Alzheimer's disease: relation of histologic markers to dementia severity, age, sex, and apolipoprotein E genotype. Arch Neurol. 1998;55:326–335. doi: 10.1001/archneur.55.3.326. [DOI] [PubMed] [Google Scholar]
- Blurton-Jones M, Kitazawa M, Martinez-Coria H, Castello NA, Müller FJ, Loring JF, Yamasaki TR, Poon WW, Green KN, LaFerla FM. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc Natl Acad Sci U S A. 2009;106(32):13594–13599. doi: 10.1073/pnas.0901402106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E, Bohl J. Staging of Alzheimer-related cortical destruction. Eur Neurol. 1993;33:403–408. doi: 10.1159/000116984. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Bruner E, Jacobs HI. Alzheimer's disease: the downside of a highly evolved parietal lobe? J Alzheimers Dis. 2013;35(2):227–240. doi: 10.3233/JAD-122299. [DOI] [PubMed] [Google Scholar]
- Buée L, Bussière T, Buée-Scherrer V, Delacourte A, Hof PR. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res Brain Res Rev. 2000;33(1):95–130. doi: 10.1016/s0165-0173(00)00019-9. [DOI] [PubMed] [Google Scholar]
- Castellani RJ, Lee HG, Zhu X, Nunomura A, Perry G, Smith MA. Neuropathology of Alzheimer disease: pathognomonic but not pathogenic. Acta Neuropathol (Berl) 2006;111:503–509. doi: 10.1007/s00401-006-0071-y. [DOI] [PubMed] [Google Scholar]
- Connor B, Young D, Lawlor P, Gai W, Waldvogel H, Faull RL, Dragunow M. Trk receptor alterations in Alzheimer's disease. Brain Res Mol Brain Res. 1996;42(1):1–17. doi: 10.1016/s0169-328x(96)00040-x. [DOI] [PubMed] [Google Scholar]
- Connor B, Young D, Yan Q, Faull RL, Synek B, Dragunow Brain-derived neurotrophic factor is reduced in Alzheimer's disease. Brain Res Mol Brain Res. 1997;49(1-2):71–81. doi: 10.1016/s0169-328x(97)00125-3. [DOI] [PubMed] [Google Scholar]
- Corrada MM, Berlau DJ, Kawas CH. A Population-Based Clinicopathological Study in the Oldest-Old: The 90+ Study. Curr Alzheimer Res. 2012;9(6):709–717. doi: 10.2174/156720512801322537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer's disease: correlation with cognitive severity. Ann Neurol. 1990;27(5):457–464. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- Deshpande A, Win KM, Busciglio J. Tau isoform expression and regulation in human cortical neurons. FASEB J. 2008;22(7):2357–2367. doi: 10.1096/fj.07-096909. [DOI] [PubMed] [Google Scholar]
- Dolan D, Troncoso J, Resnick SM, Crain BJ, Zonderman AB, O'Brien RJ. Age, Alzheimer's disease and dementia in the Baltimore Longitudinal Study of Ageing. Brain. 2010;133:2225–2233. doi: 10.1093/brain/awq141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahnestock M. Brain-derived neurotrophic factor: the link between amyloid-β and memory loss. Future Neurology. 2011;6(5):627–639. [Google Scholar]
- Fahnestock M, Marchese M, Head E, Pop V, Michalski B, Milgram WN, Cotman CW. BDNF increases with behavioral enrichment and an antioxidant diet in the aged dog. Neurobiol Aging. 2012;33(3):546–554. doi: 10.1016/j.neurobiolaging.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahnestock M, Michalski B, Xu B, Coughlin MD. The precursor pro-nerve growth factor is the predominant form of nerve growth factor in brain and is increased in Alzheimer's disease. Mol Cell Neurosci. 2001;18(2):210–220. doi: 10.1006/mcne.2001.1016. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Marín C, Rey MJ, et al. BDNF and full-length and truncated TrkB expression in Alzheimer disease. Implications in therapeutic strategies. J Neuropathol Exp Neurol. 1999;58:729–739. doi: 10.1097/00005072-199907000-00007. [DOI] [PubMed] [Google Scholar]
- Garzon D, Yu G, Fahnestock M. A new brain-derived neurotrophic factor transcript and decrease in brain-derived neurotrophic factor transcripts 1, 2 and 3 in Alzheimer's disease parietal cortex. J Neurochem. 2002;82(5):1058–1064. doi: 10.1046/j.1471-4159.2002.01030.x. [DOI] [PubMed] [Google Scholar]
- Garzon DJ, Fahnestock M. Oligomeric amyloid decreases basal levels of brain-derived neurotrophic factor (BDNF) mRNA via specific downregulation of BDNF transcripts IV and V in differentiated human neuroblastoma cells. J Neurosci. 2007;27(10):2628–2635. doi: 10.1523/JNEUROSCI.5053-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg SD, Che S, Wuu J, et al. Downregulation of trk but not p75NTR gene expression in single cholinergic basal forebrain neurons mark the progression of Alzheimer's disease. J Neurochem. 2006b;97:475–478. doi: 10.1111/j.1471-4159.2006.03764.x. [DOI] [PubMed] [Google Scholar]
- Ginsberg SD, Che S, Counts SE, Mufson EJ. Shift in the ratio of three-repeat tau and four-repeat tau mRNAs in individual cholinergic basal forebrain neurons in mild cognitive impairment and Alzheimer's disease. J Neurochem. 2006a;96(5):1401–1408. doi: 10.1111/j.1471-4159.2005.03641.x. [DOI] [PubMed] [Google Scholar]
- Goedert M, Jakes R. Expression of separate isoforms of human tau protein: correlation with the tau pattern in brain and effects on tubulin polymerization. EMBO J. 1990;9(13):4225–4230. doi: 10.1002/j.1460-2075.1990.tb07870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer's disease. Neuron. 1989;3(4):519–526. doi: 10.1016/0896-6273(89)90210-9. [DOI] [PubMed] [Google Scholar]
- Goedert M, Wischik CM, Crowther RA, Walker JE, Klug A. Cloning and sequencing of the cDNA encoding a core protein of the paired helical filament of Alzheimer disease: identification as the microtubule-associated protein tau. Proc Natl Acad Sci U S A. 1988;85(11):4051–4055. doi: 10.1073/pnas.85.11.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold G, Bouras C, Kovari E, Canuto A, Glaría BG, Malky A, Hof PR, Michel JP, Giannakopoulos P. Clinical validity of braak neuropathological staging in the oldest-old. Acta Neuropathol (Berl) 2000;99:579–582. doi: 10.1007/s004010051163. discussion 83-84. [DOI] [PubMed] [Google Scholar]
- Gong Y, Chang L, Viola KL, Lacor PN, Lambert MP, Finch CE, Krafft GA, Klein WL. Alzheimer's disease-affected brain: presence of oligomeric A beta ligands (ADDLs) suggests a molecular basis for reversible memory loss. Proc Natl Acad Sci U S A. 2003;100(18):10417–10422. doi: 10.1073/pnas.1834302100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode BL, Chau M, Denis PE, Feinstein SC. Structural and functional differences between 3-repeat and 4-repeat tau isoforms. Implications for normal taufunction and the onset of neurodegenetative disease. J Biol Chem. 2000;275(49):38182–38189. doi: 10.1074/jbc.M007489200. [DOI] [PubMed] [Google Scholar]
- Guillozet-Bongaarts AL, Garcia-Sierra F, Reynolds MR, Horowitz PM, Fu Y, Wang T, Cahill ME, Bigio EH, Berry RW, Binder LI. Tau truncation during neurofibrillary tangle evolution in Alzheimer's disease. Neurobiol Aging. 2005;26(7):1015–1022. doi: 10.1016/j.neurobiolaging.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Haroutunian V, Schnaider-Beeri M, Schmeidler J, Wysocki M, Purohit DP, Perl DP, Libow LS, Lesser GT, Maroukian M, Grossman HT. Role of the neuropathology of Alzheimer disease in dementia in the oldest-old. Arch Neurol. 2008;65(9):1211–1217. doi: 10.1001/archneur.65.9.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hock C, Heese K, Hulette C, Rosenberg C, Otten U. Region-specific neurotrophin imbalances in Alzheimer disease: decreased levels of brain-derived neurotrophic factor and increased levels of nerve growth factor in hippocampus and cortical areas. Arch Neurol. 2000;57(6):846–851. doi: 10.1001/archneur.57.6.846. [DOI] [PubMed] [Google Scholar]
- Hong M, Zhukareva V, Vogelsberg-Ragaglia V, Wszolek Z, Reed L, Miller BI, Geschwind DH, Bird TD, McKeel D, Goate A, Morris JC, Wilhelmsen KC, Schellenberg GD, Trojanowski JQ, Lee VM. Mutation-specific functional impairments in distinct tau isoforms of hereditary FTDP-17. Science. 1998;282(5395):1914–1917. doi: 10.1126/science.282.5395.1914. [DOI] [PubMed] [Google Scholar]
- Holsinger RM, Schnarr J, Henry P, Castelo VT, Fahnestock M. Quantitation of BDNF mRNA in human parietal cortex by competitive reverse transcription-polymerase chain reaction: decreased levels in Alzheimer's disease. Brain Res Mol Brain Res. 2000;76(2):347–354. doi: 10.1016/s0169-328x(00)00023-1. [DOI] [PubMed] [Google Scholar]
- Kapaki EN, Paraskevas GP, Tzerakis NG, Sfagos C, Seretis A, Kararizou E, Vassilopoulos D. Cerebrospinal fluid tau, phospho-tau181 and beta-amyloid1-42 in idiopathic normal pressure hydrocephalus: a discrimination from Alzheimer's disease. Eur J Neurol. 2007;14(2):168–173. doi: 10.1111/j.1468-1331.2006.01593.x. [DOI] [PubMed] [Google Scholar]
- Kawarabayashi T, Younkin LH, Saido TC, Shoji M, Ashe KH, Younkin SG. Age-dependent changes in brain, CSF, and plasma amyloid (beta) protein in the Tg2576 transgenic mouse model of Alzheimer's disease. J Neurosci. 2001;21(2):372–381. doi: 10.1523/JNEUROSCI.21-02-00372.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawas CH, Segal J, Stewart WF, Corrada M, Thal LJ. A validation study of the Dementia Questionnaire. Arch Neurol. 1994;51:901–906. doi: 10.1001/archneur.1994.00540210073015. [DOI] [PubMed] [Google Scholar]
- Kawas CH. Alzheimer's and dementia in the oldest old: a century of challenges. Curr AD Res. 2006;3:411–419. doi: 10.2174/156720506779025233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawas CH, Kim RC, Sonnen JA, Bullain SS, Trieu T, Corrada MM. Multiple pathologies are common and related to dementia in the oldest-old: The 90+ Study. Neurology. 2015;85:535–542. doi: 10.1212/WNL.0000000000001831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosik KS, Orecchio LD, Bakalis S, Neve RL. Developmentally regulated expression of specific tau sequences. Neuron. 1989;2(4):1389–1397. doi: 10.1016/0896-6273(89)90077-9. [DOI] [PubMed] [Google Scholar]
- Kuo YM, Emmerling MR, Vigo-Pelfrey C, Kasunic TC, Kirkpatrick JB, Murdoch GH, Ball MJ, Roher AE. Water-soluble Abeta (N-40, N-42) oligomers in normal and Alzheimer disease brains. J Biol Chem. 1996;271(8):4077–4081. doi: 10.1074/jbc.271.8.4077. [DOI] [PubMed] [Google Scholar]
- Lee HG, Castellani RJ, Zhu X, Perry G, Smith MA. Amyloid-beta in Alzheimer's disease: the horse or the cart? Pathogenic or protective? Int J Exp Path. 2005;86:133–138. doi: 10.1111/j.0959-9673.2005.00429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee VM, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annu Rev Neurosci. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- Lesné S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440(7082):352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- Lewis SA, Wang DH, Cowan NJ. Microtubule-associated protein MAP2 shares a microtubule binding motif with tau protein. Science. 1988;242(4880):936–939. doi: 10.1126/science.3142041. [DOI] [PubMed] [Google Scholar]
- Li J, Gould TD, Yuan P, Manji HK, Chen G. Post-mortem interval effects on the phosphorylation of signaling proteins. Neuropsychopharmacology. 2003;28(6):1017–1025. doi: 10.1038/sj.npp.1300112. [DOI] [PubMed] [Google Scholar]
- Lu B, Nagappan G, Lu Y. BDNF and synaptic plasticity, cognitive function, and dysfunction. Handbook Exp Pharmacol. 2014;220:223–250. doi: 10.1007/978-3-642-45106-5_9. [DOI] [PubMed] [Google Scholar]
- Luberg K, Wong J, Weickert CS, Timmusk T. Human TrkB gene: novel alternative transcripts, protein isoforms and expression pattern in the prefrontal cerebral cortex during postnatal development. J Neurochem. 2010;113(4):952–964. doi: 10.1111/j.1471-4159.2010.06662.x. [DOI] [PubMed] [Google Scholar]
- Matsuo ES, Shin RW, Billingsley ML, Van deVoorde A, O'Connor M, Trojanowski JQ, Lee VM. Biopsy-derived adult human brain tau is phosphorylated at many of the same sites as Alzheimer's disease paired helical filament tau. Neuron. 1994;13(4):989–1002. doi: 10.1016/0896-6273(94)90264-x. [DOI] [PubMed] [Google Scholar]
- McDonald JM, Savva GM, Brayne C, Welzel AT, Forster G, Shankar GM, Selkoe DJ, Ince PG, Walsh DM. Medical Research Council Cognitive Function and Ageing Study. The presence of sodium dodecyl sulphate-stable Abeta dimers is strongly associated with Alzheimer-type dementia. Brain. 2010;133:1328–1341. doi: 10.1093/brain/awq065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan P, Korvatska E, Poorkaj P, Evstafjeva Z, Robinson L, Greenup L, Leverenz J, Schellenberg GD, D’Souza I. Tau isoform regulation is region- and cell-specific in mouse brain. J Comp Neurol. 2008;511(6):788–803. doi: 10.1002/cne.21867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalski B, Fahnestock M. Pro-brain-derived neurotrophic factor is decreased in parietal cortex in Alzheimer's disease. Brain Res Mol Brain Res. 2003;111(1-2):148–154. doi: 10.1016/s0169-328x(03)00003-2. [DOI] [PubMed] [Google Scholar]
- Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Murer MG, Yan Q, Raisman-Vozari R. Brain-derived neurotrophic factorin the control human brain, and in Alzheimer's disease and Parkinson's disease. Prog Neurobiol. 2001;63:71–124. doi: 10.1016/s0301-0082(00)00014-9. [DOI] [PubMed] [Google Scholar]
- Nagahara AH, Merrill DA, Coppola G, Tsukada S, Schroeder BE, Shaked GM, Wang L, Blesch A, Kim A, Conner JM, Rockenstein E, Chao MV, Koo EH, Geschwind D, Masliah E, Chiba AA, Tuszynski MH. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer's disease. Nat Med. 2009;15(3):331–337. doi: 10.1038/nm.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy Z, Esiri MM, Jobst KA, Morris JH, King EM, McDonald B, Litchfield S, Smith A, Barnetson L, Smith AD. Relative roles of plaques and tangles in the dementia of Alzheimer's disease: correlations using three sets of neuropathological criteria. Dementia (Basel, Switzerland) 1995;6:21–31. doi: 10.1159/000106918. [DOI] [PubMed] [Google Scholar]
- Nelson PT, Jicha GA, Schmitt FA, Liu H, Davis DG, Mendiondo MS, Abner EL, Markesbery WR. Clinicopathologic correlations in a large Alzheimer disease center autopsy cohort: neuritic plaques and neurofibrillary tangles “do count” when staging disease severity. J Neuropathol Exp Neurol. 2007;66:1136–1146. doi: 10.1097/nen.0b013e31815c5efb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka T, Tagawa K, Ito H, Okazawa H. Dynamic changes of the phosphoproteome in postmortem mouse brains. PLoS One. 2011;6(6):e21405. doi: 10.1371/journal.pone.0021405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S, Wuu J, Mufson EJ, Fahnestock M. Precursor form of brain-derived neurotrophic factor and mature brain-derived neurotrophic factor are decreased in the pre-clinical stages of Alzheimer's disease. J Neurochem. 2005;93(6):1412–1421. doi: 10.1111/j.1471-4159.2005.03135.x. [DOI] [PubMed] [Google Scholar]
- Phillips HS, Hains JM, Armanini M, Laramee GR, Johnson SA, Winslow JW. BDNF mRNA is decreased in the hippocampus of individuals with Alzheimer's disease. Neuron. 1991;7:695–702. doi: 10.1016/0896-6273(91)90273-3. [DOI] [PubMed] [Google Scholar]
- Robinson JL, Geser F, Corrada MM, Berlau DJ, Arnold SE, Lee VM, Kawas CH, Trojanowski JQ. Neocortical and hippocampal amyloid-β and tau measures associate with dementia in the oldest-old. Brain. 2011;134:3708–3715. doi: 10.1093/brain/awr308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JL, Molina-Porcel L, Corrada MM, Raible K, Lee EB, Lee VM, Kawas CH, Trojanowski JQ. Perforant path synaptic loss correlates with cognitive impairment and Alzheimer's disease in the oldest-old. Brain. 2014;137:2578–2587. doi: 10.1093/brain/awu190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- Savaskan E, Müller-Spahn F, Olivieri G, Bruttel S, Otten U, Rosenberg C, Hulette C, Hock C. Alterations in trk A, trk B and trk C receptor immunoreactivities in parietal cortex and cerebellum in Alzheimer's disease. Eur Neurol. 2000;44(3):172–180. doi: 10.1159/000008229. [DOI] [PubMed] [Google Scholar]
- Savva GM, Wharton SB, Ince PG, Forster G, Matthews FE, Brayne C. Age, neuropathology, and dementia Medical Research Council Cognitive Function and Ageing Study. N Engl J Med. 2009;360(22):2302–2309. doi: 10.1056/NEJMoa0806142. [DOI] [PubMed] [Google Scholar]
- Siegel GJ, Chauhan NB. Neurotrophic factors in Alzheimer's and Parkinson's disease brain. Brain Res Brain Res Rev. 2000;33:199–227. doi: 10.1016/s0165-0173(00)00030-8. [DOI] [PubMed] [Google Scholar]
- Steinerman JR, Irizarry M, Scarmeas N, Raju S, Brandt J, Albert M, Blacker D, Hyman B, Stern Y. Distinct pools of beta-amyloid in Alzheimer disease-affected brain: a clinicopathologic study. Arch Neurol. 2008;65(7):906–912. doi: 10.1001/archneur.65.7.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoilov P, Castren E, Stamm S. Analysis of the human TrkB gene genomic organization reveals novel TrkB isoforms, unusual gene length, and splicing mechanism. Biochem Biophys Res Commun. 2002;290(3):1054–1065. doi: 10.1006/bbrc.2001.6301. [DOI] [PubMed] [Google Scholar]
- Tremblay C, Pilote M, Phivilay A, Emond V, Bennett DA, Calon F. Biochemical characterization of Abeta and tau pathologies in mild cognitive impairment and Alzheimer’s disease. J. Alzheimers Dis. 2007;12:377–390. doi: 10.3233/jad-2007-12411. [DOI] [PubMed] [Google Scholar]
- Vanmechelen E, Vanderstichele H, Davidsson P, Van Kerschaver E, Van Der Perre B, Sjögren M, Andreasen N, Blennow K. Quantification of tau phosphorylated at threonine 181 in human cerebrospinal fluid: a sandwich ELISA with a synthetic phosphopeptide for standardization. Neurosci Lett 5. 2000;285(1):49–52. doi: 10.1016/s0304-3940(00)01036-3. [DOI] [PubMed] [Google Scholar]
- Wong J, Higgins M, Halliday G, Garner B. Amyloid beta selectively modulates neuronal TrkB alternative transcript expression with implications for Alzheimer's disease. Neuroscience. 2012;210:363–374. doi: 10.1016/j.neuroscience.2012.02.037. [DOI] [PubMed] [Google Scholar]
- Yamada K, Nabeshima T. Brain-derived neurotrophic factor/TrkB signaling in memory processes. J Pharmacol Sci. 2003;91:267–270. doi: 10.1254/jphs.91.267. [DOI] [PubMed] [Google Scholar]