Abstract
The male sex chromosome disorder, 47,XYY syndrome (XYY), is associated with increased risk for social-emotional difficulties, attention-deficit hyperactivity disorder (ADHD) and autism spectrum disorder (ASD). We hypothesize that increased Y chromosome gene copy number in XYY leads to overexpression of Y-linked genes related to brain development and function, thereby increasing risk for these phenotypes. We measured expression in blood of two Y genes NLGN4Y and RPS4Y in 26 boys with XYY and 11 male controls and evaluated whether NLGN4Y expression correlates with anxiety, ADHD, depression and autistic behaviors (from questionnaires) in boys with XYY. The XYY cohort had increased risk of ASD behaviors on the social responsiveness scale (SRS) and increased attention deficits on the Conners’ DSM-IV inattention and hyperactive scales. In contrast, there was no increase in reported symptoms of anxiety or depression by the XYY group. Peripheral expression of two Y genes in boys with XYY vs. typically developing controls was increased twofold in the XYY group. Results from the SRS total and autistic mannerisms scales, but not from the attention, anxiety or depression measures, correlated with peripheral expression of NLGN4Y in boys with XYY. Males with XYY have social phenotypes that include increased risk for autism-related behaviors and ADHD. Expression of NLGN4Y , a gene that may be involved in synaptic function, is increased in boys with XYY, and the level of expression correlates with overall social responsiveness and autism symptoms. Thus, further investigation of NLGN4Y as a plausible ASD risk gene in XYY is warranted.
Keywords: Neuroligin, NLGN4Y, social function, XYY
The male sex chromosome aneuploidy disorder 47,XYY (XYY syndrome) is associated with an increased risk for neurodevelopmental phenotypes, including developmental delays, attention-deficit hyperactivity disorder (ADHD), learning disabilities, social-emotional difficulties and autism spectrum disorders (ASDs) (Bardsley et al. 2013; Bishop & Scerif 2011; Cordeiro et al. 2012; Geerts et al. 2003; Margari et al. 2014; Robinson et al. 1990; Ross et al. 2009, 2012; Tartaglia et al. 2012). Additional neurocognitive phenotypes in XYY include increased difficulties with language and an increased frequency of seizure disorders (present in 13%) (Bardsley et al. 2013). Consecutive newborn screening series show that XYY occurs in 1 in 1000 males, but it is diagnosed much less frequently, most likely because associated features such as tall stature or developmental delays are not distinctive (Robinson et al. 1990). Testicular function is generally normal, unlike in other sex chromosome disorders such as 47,XXY Klinefelter syndrome (KS). In addition, XYY is not typically associated with major cognitive impairment or intellectual disability. General intelligence is typically normal or only mildly diminished, although learning disabilities are common (Bender et al. 1984; Leggett et al. 2010; Netley 1986; Robinson et al. 1985). While the behavioral phenotypes of XYY and XXY share an increased risk for language and learning disabilities, the rate of ASD in males with XYY ranges from 19% to 50% in XYY cohorts diagnosed prenatally or postnatally, compared with 5–10% in XXY (Bishop et al. 2011; Cordeiro et al. 2012).
To date, there have been no investigations of potential pathogenic genetic mechanisms of the social behavior findings and increased ASD risk in XYY. However, Bishop et al. (2011) proposed that an extra copy of NLGN4Y might be associated with elevated risk of autism in boys with XYY, including in a prenatally diagnosed cohort. XYY syndrome represents a novel, genetically homogeneous model for elucidating Y chromosome-related genetic influences on behavioral phenotypes such as ASD. Specifically, we hypothesize that the pathogenesis of social findings in XYY is related to the extra Y chromosome and associated overexpression of Y chromosome genes. An essential requirement for a gene dosage-dependent phenotypic mechanism is copy number-dependent expression of the gene. As an initial test of the Y gene overexpression hypothesis, we measured peripheral blood expression of the gene NLGN4Y , an ASD candidate gene by virtue of its high similarity to the potential ASD gene NLGN4X , in boys with XYY and age-matched typically developing (TD) male controls. For comparison, we also measured expression of a ubiquitously expressed ribosomal protein gene, RPS4Y . We predicted that both genes would have twofold increased expression in boys with XYY compared to TD control boys. Second, we evaluated whether NLGN4Y expression correlated with behavioral features of XYY, including ADHD symptoms, anxiety, depression and autistic behaviors. We hypothesized that the expression of NLGN4Y would correlate with autism-related behaviors.
Materials and Methods
Boys with karyotype 47,XYY, aged 4–14 years (n=26), were evaluated at Thomas Jefferson University. Subjects were recruited from a broad geographic and socioeconomic distribution through the support of the national XYY advocacy organization (KS&A), by direct referral through an established referral network of university and community-based pediatricians, and through the genetics clinic at A. I. duPont Hospital for Children. All boys with XYY had postnatal karyotypes confirming their non-mosaic XYY diagnoses. Ascertainment bias was minimized because all subjects were recruited on the basis of prior diagnoses of XYY without consideration of other diagnoses. A substantial proportion (10/21, 48%) had been diagnosed prenatally, which would also reduce bias.
Age-matched, TD male controls (n=11) were recruited during the same time interval as the XYY cohort from study fiyers and our pediatrics clinics, had no history of previous psychological or special educational treatment, and were not taking any medications for psychiatric diagnoses. These controls were not karyotyped but are presumed to be 46,XY on the basis of the low prevalence (<0.5%) of sex chromosome abnormalities in normal populations.
The study was approved by the Human Studies Committee at Thomas Jefferson University and informed consent and assent (age-appropriate) was obtained from all participating families.
Socioeconomic status
Socioeconomic status was derived from the Hollingshead 2-Factor Index of Social Status based on education and occupation of parents for children (Hollingshead & Redlich 1958).
Study evaluation
Participants (XYY and TD controls) were evaluated with a protocol that included medical and developmental-behavioral history, physical examination, cognitive testing and completion of standardized behavioral questionnaires (two parent questionnaires and two child questionnaires). Blood was also collected from all participants for genetic testing.
Cognitive evaluation
Subjects were individually administered the differential ability scales (DAS-2) (Elliott 1983) to assess cognitive abilities. Raw scores were converted to standard scores (mean of 100, SD of 15), based on the age- and sex-based norms for children aged 4–17 years. Index scores examined were general conceptual ability (full-scale IQ), verbal cluster (verbal IQ) and non-verbal cluster (performance IQ, non-verbal spatial ability).
Parent questionnaires
The social responsiveness scale
The social responsiveness scale (SRS) (Constantino 2005) assesses symptoms reported by parents over the past 6 months in five domains: social awareness, social cognition, social communication, social motivation and autistic mannerisms in children aged 4–18 years. The SRS is a validated screening tool for autism and scores in the severe range are strongly associated with the clinical diagnosis of ASD (Constantino 2005). Raw scores are converted into sex-normed t-scores, with a mean of 50 and an SD of 10 and are classified into categories of normal (0–59), mild to moderate (60–75) or severe (76 or higher), to assist in determining the presence and degree of social deficits and whether symptoms may be consistent with those observed in individuals with ASD.
Conners’ parent rating scale – revised
Conners’ parent rating scale – revised long version (CPRS-R) (Conners 1997) is a standardized (age and sex) measure assessing parental report of attention problems, hyperactivity, impulsivity and other behavioral symptoms associated with ADHD in children aged 3–17 years. Subscales include oppositional behaviors, cognitive problems/inattention, hyperactivity, anxious-shy, perfectionism, social problems and psychosomatic symptoms. Three continuous Diagnostic and Statistical Manual (DSM)-IV-based ADHD scores (inattentive, hyperactive/impulsive and combined) are derived by summing the total points across appropriate sets of item and converting to t-scores. Scores of 6 or more of the 18 that comprise the DSM-IV ADHD criteria were tallied for inattentive symptoms or hyperactive-impulsive symptoms.
Child self-report questionnaires
Participants were asked to complete two self-report questionnaires to assess symptoms of anxiety and depression. If the children were unable to read the questionnaires, they were read to them.
Children’s depression inventory
Children’s depression inventory (CDI) (Kovacs 2003) is a self-report measure of behavioral signs and symptoms of depression in children aged 6–17 years with well-established reliability, internal consistency and validity. Total CDI score refiects the presence of overall depressive symptoms. The CDI is reported as t-scores (mean=50, SD=10) normed for age and sex, and total CDI score refiects the presence of overall depressive symptoms.
Revised child’s manifest anxiety scale
Revised child’s manifest anxiety scale (RCMAS) (Reynolds & Richmond 1978) is an instrument which measures self-reported anxiety symptoms for children aged 6–19 years. The 37 items measure subjective, motor and physiological anxiety symptoms. Scales include total anxiety, physiological, worry/oversensitivity and social concerns and are reported as t-scores (mean=50, SD=10) normed for age and sex.
Genetic testing
Blood was collected from boys with XYY and TD males using the PAXGene system (QIAGEN, Valencia, CA, USA) and sent via overnight courier to UT Southwestern for RNA isolation according to the manufacturer’s protocols. RNA was reverse-transcribed to cDNA using the LifeTechnologies Applied Biosystems, Foster City, CA, USA, High Capacity RNA-to-cDNA kit (#4387406). NLGN4Y and RPS4Y transcript levels were quantitated with LifeTechnologies Applied Biosystems Taqman assays Hs00382154_m1 and Hs00606158_m1, respectively, and normalized to the control gene, HPRT1 (#4326321E). Assays were run in triplicate on an Applied Biosystems 7900 HT Real Time instrument, and samples with replicate CV >20% were excluded from statistical analyses. A single RNA standard prepared from pooled human male brain tissues (a gift from Dr Carol Tamminga, UT Southwestern) was used to generate standard curves for assays so that expression data collected from different plates could be merged.
Statistical methods
All results are presented as mean ± SD scores, standard scores or t-scores. Statistical comparisons included t-tests, Pearson correlations and Chi-squared test comparisons. Correlations between behavioral findings and gene expression were evaluated in the XYY group only because the number of controls (11) was too low to analyze separately. Results were considered statistically significant at P ≤ 0.05. In addition, in order to compare correlations of NLGN4Y and RPS4Y expression to behavioral findings in the SRS results within the XYY population, Hotelling’s t-test to compare correlated correlations, which were based on the bivariate correlations, was used.
Results
Participants (XYY and age-matched male controls)
A total of 26 boys with XYY were evaluated in this study. Their mean ± SD age (range) was 9.6 ± 2.9 years (4.0–14.4 years). Twenty five of the 26 (96%) boys evaluated were Caucasian. Eleven boys were diagnosed before 2 years of age, including nine by routine prenatal testing and two in infancy due to developmental delays. Fourteen boys were diagnosed postnatally between ages 2 and 12 years: five for language delay and/or behavior issues, seven for miscellaneous reasons (other developmental delay, dysmorphic features and mother’s request) and one was diagnosed after age 12 years for learning issues. Four of the 26 (15%) participants had a history of seizures – two with a history of absence seizures and two had significant febrile seizures in childhood. None were receiving seizure medications at the time of study evaluation. One patient had previous diagnosis of Tourette’s syndrome, and 16 of the 25 had mild resting or intention tremors. Physical, cognitive and behavioral results of a subset of these XYY subjects and controls were previously reported (Bardsley et al. 2013; Cordeiro et al. 2012; Ross et al. 2012).
We also studied 11 TD boys, aged 4.2–13.8 years. None had any previous major medical or psychological diagnoses, and none were receiving medications. Eight of the 11 were Caucasian (Table 1).
Table 1.
Patient demographics and cognitive test results
| XYY | TD controls | P-value* | |
|---|---|---|---|
| n | 26 | 11 | |
| Age | 9.6±2.9 | 8.4±3.1 | 0.29 |
| Socioeconomic status | 53±10 | 49±8 | 0.35 |
| Height SDS† | 0.9±1.1 | 0.6±0.9 | 0.36 |
| Weight SDS | 0.8±0.9 | 1.1±0.6 | 0.44 |
| HC SDS | 1.4±1.7 | 1.4±1.1 | 0.9 4 |
| DAS verbal | 88±19 | 103±16 | 0.03 |
| DAS non-verbal | 92±21 | 108±19 | 0.03 |
| General conceptual ability | 91±18 | 107±18 | 0.02 |
XYY vs. controls.
Standard Deviation Score
Patient demographics and cognitive test results
Mean age was similar in both groups, but the controls were about 1 year younger than the XYY subjects. Mean height weight, and head circumference SDS (Table 1) were similar in both groups. The control subjects had, on average, significantly higher cognitive scores on the DAS verbal, non-verbal and general conceptual ability scales (Table 1).
Behavioral questionnaires in XYY vs. controls
Parents completed the SRS and the CPRS-R. The children completed the RCMAS and the CDI (Table 2). The sample size for each assessment varies because some participants were not within the normative ages of the assessments and some data were incomplete or missing. The behavioral results for the TD controls are presented for the reader without statistical comparison with the XYY group, based on the small numbers of subjects.
Table 2.
Behavioral questionnaires in XYY vs. controls (mean±SD)
| XYY | TD controls | |
|---|---|---|
| SRS t-scores (n) | 18 | |
| Total | 66±18 | 50±10 |
| Social awareness | 66±15 | 52±9 |
| Social cognition | 66±19 | 55±13 |
| Social communication | 64±18 | 48±7 |
| Social motivation | 59±14 | 49±7 |
| Autistic mannerisms | 67±16 | 48±10 |
| Conners’ t-scores (n) | 25 | 11 |
| Oppositional | 64±14 | 52±10 |
| Cognitive problems/inattention | 65±10 | 50±11 |
| Hyperactivity | 64±14 | 50±8 |
| Anxiety-shy | 60±13 | 52±9 |
| Perfectionism | 56±14 | 50±9 |
| Social problems | 67±16 | 51±11 |
| Psychosomatic | 59±14 | 51±11 |
| DSM-IV-inattentive | 64±11 | 48±11 |
| DSM-IV hyperactive-impulsive | 63±13 | 52±9 |
| DSM-IV total | 65±11 | 49±11 |
| CDI t-scores (n) | 16 | |
| Total | 48±11 | 45±8 |
| RCMAS t-scores (n) | 17 | 7 |
| Total anxiety | 52±13 | 49±10 |
Social responsiveness scale
The t-scores of different domains of social functioning and autistic mannerisms are compared in boys with XYY vs. TD controls in Table 2. Mean t-scores on the SRS were increased in the XYY group as a whole, indicating increased social skills difficulties and autistic behaviors. Based on SRS total t-scores, 5 of the 18 in the XYY group were in the mild-moderate range and 7 were in the severe range. One of the six controls had a total t-score in the mild-moderate range. All mean SRS subscales t-scores were also elevated in the XYY group, particularly autistic mannerisms, where 7 of 18 in the XYY group were in the mild-moderate range and 7 were in the severe range. One of the eight controls had an autistic mannerisms t-score in the mild-moderate range.
Conners’ rating scale
On the basis of the Conners’ rating scale, 6 of the 26 boys in the XYY group had symptoms consistent with DSM-IV criteria for inattentive subtype, two for hyperactive-impulsive subtype and one for combined subtype. For controls, one of the 11 had possible diagnosis of DSM-IV inattentive subtype, and none met criteria for hyperactive-impulsive or combined subtype. Mean t-scores on the DSM-IV ADHD scales for the XYY group were, on average, greater than 1 SD over the mean and higher than the TD control group (Table 2). For the subscales, mean t-scores were generally higher in the XYY group vs. TD controls for the oppositional, cognitive problems/inattention, hyperactivity and social problems subscales, but not for the anxiety-shy, perfectionism or psychosomatic subscales.
Child self-report anxiety and depression behavioral questionnaire results
On responses from self-report questionnaires of anxiety (RCMAS) and depression (CDI) (Table 2), the XYY group reported themselves as in the normal range for total RCMAS and CDI scores and their subscales, thereby indicating an overall lack of significant anxiety or depressive characteristics on these instruments. Likewise, mean RCMAS and CDI t-scores were similar to findings from the TD control group.
NLGN4Y and RPS4Y expression results
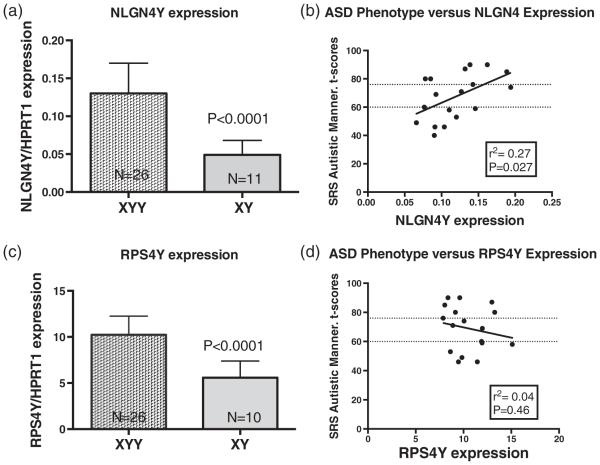
We measured expression in peripheral blood of two Y genes, NLGN4Y and RPS4Y , in 26 boys with XYY and 11 age-matched, healthy TD male controls. For both genes, the mean steady-state mRNA level, normalized to non-Y housekeeping gene HPRT1, was increased approximately twofold (P < 0.0001) in XYY subjects compared with controls (Fig. 1). The twofold increase in expression of Y genes in XYY males was consistent despite the fact that the two genes were expressed at very different levels, with RPS4Y having much higher abundance, as refiected by the Y axes (Fig. 1). Expression of both genes was similar in prenatally and postnatally diagnosed XYY cohorts, in XYY cohorts with and without seizures, and with and without tremors (Table 3). Mean levels of NLGN4Y were increased, on average, in the four boys with XYY with a positive seizure history, without statistical significance. Expression of RPS4Y was similar in both groups.
Figure 1. NLGN4Y and RPS4Y Expression results.
(a) Mean + SD expression of Y chromosome gene, NLGN4Y in peripheral blood of boys with XYY vs. XY controls. (b) NLGN4Y expression levels vs. SRS autistic mannerisms t-scores. (c) RPS4Y expression levels. (d) RPS4Y expression levels vs. SRS autistic mannerisms t-scores. For (b) and (d), T-score cut-offs of mild to moderate (60–75) or severe (76 or higher) ratings are indicated with dotted lines.
Table 3.
Mean (±SD) NLGN4Y/RPS4Y expression in XYY subcohorts
| NLGN4Y | RPS4Y | P-value* | ||
|---|---|---|---|---|
| XYY (n=26) | 0.13±0.04 | 10.20±2.00 | †0.0001, | ‡0.0001 |
| TD male controls (n=9) |
0.05±0.02 | 5.16±1.7 6 | ||
| XYY by Dx (n=26) | 0.13±0.04 | 10.20±2.04 | ||
| Prenatal (n =9) | 0.12±0.04 | 10.69±2.17 | †0.48, | ‡0.45 |
| Postnatal (n=17) | 0.13±0.04 | 10.01±1.89 | ||
|
XYY by seizures
(n=26) |
||||
| With seizures (n=4) | 0.15±0.05 | 9.93±2.31 | †0.46, | ‡0.90 |
| Without seizures (n=22) |
0.13±0.04 | 10.28±1.95 | ||
|
XYY by tremors
(n=26) |
||||
| With tremors (n=16) |
0.13±0.04 | 10.6±2.3 | †0.99, | ‡0.95 |
| Without tremors (n=9) |
0.13±0.04 | 10.1±1.9 | ||
t-Tests (XYY vs. TD, XYY: prenatal vs. postnatal), XYY: seizures (yes or no), XYY: tremors (yes or no).
NLGN4Y.
RPS4Y.
We next evaluated whether total scores on the SRS, Conners’ rating scale, CDI and RCMAS were related to NLGN4Y or RPS4Y expression in the XYY cohort (Table 4). Expression of NLGN4Y , but not RPS4Y , correlated significantly with the total SRS t-score (r=0.48, P=0.04 vs. r=−0.24, P=0.35) and with the SRS autistic mannerisms symptom t-scores (r=0.52, P=0.03 vs. r=−0.19, P=0.46) (Fig. 1). Statistical comparison of two correlation coefficients using Hotelling’s t-test was significantly different for SRS autistic mannerisms (P=0.038) and approached significance for SRS total (P=0.07). For the Conners’ rating scale, CDI and RCMAS, none of the total or subscale scores were correlated with NLGN4Y or RPS4Y expression. None of the other factors including age, verbal function, general conceptual ability or prenatal vs. postnatal diagnosis (Table 4) correlated with NLGN4Y or RPS4Y expression.
Table 4.
Correlation of cognitive and behavioral results and NLGN4Y/RPS4Y expression in boys with XYY
|
NLGN4 r- value |
P- value* |
RPS4Y r- value |
P- value* |
|
|---|---|---|---|---|
| SRS t-scores total (n=18) | 0.48 | 0.04 | −0.24 | 0.35 |
| SRS t-scores Autistic Mannerisms (18) |
0.52 | 0.03 | −0.19 | 0.46 |
| Conners’ DSM-4 inattention t-scores (25) |
0.01 | 0.96 | 0.20 | 0.35 |
| Conners’ DSM-4 hyperactive t-scores (25) |
0.06 | 0.76 | −0.08 | 0.71 |
| Conners’ DSM-4 total t-scores (25) |
0.05 | 0.82 | 0.08 | 0.71 |
| CDI t-scores total (16) | −0.13 | 0.64 | −0.08 | 0.76 |
| RCMAS t-scores total (17) | 0.23 | 0.37 | −0.32 | 0.22 |
| DAS verbal cluster (26) | −0.11 | 0.62 | 0.02 | 0.93 |
| DAS general conceptual ability (26) |
0.12 | 0.59 | −0.10 | 0.67 |
| Age (26) | 0.27 | 0.18 | −0.24 | 0.25 |
| Prenatal vs. postnatal Dx (26) |
0.09 | 0.66 | −0.16 | 0.43 |
Pearson correlation.
Discussion
This cross-sectional study evaluated behavioral features in 26 boys with XYY, using the SRS to measure autistic features: the Conners’ rating scale for ADHD symptoms, the RCMAS for anxiety symptoms and the CDI for depressive symptoms. Our results support previously published studies which show an increased risk of ASD and ADHD symptoms in boys with XYY compared to TD boys (Bardsley et al. 2013; Bishop & Scerif 2011; Cordeiro et al. 2012; Margari et al. 2014; Ross et al. 2012). ASD-related symptoms, as reported on the SRS, and attention-related behaviors on the Conners’ rating scale including DSM-IV inattention and hyperactive were increased in the XYY group as a whole (based on t-scores), and compared with the TD control group. In contrast, on the RCMAS and the CDI, there was no increase in reported symptoms of anxiety or depression reported by boys in the XYY group as a whole, or compared with the control group. While anxiety and depression symptoms have previously been reported to be more common in XYY, this association is less strongly associated with XYY than the ADHD-related behaviors (inattention, hyperactivity and impulsivity) and ASD symptoms (Bardsley et al. 2013; Bishop & Scerif 2011; Cordeiro et al. 2012; Margari et al. 2014; Ross et al. 2012).
Analysis of peripheral expression of two Y chromosome genes in boys with XYY vs. TD control boys showed that expression was increased twofold in XYY. This was predicted given the presence of a second Y chromosome, but the literature surprisingly lacks evidence showing copy number-dependent expression of Y chromosome genes in vivo. A novel finding in this study is that measures of autistic behaviors (the total SRS and SRS autistic mannerism T-scores) correlated with peripheral expression of the gene NLGN4Y in boys with XYY, while measures of attention, anxiety and depression did not.
Approximately 19–50% of males with XYY (Bardsley et al. 2013; Bishop & Scerif 2011; Lee et al. 2012) satisfy diagnostic criteria for ASD, and XYY males are an order of magnitude more prevalent in idiopathic ASD cohorts screened for cytogenetic or copy number variation (CNV) than in the general population (Rosenfeld et al. 2010). Both small and large Y chromosome duplications have been reported in 0.5–12% of cytogenetically screened autism populations (Li et al. 1993; Vorstman et al. 2006; Wassink et al. 2001). Other phenotypic features in XYY that overlap with the idiopathic ASD population include difficulties with language, and an increased risk of impulsivity and oppositional behaviors (Robinson et al. 1990; Ross et al. 2012; Theilgaard 1984; Welch 1985), as well as seizure disorders (present in 13%) (Bardsley et al. 2013).
Heritability studies have shown that genetic factors are important in studying behavioral phenotypes, but dissecting out the relationships among genes and behavioral phenotypes such as ASD is confounded by both genetic heterogeneity and the paucity of neurobiological models. These problems can be circumvented by studying clinical phenotypes and biomarkers for genetically defined subgroups such as XYY. We and others (Bishop et al. 2011) hypothesized that increased Y chromosome gene copy number in XYY leads to overexpression of one or more Y-linked genes related to brain development and function, thereby increasing risk for ASD in XYY. The 4:1 male predominance for ASD (Newschaffer et al. 2007) also suggests involvement of sex-linked genes.
One Y gene in particular, neuroligin 4Y (NLGN4Y ), is an intriguing XYY ASD candidate (Bishop & Scerif 2011). Neuroligin 4 encodes a member of the neuroligin family of trans-synaptic cell adhesion molecules that bind neurexins and stabilize excitatory and inhibitory synaptic activity (Hoon et al. 2011; Jamain et al. 2003; Zhang et al. 2009). Genetic studies have implicated synaptic molecules, including neuroligins and neurexins, in the pathogenesis of ASD in some studies (Daoud et al. 2009; Jamain et al. 2003; Powell & Boucard 2010) but not in others (Blasi et al. 2006; Gauthier et al. 2005; Vincent et al. 2004; Ylisaukko-oja et al. 2005). Included among these are neuroligin 4X (NLGN4X ) and neuroligin 4Y (NLGN4Y ), mutations of which have been found in rare sporadic and familial forms of ASD (Jamain et al. 2003; Laumonnier et al. 2004; Yan et al. 2008).
Evidence supporting a role of individual Y genes in ASD is inconclusive (Baron-Cohen et al. 2011). Genes in the non-recombining region of the Y chromosome (95% of Y) cannot be studied by linkage and have generally not been included in ASD genome-wide association studies or focused CNV studies. There is evidence that mutations in non-coding promoter sequences or CNV of NLGN4X or other neuroligin genes, leading to gain of function, can alter synaptic function. For example, neuroligin 3 duplication cosegregated with ASD in an extended family (Kaya et al. 2012), de novo sequence variation in the NLGN4X promoter was associated with increased gene expression in an ASD subject (Daoud et al. 2009) and neuroligin 1 or 2 overexpression in mice, either by transgenic manipulation or as a result of increased expression of eIF4E or knockout of eIF4E-binding protein 2, led to ASD-like behavioral phenotypes (Dahlhaus et al. 2010; Gkogkas et al. 2013). XY males express two neuroligin genes (NLGN4X and NLGN4Y ), whereas XX females express only one copy of NLGN4X due to X-inactivation (Castagne et al. 2011; Talebizadeh et al. 2006), suggesting that greater total neuroligin 4 levels in males vs. females may contribute to the marked male bias of idiopathic ASD (Baron-Cohen et al. 2011; Bishop & Scerif 2011). These Y chromosome-related mechanisms may also be relevant in the context of the 4:1 male:female predominance of idiopathic ASD (Newschaffer et al. 2007).
Our model for neuroligin 4 overexpression in the pathogenesis of ASD in XYY assumes that NLGN4X and NLGN4Y have overlapping expression patterns and serve redundant functions. While the mRNA expression patterns of NLGN4Y and NLGN4X are highly similar in the adult brain (Allen Brain Atlas), their developmental expression patterns are unknown. Moreover, NLGN4Y and NLGN4X expression has been examined only at the RNA and not at the protein level. It is also possible that there are subtle differences in the synaptic function of neuroligin 4X vs. 4Y. Last, an antisense transcript from the NLGN4Y locus in human embryonic stem cells has been described (Brandenberger et al. 2004). Whether this transcript affects neuroligin 4Y protein levels is currently unknown.
Study limitations relate to the control sample being small, and to the potential for ascertainment bias in the XYY sample. If boys with XYY were diagnosed postnatally on the basis of developmental or behavioral issues, this could create a sampling bias for more severe behavioral features in our sample. Milder behavioral findings would perhaps have been found in a larger, prenatally diagnosed XYY cohort; however, subsets of boys with XYY ascertained without bias from newborn screening studies or prenatally also have behavior findings, including ASD (Bishop et al. 2011; Bender et al. 1984, 1993), suggesting that the association with the karyotype is genuine. Also, our main result showing the association between NLGN4Y expression and autistic symptoms was not affected by whether diagnosis was prenatal or postnatal.
Another limitation of this study is that our results were obtained from peripheral blood and may not refiect expression in brain. However, gene expression patterns of many genes in peripheral blood leukocytes and derivative lymphoblastoid cell lines are highly heritable and stable over time and can be used as intermediate phenotypes (Cheung et al. 2005). Studies are beginning to relate expression of genes relevant to ASD, e.g. FMR1 and MECP2, in blood-derived lymphoblastoid cell lines and peripheral blood lymphocytes (Glatt et al. 2012) to ASD phenotypes. It was interesting to note that there is significant individual variation in expression of the Y genes tested, particularly among XYY subjects, which could relate to phenotypic variation. For NLGN4Y , expression in XYY was slightly more than double that in the XY cohort. In contrast, for RPS4Y, expression in XYY was about double the level in the XY cohort. In general, NLGN4Y is expressed in blood in lower levels than RPS4Y, by an order of magnitude. Thus, increased variation in NLGN4Y expression could be related to nonlinearity of the relationship between copy number and RNA expression, measurement error related to lower signal to noise ratio, stochastic effects of low-abundance gene transcription or to statistical outliers in small sample sizes. Another limitation is that we used a parent-report questionnaire for assessment of ASD symptoms. Our goal in future studies is to expand these findings using gold standard ASD diagnostic tools in a larger cohort of males with XYY.
Last, association studies relating peripheral gene expression to behavioral phenotypes do not parse causes vs. symptoms of ASD. Nonetheless, by integrating genetic and behavioral findings, prediction of behavioral and clinical outcome can be anticipated, shedding light on underlying gene – behavior relationships and the neurobiological pathways underlying such associations in XYY.
In summary, XYY represents a novel, genetically homogeneous model for elucidating Y chromosome-related genetic influences on ASD. In light of evidence that sex-differentiating mechanisms play an important role in the development of ASD (Baron-Cohen et al. 2011; Gillberg et al. 1984), the strong likelihood that increased Y-chromosome dosage and increased risk of ASD are interrelated warrants further investigation. XXY syndrome is also of great interest in understanding the influence of the sex chromosomes on male brain development and behavior because XYY is associated with normal testicular function, in contrast to males with an extra X chromosome XXY, KS, who have both an extra sex chromosome and testicular failure.
Conclusions
These results support previous studies showing an increased risk of autism-related behaviors and ADHD symptoms in children with XYY syndrome. Further, expression of NLGN4Y , a gene that may be involved in synaptic function, is increased in boys with XYY vs. XY controls, and the level of expression correlates with more severe autism symptom scores, but not with attention, anxiety or depression symptoms. Thus, further investigation of NLGN4Y as a plausible ASD risk gene in XYY is warranted. XYY is a compelling genetic model for increased autism risk and may provide insight into the molecular basis for the male bias observed in the general ASD population.
Acknowledgments
We thank Karen Kowal, Purita Ramos, and Terry Gemelli for their excellent assistance during the study. This study was supported by Delaware Health Science Alliance Pilot Award (J.L.R.). We also thank Dr Harvey Kushner for providing statistical analysis for this manuscript. This work was also supported by NIH/NCATS Colorado CTSI grant number UL1 TR001082 and NIH/NINDS K23NS070337 (NRT). Contents are the authors’ sole responsibility and do not necessarily represent official NIH views.
Footnotes
No authors have a conflict of interest.
References
- Bardsley MZ, Kowal K, Levy C, Gosek A, Ayari N, Tartaglia N, Lahlou N, Winder B, Grimes S, Ross JL. 47,XYY syndrome: clinical phenotype and timing of ascertainment. J Pediatr. 2013;163:1085–1094. doi: 10.1016/j.jpeds.2013.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Lombardo MV, Auyeung B, Ashwin E, Chakrabarti B, Knickmeyer R. Why are autism spectrum conditions more prevalent in males? PLoS Biol. 2011;9:e1001081. doi: 10.1371/journal.pbio.1001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender BG, Puck MH, Salbenblatt JA, Robinson A. The development of four unselected 47,XYY boys. Clin Genet. 1984;25:435–445. doi: 10.1111/j.1399-0004.1984.tb02013.x. [DOI] [PubMed] [Google Scholar]
- Bender BG, Linden MG, Robinson A. Neuropsychological impairment in 42 adolescents with sex chromosome abnormalities. Am J Med Genet. 1993;48:169–173. doi: 10.1002/ajmg.1320480312. [DOI] [PubMed] [Google Scholar]
- Bishop DV, Scerif G. Klinefelter syndrome as a window on the aetiology of language and communication impairments in children: the neuroligin-neurexin hypothesis. Acta Paediatr. 2011;100:903–907. doi: 10.1111/j.1651-2227.2011.02150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop DV, Jacobs PA, Lachlan K, Wellesley D, Barnicoat A, Boyd PA, Fryer A, Middlemiss P, Smithson S, Metcalfe K, Shears D, Leggett V, Nation K, Scerif G. Autism, language and communication in children with sex chromosome trisomies. Arch Dis Child. 2011;96:954–959. doi: 10.1136/adc.2009.179747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi F, Bacchelli E, Pesaresi G, Carone S, Bailey AJ, Maestrini E. Absence of coding mutations in the X-linked genes neuroligin 3 and neuroligin 4 in individuals with autism from the IMGSAC collection. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:220–221. doi: 10.1002/ajmg.b.30287. [DOI] [PubMed] [Google Scholar]
- Brandenberger R, Wei H, Zhang S, Lei S, Murage J, Fisk GJ, Li Y, Xu C, Fang R, Guegler K, Rao MS, Mandalam R, Lebkowski J, Stanton LW. Transcriptome characterization elucidates signaling networks that control human ES cell growth and differentiation. Nat Biotechnol. 2004;22:707–716. doi: 10.1038/nbt971. [DOI] [PubMed] [Google Scholar]
- Castagne R, Zeller T, Rotival M, Szymczak S, Truong V, Schillert A, Tregouet DA, Munzel T, Ziegler A, Cambien F, Blankenberg S, Tiret L. Influence of sex and genetic variability on expression of X-linked genes in human monocytes. Genomics. 2011;98:320–326. doi: 10.1016/j.ygeno.2011.06.009. [DOI] [PubMed] [Google Scholar]
- Cheung VG, Spielman RS, Ewens KG, Weber TM, Morley M, Burdick JT. Mapping determinants of human gene expression by regional and genome-wide association. Nature. 2005;437:1365–1369. doi: 10.1038/nature04244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners C. Conners’ Rating System - Revised Parent and Teacher Technical Manual, Multi-Health Systems. Tonawanda, NY: 1997. [Google Scholar]
- Constantino J. Social Responsiveness Scale (SRS) Western Psychological Services; Los Angeles, CA: 2005. [Google Scholar]
- Cordeiro L, Tartaglia N, Roeltgen D, Ross J. Social deficits in male children and adolescents with sex chromosome aneuploidy: a comparison of XXY, XYY, and XXYY syndromes. Res Dev Disabil. 2012;33:1254–1263. doi: 10.1016/j.ridd.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlhaus R, Hines RM, Eadie BD, Kannangara TS, Hines DJ, Brown CE, Christie BR, El-Husseini A. Overexpression of the cell adhesion protein neuroligin-1 induces learning deficits and impairs synaptic plasticity by altering the ratio of excitation to inhibition in the hippocampus. Hippocampus. 2010;20:305–322. doi: 10.1002/hipo.20630. [DOI] [PubMed] [Google Scholar]
- Daoud H, Bonnet-Brilhault F, Vedrine S, Demattei MV, Vourc’h P, Bayou N, Andres CR, Barthelemy C, Laumonnier F, Briault S. Autism and nonsyndromic mental retardation associated with a de novo mutation in the NLGN4X gene promoter causing an increased expression level. Biol Psychiatry. 2009;66:906–910. doi: 10.1016/j.biopsych.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Elliott CD. Differential Ability Scales-Introductory and Technical Handbook. Harcourt, Brace, Jovanovich; San Diego, CA: 1983. [Google Scholar]
- Gauthier J, Bonnel A, St-Onge J, Karemera L, Laurent S, Mottron L, Fombonne E, Joober R, Rouleau GA. NLGN3/NLGN4 gene mutations are not responsible for autism in the Quebec population. Am J Med Genet B Neuropsychiatr Genet. 2005;132B:74–75. doi: 10.1002/ajmg.b.30066. [DOI] [PubMed] [Google Scholar]
- Geerts M, Steyaert J, Fryns JP. The XYY syndrome: a follow-up study on 38 boys. Genet Couns. 2003;14:267–279. [PubMed] [Google Scholar]
- Gillberg C, Winnergard I, Wahlstrom J. The sex chromosomes--one key to autism? An XYY case of infantile autism. Appl Res Ment Retard. 1984;5:353–360. doi: 10.1016/s0270-3092(84)80056-9. [DOI] [PubMed] [Google Scholar]
- Gkogkas CG, Khoutorsky A, Ran I, Rampakakis E, Nevarko T, Weatherill DB, Vasuta C, Yee S, Truitt M, Dallaire P, Major F, Lasko P, Ruggero D, Nader K, Lacaille JC, Sonenberg N. Autism-related deficits via dysregulated eIF4E-dependent translational control. Nature. 2013;493:371–377. doi: 10.1038/nature11628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatt SJ, Tsuang MT, Winn M, Chandler SD, Collins M, Lopez L, Weinfeld M, Carter C, Schork N, Pierce K, Courchesne E. Blood-based gene expression signatures of infants and toddlers with autism. J Am Acad Child Adolesc Psychiatry. 2012;51:934–944. doi: 10.1016/j.jaac.2012.07.007. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB, Redlich F. Social Class and Mental Illness. John Wiley; New York, NY: 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoon M, Soykan T, Falkenburger B, Hammer M, Patrizi A, Schmidt KF, Sassoe-Pognetto M, Lowel S, Moser T, Taschen-berger H, Brose N, Varoqueaux F. Neuroligin-4 is localized to glycinergic postsynapses and regulates inhibition in the retina. Proc Natl Acad SciUSA. 2011;108:3053–3058. doi: 10.1073/pnas.1006946108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamain S, Quach H, Betancur C, Rastam M, Colineaux C, Gillberg IC, Soderstrom H, Giros B, Leboyer M, Gillberg C, Bourgeron T. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet. 2003;34:27–29. doi: 10.1038/ng1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya N, Colak D, Albakheet A. A novel X-linked disorder with developmental delay and autistic features. Ann Neurol. 2012;71:498–508. doi: 10.1002/ana.22673. [DOI] [PubMed] [Google Scholar]
- Kovacs MAMS. CDI Children’s Depression Inventory Technical Manual Update. MHS; North Tonawanda, NY: 2003. [Google Scholar]
- Laumonnier F, Bonnet-Brilhault F, Gomot M, Blanc R, David A, Moizard MP, Raynaud M, Ronce N, Lemonnier E, Calvas P, Laudier B, Chelly J, Fryns JP, Ropers HH, Hamel BC, Andres C, Barthelemy C, Moraine C, Briault S. X-linked mental retardation and autism are associated with a mutation in the NLGN4 gene, a member of the neuroligin family. Am J Hum Genet. 2004;74:552–557. doi: 10.1086/382137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee NR, Wallace GL, Adeyemi EI, Lopez KC, Blumenthal JD, Clasen LS, Giedd JN. Dosage effects of X and Y chromosomes on language and social functioning in children with supernumerary sex chromosome aneuploidies: implications for idiopathic language impairment and autism spectrum disorders. J Child Psychol Psychiatry. 2012;53:1072–1081. doi: 10.1111/j.1469-7610.2012.02573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggett V, Jacobs P, Nation K, Scerif G, Bishop DV. Neurocognitive outcomes of individuals with a sex chromosome trisomy: XXX, XYY, or XXY: a systematic review*. Dev Med Child Neurol. 2010;52:119–129. doi: 10.1111/j.1469-8749.2009.03545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SY, Chen YC, Lai TJ, Hsu CY, Wang YC. Molecular and cytogenetic analyses of autism in Taiwan. Hum Genet. 1993;92:441–445. doi: 10.1007/BF00216447. [DOI] [PubMed] [Google Scholar]
- Margari L, Lamanna AL, Craig F, Simone M, Gentile M. Autism spectrum disorders in XYY syndrome: two new cases and systematic review of the literature. Eur J Pediatr. 2014;173:277–283. doi: 10.1007/s00431-014-2267-9. [DOI] [PubMed] [Google Scholar]
- Netley CT. Summary overview of behavioural development in individuals with neonatally identified X and Y aneuploidy. Birth Defects Orig Artic Ser. 1986;22:293–306. [PubMed] [Google Scholar]
- Newschaffer CJ, Croen LA, Daniels J, Giarelli E, Grether JK, Levy SE, Mandell DS, Miller LA, Pinto-Martin J, Reaven J, Reynolds AM, Rice CE, Schendel D, Windham GC. The epidemiology of autism spectrum disorders. Annu Rev Public Health. 2007;28:235–258. doi: 10.1146/annurev.publhealth.28.021406.144007. [DOI] [PubMed] [Google Scholar]
- Powell CM, Boucard AA. Neuroligins and neurexins: synaptic bridges implicated in autism. In: Blatt GJ, editor. The Neurochemical Basis of Autism. Springer; New York, Heidelberg: 2010. [Google Scholar]
- Reynolds CR, Richmond BO. What I think and feel: a revised measure of children’s manifest anxiety. J Abn Child Psychology. 1978;6:271–280. doi: 10.1007/BF00919131. [DOI] [PubMed] [Google Scholar]
- Robinson A, Bender BG, Puck MH, Salbenblatt JA. Growth and Development of Children with a 47,XYY karyotype. Alan R. Liss, Inc.; New York, NY: 1985. [Google Scholar]
- Robinson A, Bender BG, Linden MG, Salbenblatt JA. Sex chromosome aneuploidy: the Denver Prospective Study. Birth Defects Orig Artic Ser. 1990;26:59–115. [PubMed] [Google Scholar]
- Rosenfeld JA, Ballif BC, Torchia BS, Sahoo T, Ravnan JB, Schultz R, Lamb A, Bejjani BA, Shaffer LG. Copy number variations associated with autism spectrum disorders contribute to a spectrum of neurodevelopmental disorders. Genet Med. 2010;12:694–702. doi: 10.1097/GIM.0b013e3181f0c5f3. [DOI] [PubMed] [Google Scholar]
- Ross JL, Zeger MP, Kushner H, Zinn AR, Roeltgen DP. An extra X or Y chromosome: Contrasting the cognitive and motor phenotypes in childhood in boys with 47,XYY syndrome or 47,XXY Klinefelter syndrome. Dev Disabil Res Rev. 2009;15:309–317. doi: 10.1002/ddrr.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross JL, Roeltgen DP, Kushner H, Zinn AR, Reiss A, Bardsley MZ, Mccauley E, Tartaglia N. Behavioral and social phenotypes in boys With 47,XYY syndrome or 47,XXY Klinefelter syndrome. Pediatrics. 2012;129:769–778. doi: 10.1542/peds.2011-0719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talebizadeh Z, Simon SD, Butler MG. X chromosome gene expression in human tissues: male and female comparisons. Genomics. 2006;88:675–681. doi: 10.1016/j.ygeno.2006.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia NR, Ayari N, Hutaff-Lee C, Boada R. Attention-deficit hyperactivity disorder symptoms in children and adolescents with sex chromosome aneuploidy: XXY, XXX, XYY, and XXYY. J Dev Behav Pediatr. 2012;33:309–318. doi: 10.1097/DBP.0b013e31824501c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theilgaard A. A psychological study of the personalities of XYY- and XXY-men. Acta Psychiatr Scand Suppl. 1984;315:1–133. [PubMed] [Google Scholar]
- Vincent JB, Kolozsvari D, Roberts WS, Bolton PF, Gurling HM, Scherer SW. Mutation screening of X-chromosomal neuroligin genes: no mutations in 196 autism probands. Am J Med Genet B Neuropsychiatr Genet. 2004;129B:82–84. doi: 10.1002/ajmg.b.30069. [DOI] [PubMed] [Google Scholar]
- Vorstman JA, Staal WG, Van Daalen E, Van Engeland H, Hochstenbach PF, Franke L. Identification of novel autism candidate regions through analysis of reported cytogenetic abnormalities associated with autism. Mol Psychiatry. 2006;11:18–28. doi: 10.1038/sj.mp.4001781. [DOI] [PubMed] [Google Scholar]
- Wassink TH, Piven J, Patil SR. Chromosomal abnormalities in a clinic sample of individuals with autistic disorder. Psychiatr Genet. 2001;11:57–63. doi: 10.1097/00041444-200106000-00001. [DOI] [PubMed] [Google Scholar]
- Welch J. Clinical Aspects of the XYY Syndrome. Alan R. Liss, Inc.; New York, NY: 1985. [Google Scholar]
- Yan J, Feng J, Schroer R, Li W, Skinner C, Schwartz CE, Cook EH, Jr, Sommer SS. Analysis of the neuroligin 4Y gene in patients with autism. Psychiatr Genet. 2008;18:204–207. doi: 10.1097/YPG.0b013e3282fb7fe6. [DOI] [PubMed] [Google Scholar]
- Ylisaukko-Oja T, Rehnstrom K, Auranen M, Vanhala R, Alen R, Kempas E, Ellonen P, Turunen JA, Makkonen I, Riikonen R, Nieminen-Von Wendt T, Von Wendt L, Peltonen L, Jarvela I. Analysis of four neuroligin genes as candidates for autism. Eur J Hum Genet. 2005;13:1285–1292. doi: 10.1038/sj.ejhg.5201474. [DOI] [PubMed] [Google Scholar]
- Zhang C, Milunsky JM, Newton S, Ko J, Zhao G, Maher TA, Tager-Flusberg H, Bolliger MF, Carter AS, Boucard AA, Powell CM, Sudhof TC. A neuroligin-4 missense mutation associated with autism impairs neuroligin-4 folding and endoplasmic reticulum export. J Neurosci. 2009;29:10843–10854. doi: 10.1523/JNEUROSCI.1248-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]