Abstract
Nonobstructive coronary artery disease (CAD) in women is associated with adverse cardiovascular (CV) outcomes; however, information regarding genetic variants that predispose women to nonobstructive CAD is lacking. Women from the Women's Ischemia Syndrome Evaluation (WISE) Study and the St. James Women Take Heart (WTH) Study were genotyped with the Cardio-MetaboChip. WISE enrolled women with symptoms and signs of ischemia referred for coronary angiography; WTH enrolled asymptomatic, community-based women without heart disease. Analyses were conducted with a case (WISE) - control (WTH) design and multivariate logistic regression models to investigate genetic variation associated with likelihood of nonobstructive CAD. One genetic marker, single nucleotide polymorphism (SNP) rs2301753 on chromosome 6 in RNF39, achieved chip-wide significance for nonobstructive CAD (P < 9.5 × 10−7). After adjusting for baseline characteristics, we found no variants achieved chip-wide significance. However, SNP rs2301753 on chromosome 6 in RNF39 was associated with reduced likelihood of nonobstructive CAD [odds ratio (OR) 0.42 and 95% confidence interval (CI) of 0.29 to 0.68], at a nominal level of P = 5.6 × 10−6, while SNP rs12818945 in the ATP2B1 locus on chromosome 12 was associated with increased odds for nonobstructive CAD (OR 2.38 and 95% CI of 1.63 to 3.45) and nominal P = 5.8 × 10−6. The functions of RNF39 and ATP2B1 raise the possibility that genes involved in cardio-dysfunction may contribute to nonobstructive CAD in Caucasian women and may provide insights into novel approaches for therapy and prevention. If replicated, incorporation of these genetic variants into diagnostic evaluation may identify women at high risk for nonobstructive CAD.
Keywords: genetics, nonobstructive coronary artery disease, cardio-metabochip, sex
metabolic conditions such as prediabetes, diabetes, prehypertension, hypertension, and dyslipidemia predispose individuals to ischemic heart disease, which is the leading cause of death in both men and women in United States (14). While ischemic heart disease due to obstructive coronary artery disease (CAD) is well studied (29), nonobstructive CAD also contributes to cardiovascular (CV) morbidity and mortality (1, 12, 16) but is less well characterized particularly in women, as nonobstructive CAD risk is often underdiagnosed and underestimated (18, 19). In women compared with men, there is more remodeling of the coronary arteries and more endothelial dysfunction, increasing their risk for developing nonobstructive CAD (2, 35). Nonobstructive CAD is a prevalent condition in women and is associated with an increase in risk for ischemia-related adverse outcomes including mortality (15). Furthermore, findings from the Women's Ischemia Syndrome Evaluation (WISE) Study have previously shown that myocardial ischemia is linked with metabolic traits and endothelial dysfunction (18, 20, 30).
Whether there are genetic factors that predispose women to pathological metabolic conditions that are mechanistically related to nonobstructive CAD is not known. This study was designed to investigate genetic variants associated with nonobstructive CAD in Caucasian women by utilizing clinical data and biological material collected from two large and complementary cohorts, the WISE (25), which enrolled women with chest pain and/or suspected myocardial ischemia who were ultimately diagnosed with nonobstructive CAD, and the St. James Women Take Heart (WTH) Study (11), which enrolled asymptomatic women without known heart disease. These phenotypically defined cohorts represent a unique opportunity to investigate specific associations between confirmed metabolic cardiovascular genetic variants and clinically defined nonobstructive CAD that would not be possible in more diverse or clinically less well-characterized populations.
Given the high prevalence of adverse metabolic conditions that associate with nonobstructive CAD in women, we used the custom-designed Cardio-MetaboChip, which includes single nucleotide polymorphisms (SNPs) related to metabolic traits as well as CV disease, to examine potential genetic variants that might associate with nonobstructive CAD phenotype in women.
METHODS AND MATERIALS
General design.
The general design is a case controlled study, where cases were women enrolled in the WISE study who presented with chest pain and/or suspected myocardial ischemia and were ultimately diagnosed with nonobstructive CAD and controls were women enrolled in the WTH study, who were asymptomatic without known heart disease. Demographic and clinical measurements from these cohorts were available for use in the current analysis.
WISE.
The WISE is a prospective study of women who underwent clinically indicated coronary angiography for signs and symptoms of myocardial ischemia, with the objective of improving strategies for diagnosing CAD in women. Of the 935 women originally enrolled in WISE between 1997 and 2000, 512 Caucasians were genotyped with the Cardio-MetaboChip. At the baseline visit, demographic information was collected, the women underwent a physical examination, and a medical history that included information regarding CV symptoms was obtained. Coronary angiography was quantitatively and qualitatively evaluated for the presence and extent of CAD by the WISE angiographic core laboratory (masked to historical data) as previously described (33), to classify CAD status (25). Women with obstructive CAD defined as ≥50% stenosis in any epicardial coronary artery were excluded. Caucasian women with signs and symptoms of ischemia and with nonobstructive CAD (defined as <50% stenosis in any coronary artery) were included in this analysis (10).
St. James WTH.
WTH is a prospective study dedicated to evaluating risk factors for heart disease in asymptomatic women volunteers from the greater Chicago metropolitan area. A total of 5,932 participants were enrolled from 1992 to 2000 (11), and 1,036 Caucasian women were genotyped on the Cardio-MetaboChip. All women were free of diagnosis of any CV disease and were able to walk on a treadmill at a moderate pace. Women were excluded if they were pregnant, had experienced typical anginal symptoms or myocardial infarction within the previous 3 mo, weighed >147 kg, or had a blood pressure (BP) ≥170/110 mmHg. Women from WTH with signs/symptoms of CAD based on exercise test were excluded from this study. All participants underwent a physical examination and resting electrocardiogram and supine BP measurements.
Genotyping.
All women enrolled in the WISE and WTH provided voluntary informed consent for participation and for collection of a DNA sample. All DNA samples were genotyped using the Cardio-MetaboChip, which is a custom Illumina genotyping array with ∼200,000 selected SNPs of interest from loci identified in genome-wide association studies (GWAS) for metabolic and atherosclerotic/CV disease traits (37). Content on the chip was selected on the basis of large-scale meta-analysis of relevant traits (including up to 100,000 individuals) and of HapMap and 1000 Genomes Project SNP content. The chip was designed by collaborating representatives of the CARDIoGRAM (coronary artery disease), DIAGRAM (Type 2 diabetes), GIANT (height and weight), MAGIC (glycemic traits), Lipids (lipids), ICBP-GWAS (blood pressure), and QT-IGC (QT interval) GWAS meta-analysis consortia (37). After removing SNPs with call rate <0.05, monomorphic SNPs with minor allele frequency (MAF) <0.0001, and SNPs not in Hardy-Weinberg equilibrium (P < 0.0001), we were left with a total of 121,313 SNPs available. Linkage disequilibrium (LD) pruning with cutoff r2 = 0.5 yielded 64,687 unlinked SNPs. Because our goal was to identify more common genetic variants associated with nonobstructive CAD, we removed the rare genetic variants, defined as an MAF of <3%, which further reduced the total number of SNPs included in the association analyses to 52,371 and yielded a suggested P value of 9.5 × 10−7 (0.05/52,371) for chip-wide significance. A P value of 1 × 10−4 was considered suggestive of significance.
Statistical analysis.
Although all participants included were Caucasian based on self-report, principal component analysis was performed with EIGENSOFT to analyze genetically determined ancestry information. Outliers were excluded based on this ancestral analysis. Logistic regression with an additive model was conducted between the 332 WISE cases and the 1,003 WTH controls with and without adjustment for covariates, in PLINK version 1.07. Genomic inflation factor (λ) was calculated with the adjust function in PLINK. Covariates in the logistic regression model included age, body mass index (BMI), history of diabetes, and principal components for population stratification. Because the top identified signals, loci in ATP2B1, a gene involved with intracellular calcium homeostasis, and RNF39, a gene thought to contribute in an early phase of synaptic plasticity, were previously identified as markers for diastolic BP and waist-hip ratio, respectively (23, 37), and the loci were identified in our analysis as associated with nonobstructive CAD, we conducted separate sensitivity analyses in additional logistic regression models that included waist-hip ratio and diastolic BP. For the sensitivity analysis of the ATP2B1 signal and diastolic BP, we included use of antihypertensive medications as a covariate in the model, and we also tested the association in a smaller cohort that excluded women who reported using antihypertensive medications. Lastly, we performed univariate analyses of our top genetic signals and other characteristics (history of hypertension, family history of ischemic heart disease, and smoking status) that differed significantly at baseline to confirm an association with nonobstructive CAD.
Haploview 4.2 was employed to generate Manhattan plots and run haplotype analysis. Regional plots were generated by LocusZoom (https://statgen.sph.umich.edu/locuszoom). The Student t-test was used to examine the differences for continuous characteristics, while χ2-tests were employed to estimate the differences for dichotomous characteristics, comparing the groups. A power calculation was performed with Quanto using log-additive model. Power estimation with a two-sided alpha level of 9.5 × 10−7 in our datasets suggests that we have 80% power to detect genetic variants with odds ratio (OR) of 2.6 for MAF = 0.05, 2.1 for MAF = 0.10 and 1.8 for MAF = 0.20.
RESULTS
Clinical Characteristics of the Cohorts
WISE.
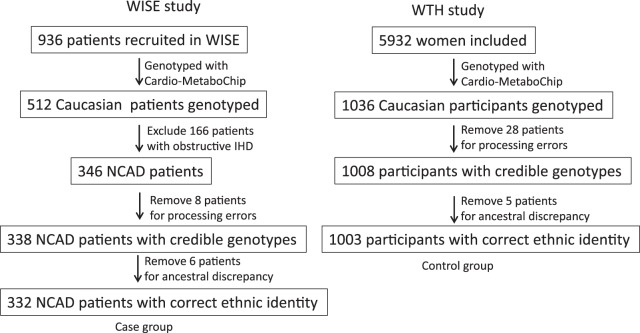
Of the 512 Caucasian women with available genotype data, 346 met the definition of nonobstructive CAD (≤50% stenosis) and were included in the genetic analysis. After quality control procedures, 14 women were excluded: eight due to likely relatedness and six whose ancestry identifiers did not cluster with CEU samples (Centre d'Etude du Polymorphisme Humain people from Utah) in HapMap 3, leaving 332 women included in the final WISE dataset for genetic analysis (Fig. 1).
Fig. 1.
Flow diagram of WISE and WTH participants enrolled for the analysis. The numbers of participants excluded and used in our analysis from the 2 cohorts are indicated. WISE, Women's Ischemia Syndrome Evaluation; WTH, St. James Women Take Heart; NCAD, nonobstructive coronary artery disease; IHD, ischemic heart diseases.
WTH.
There were 1,036 Caucasian women with available genotype data. After quality control procedures, 33 women were excluded: 28 women due to likely relatedness and five women whose ancestry identifiers did not cluster with CEU samples in HapMap 3, yielding 1,003 women included in the final WTH dataset for analysis (Fig. 1).
Pertinent baseline characteristics of the women included in this genotyping study are summarized in Table 1. As expected, since they were without known CV disease, the WTH participants were younger and leaner than those from the WISE cohort. Also as expected the prevalence of diabetes, hypertension and smoking were significantly greater in the WISE participants than those in the WTH (P < 0.0001 for all). Neither HDL cholesterol nor LDL cholesterol differed between the cohorts, which may be due to the use of lipid-lowering drugs in both cohorts.
Table 1.
Demographic baselines and clinical characteristics
| Demographic Baselines | WISE (n = 332) | WTH (n = 1003) | P Value |
|---|---|---|---|
| Mean age ± SD, yr | 55.8 ± 10.3 | 53.3 ± 10.9 | 0.0002 |
| Mean BMI ± SD, kg/m2 | 29.4 ± 6.7 | 27.2±.6 | <0.0001 |
| Diabetes, % | 12.4 | 3.5 | <0.0001 |
| Hypertension, % | 49.1 | 16.1 | <0.0001 |
| Family history of IHD, n, % | 228, 68.8 | 464, 46.3 | <0.0001 |
| Mean HDL-C ± SD, mg/dl | 53.6 ± 15.4 | 51.7 ± 14.9 | 0.10 |
| Mean LDL-C ± SD, mg/dl | 126.1 ± 45.3 | 124.0 ± 32.8 | 0.47 |
| Mean SBP ± SD, mmHg | 134.1 ± 20.2 | 129.3 ± 19.7 | 0.0002 |
| Mean DBP ± SD, mmHg | 76.3 ± 10.4 | 81.4 ± 11.2 | <.0001 |
| Mean WHR ± SD | 0.78 ± 0.08 | 0.84 ± 0.11 | <.0001 |
| Smoking, % | 30.1 | 13.6 | <0.0001 |
| Nonobstructive CAD | 60.2% (<20% stenosis); 39.8% (20–49% stenosis) | NA | <0.0001 |
Continuous characteristics are presented as means ± SD; Categorical characteristics are presented as percentage.
WISE, Women's Ischemia Syndrome Evaluation Study; WTH, St. James Women Take Heart Study; BMI, body mass index; IHD, ischemic heart diseases; HDL-C, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol; SBP, systolic blood pressure; DBP, diastolic blood pressure; WHR, waist-hip ratio; CAD, coronary artery disease. NA, not available.
Unadjusted Association Analysis
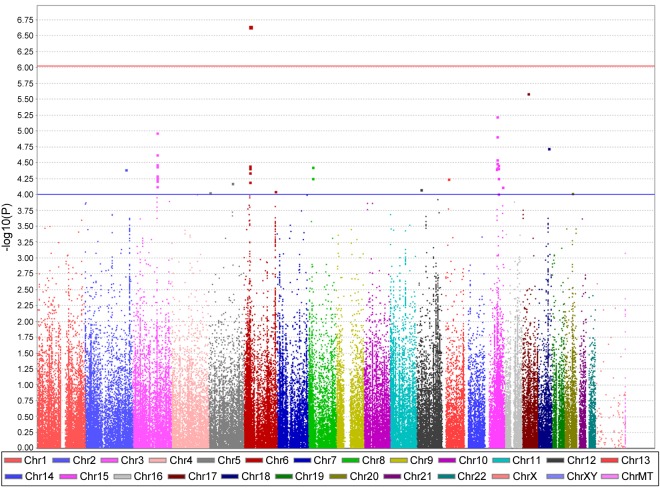
Using the SNP genotype characterized by the Cardio-MetaboChip, we first performed a logistic regression between the 332 WISE cases and 1,003 WTH controls, without adjusting for any covariates. As shown in the Manhattan plot (Fig. 2), three SNP clusters (on chromosome 6, 12, and 15) are noteworthy. One SNP, rs2301753 in RNF39 on chromosome 6, reached the specified level of statistical significance for the association with nonobstructive CAD in Caucasian women, with a P value of 7.3 × 10−7 (Table 2). A functional search revealed that rs2301753 represents a missense variant (C>A), altering the RNF39 coding sequence at position #304 from alanine to glutamic acid. The top SNP cluster on chromosome 12 was found to be in the ATP2B1 locus, while the top SNP cluster on chromosome 15 is in an intergenic region.
Fig. 2.
Manhattan plot of associations with nonobstructive CAD in selected WISE and WTH participants without adjustment. x-Axis, the chromosome position of all single nucleotide polymorphisms (SNPs); y-axis, −log10-transformed P values for the associations. Chr, chromosome.
Table 2.
Top signals of logistic regression (P < 10−4) without adjustment
| SNP | Chr | Base Pair Position | Minor Allele | MAF | Selection Category | Host Gene | OR (95% CI) | P Value |
|---|---|---|---|---|---|---|---|---|
| rs2301753 | 6 | 30147219 | A | 0.117 | WHR | RNF39 | 0.40 (0.28, 0.57) | 7.26E-07 |
| chr17:30331990 | 17 | 30331990 | A | 0.053 | QT | LIG3 | 2.41 (1.68, 3.47) | 1.91E-06 |
| chr15:61195932 | 15 | 61195932 | A | 0.040 | HDL | intergenic | 2.64 (1.76, 3.96) | 2.73E-06 |
| rs8095193 | 18 | 58834095 | A | 0.222 | QT | intergenic | 1.60 (1.30, 1.96) | 7.33E-06 |
| rs550338 | 12 | 24403304 | A | 0.238 | T2D | SOX5 | 0.61 (0.48, 0.76) | 1.16E-05 |
| rs11657937 | 17 | 56508571 | A | 0.063 | SBP | BCAS3 | 2.01 (1.47, 2.75) | 1.18E-05 |
| rs12818945 | 12 | 88542365 | A | 0.047 | DBP | ATP2B1 | 2.19 (1.53, 3.13) | 1.84E-05 |
| rs9379976 | 6 | 27406179 | G | 0.236 | MHC | intergenic | 1.53 (1.25, 1.87) | 3.18E-05 |
| rs17532886 | 16 | 50026847 | G | 0.226 | FG | intergenic | 0.62 (0.50, 0.78) | 3.19E-05 |
| rs2445887 | 5 | 78345800 | A | 0.460 | FG | DMGDH | 0.69 (0.58, 0.83) | 4.78E-05 |
| chr1:160598245 | 1 | 160598245 | G | 0.371 | QT | NOS1AP | 1.45 (1.21, 1.74) | 5.22E-05 |
| rs9266772 | 6 | 31460092 | G | 0.185 | SBP | intergenic | 1.56 (1.26, 1.93) | 5.59E-05 |
| rs2493134 | 1 | 228915982 | G | 0.436 | MICAD | AGT | 0.70 (0.58, 0.83) | 7.91E-05 |
| rs2024366 | 7 | 153663907 | G | 0.326 | SBP | DPP6 | 0.67 (0.55, 0.82) | 8.03E-05 |
| rs7328971 | 13 | 30390640 | A | 0.250 | QT | C13orf33 | 1.48 (1.22, 1.81) | 8.08E-05 |
SNP, single nucleotide polymorphism identifier; Chr, chromosome number; MAF, minor allele frequency; Host gene, gene locus of SNP; OR, odds ratio; 95% CI, 95% confidence interval; P, asymptotic P value for t-statistic. Selection category is the functional annotation of the selected SNP on the Cardio-MetaboChip.
QT, QT interval; T2D, Type 2 diabetes; MHC, major histocompatibility complex; FG, fasting glucose level; MICAD, myocardial infarction and coronary artery diseases.
Adjusted Association Analysis
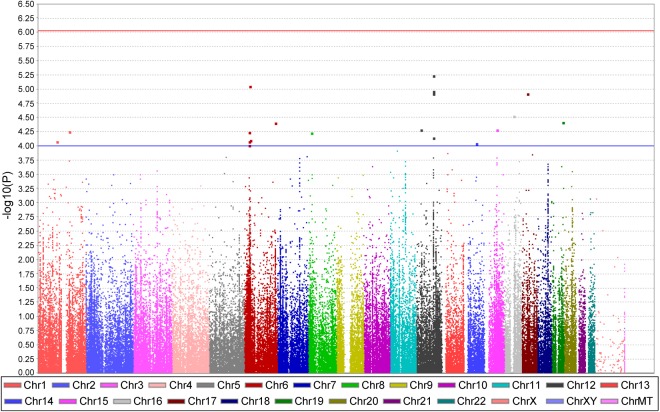
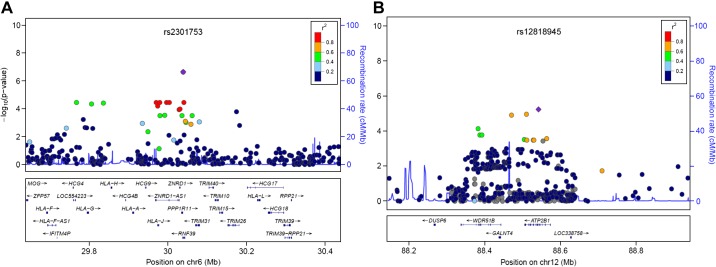
After adjusting for age, BMI, diabetes, and ancestry-informative principal components, we again conducted logistic regression analysis in the two cohorts. The genomic inflation factor (λ) is 1, suggesting the populations were similar in the two cohorts. After adjustment, as shown in Fig. 3, no signals achieved chip-wide significance, although SNP rs2301753 in RNF39 on chromosome 6 remained the top signal. The regional plot of the RNF39 locus suggests that rs2301753 is in high LD with several SNPs in RNF39 and downstream genes coding for human leukocyte antigens (HLAs) (Fig. 4A). Top signals from the adjusted analysis were also found on chromosome 12 in the ATP2B1 locus, either in an intron or near the gene, and were in high LD (Fig. 4B). Table 3 summarizes the top signals from the adjusted analysis.
Fig. 3.
Manhattan plot of associations with nonobstructive CAD in selected WISE and WTH participants. The covariates for the logistic model include age, body mass index, history of diabetes, and principal components for population correction. x-Axis, the chromosome position of all SNPs; y-axis, −log10-transformed P values for the associations.
Fig. 4.
Regional plot of SNPs in RNF39 and ATP2B1. The −log10-transformed P values for each association are shown on the y-axis, with x-axis showing physical positon on chromosome 12. SNPs are colored based on their r2 with the labeled top SNP that has the smallest P value in the region, indicated by purple diamond. Recombination rates estimated from individuals in the HapMap population are indicated by horizontal blue lines. Genes within the recombination region of the top SNPs are labeled at bottom. A: rs2301753 in RNF39 locus. B: rs12818945 in ATP2B1 locus.
Table 3.
Top signals of logistic regression (P < 10−4) in WISE and WTH participants adjusted for age, BMI, diabetes, and principal components for ancestral correction
| SNP | Chr | Base Pair Position | Minor Allele | MAF | Selection Category | Host Gene | OR (95% CI) | P Value |
|---|---|---|---|---|---|---|---|---|
| rs12818945 | 12 | 88542365 | A | 0.047 | DBP | ATP2B1 | 2.38 (1.63, 3.45) | 5.82E-06 |
| rs2301753 | 6 | 30147219 | A | 0.117 | WHR | RNF39 | 0.42 (0.29, 0.62) | 8.91E-06 |
| chr17:30331990 | 17 | 30331990 | A | 0.053 | QT | LIG3 | 2.35 (1.60, 3.44) | 1.20E-05 |
| rs17532886 | 16 | 50026847 | G | 0.226 | fastGlu | intergenic | 0.61 (0.48, 0.77) | 3.02E-05 |
| rs3852892 | 19 | 59414407 | A | 0.113 | N/A | intergenic | 1.76 (1.35, 2.31) | 3.85E-05 |
| rs6906489 | 6 | 161573429 | G | 0.047 | DBP | AGPAT4 | 0.27 (0.15, 0.51) | 3.90E-05 |
| chr15:61195932 | 15 | 61195932 | A | 0.040 | HDL | intergenic | 2.41 (1.58, 3.70) | 5.19E-05 |
| rs550338 | 12 | 24403304 | A | 0.238 | T2D | SOX5 | 0.62 (0.49, 0.78) | 5.25E-05 |
| chr1:160598245 | 1 | 160598245 | G | 0.371 | QT | NOS1AP | 1.48 (1.22, 1.78) | 5.56E-05 |
| rs9379976 | 6 | 27406179 | G | 0.236 | MHC | intergenic | 1.54 (1.25, 1.91) | 5.71E-05 |
| rs10503586 | 8 | 16792998 | G | 0.074 | T2D | intergenic | 1.92 (1.40, 2.63) | 5.83E-05 |
| chr12:88382885 | 12 | 88382885 | A | 0.031 | SBP | TUWD12 | 2.53 (1.60, 4.00) | 7.22E-05 |
| rs9266772 | 6 | 31460092 | G | 0.185 | SBP | intergenic | 1.57 (1.25, 1.96) | 8.00E-05 |
| rs12025601 | 1 | 96796873 | A | 0.249 | BMI | intergenic | 1.52 (1.23, 1.87) | 8.36E-05 |
| rs9379977 | 6 | 27406258 | G | 0.235 | MHC | intergenic | 1.53 (1.24, 1.89) | 8.37E-05 |
| rs12889954 | 14 | 63457221 | G | 0.156 | HbA1C | SYNE2 | 1.63 (1.28, 2.09) | 9.17E-05 |
Selection category is the functional annotation of the selected SNP on the Cardio-MetaboChip.
fastGlu, fasting glucose level; HbA1C, hemoglobin A1c.
Because ATP2B1 has been identified in GWAS as a hypertension marker (associated with diastolic BP) (23, 36), we also tested the association of the five SNPs in ATP2B1 (chr12:88382885, rs34205054, rs10506975, rs12818945, rs73198547) and diastolic BP in a linear regression of the combined WISE and WTH participants. We observed a consistent and similar trend for association of these ATP2B1 markers and diastolic BP (Table 4). Therefore, diastolic BP was added as a covariate in the logistic model, and SNPs rs2301753 and rs12818945 remained at the top of the list of associated signals (Table 5). Association with this ATP2B1 marker remained after including use of antihypertensive medications in the model or excluding women who reported use of antihypertensive medications (data not shown). In addition, rs2301753 was identified in GWAS as a marker for waist-hip ratio (37), and we tested this association in our dataset with a univariate model and found that rs2301753 significantly correlated with waist-hip ratio, P = 0.002. When waist-hip ratio was included in the regression model, rs2301753 remained significantly associated with reduced odds of nonobstructive CAD, with P = 4 × 10−4. Finally, when the top signals were tested in univariate regression models for other characteristics that differed at baseline among the WISE and WTH cohorts, rs2301753 and rs12818945 were consistent and remained at the level of suggestive associations for nonobstructive CAD.
Table 4.
Associations of ATP2B1 SNPs with DBP in univariate model in WISE/WTH cohorts
| SNP | Chr | Base Pair Position | Minor Allele | MAF | BETA | P |
|---|---|---|---|---|---|---|
| chr12:88382885 | 12 | 88382885 | A | 0.031 | −1.48 | 0.220 |
| rs34205054 | 12 | 88471152 | A | 0.046 | −1.72 | 0.074 |
| rs10506975 | 12 | 88510654 | G | 0.047 | −1.85 | 0.058 |
| rs12818945 | 12 | 88542365 | A | 0.047 | −1.78 | 0.068 |
| rs73198547 | 12 | 88579287 | C | 0.047 | −1.86 | 0.057 |
BETA, regression coefficient.
Table 5.
Top signals of logistic regression (P < 10−4) adjusted for age, BMI, diabetes, principal components for ancestral correction, and DBP
| SNP | Chr | Base Pair Position | Minor Allele | MAF | Selection Category | Host gene | OR (95% CI) | P |
|---|---|---|---|---|---|---|---|---|
| rs9266772 | 6 | 31460092 | G | 0.185 | SBP | intergenic | 1.67 (1.32, 2.10) | 1.50E-05 |
| rs6906489 | 6 | 161573429 | G | 0.047 | DBP | AGPAT4 | 0.25 (0.13, 0.47) | 1.85E-05 |
| rs3852892 | 19 | 59414407 | A | 0.113 | N/A | intergenic | 1.78 (1.35, 2.35) | 5.18E-05 |
| rs10503586 | 8 | 16792998 | G | 0.074 | T2D | intergenic | 1.99 (1.42, 2.77) | 5.82E-05 |
| rs2301753 | 6 | 30147219 | A | 0.117 | WHR | RNF39 | 0.45 (0.30, 0.67) | 5.95E-05 |
| chr1:160598245 | 1 | 160598245 | G | 0.371 | QT | NOS1AP | 1.49 (1.22, 1.82) | 6.69E-05 |
| rs12818945 | 12 | 88542365 | A | 0.047 | DBP | ATP2B1 | 2.23 (1.50, 3.31) | 6.97E-05 |
| rs12025601 | 1 | 96796873 | A | 0.249 | BMI | intergenic | 1.54 (1.24, 1.92) | 9.10E-05 |
Selection category is the functional annotation of the selected SNP on Cardio-MetaboChip.
DISCUSSION
This study using the Cardio-MetaboChip identified two suggestive genetic loci associated with nonobstructive CAD in well characterized cohorts of women, one on chromosome 6 and one on chromosome 12. These genetic signals were previously identified and replicated in CAD GWAS analyses, and now we have extended the association of these signals to the nonobstructive CAD phenotype. The SNPs on chromosome 6 are located in the major histocompatibility complex, a region in which the majority of genes are involved in immune response and inflammation. In contrast, the SNPS on chromosome 12 are involved in BP susceptibility.
The top signal, rs2301753 in RNF39, achieved chip-wide significance in the unadjusted analysis. This SNP causes a missense change in the RNF39 protein, from a nonpolar to a polar amino acid, indicating the potential for a substantial change in protein function, although loss of function was not confirmed by Polyphen-2 (which rated the variant as benign with a score of 0) (http://genetics.bwh.harvard.edu/pph2/). RNF39 is a protein with RING finger domain, a Cys3HisCys4 zinc finger that binds two zinc cations. Although the exact role of RNF39 in vivo remains to be established in humans, its chromosomal position, encompassed by HLA genes, indicates its potential role in immune response. In a Japanese cohort, RNF39 was associated with Behcet's disease, an autoimmune disorder causing inflammation in blood vessels (22). All of these data suggest the SNP rs2301753 could substantially affect function of RNF39 in immune response and inflammation, possibly in the vessel wall. Therefore, we hypothesize that loss of function of RNF39 caused by the rs2301753 minor allele, the allele associated in WISE, compromises or disables the buildup of immune reactions. However, this downside may have some interesting “beneficial” effect in ischemic heart disease in women. As growing evidence has pointed out, an inflammatory milieu may well be the underlying cause of the pathophysiology of nonobstructive CAD (4, 7), which in women is thought to encompass the smaller coronary arteries/arterioles and has been implicated to cause myocardial ischemia (4).
The loss of function of RNF39 may lower the risk for formation of endothelial plaque in coronary microvessels. This is consistent with our study that the rs2301753 minor allele was associated with reduced odds of nonobstructive CAD, suggesting a relative protective effect. Previous investigations from WISE and others have characterized C-reactive protein, IL-6, and serum amyloid A as inflammation risk factors contributing to nonobstructive CAD in women (17, 31, 34, 38). Furthermore, development of global measures of inflammation and simply counting the number of inflammatory markers with high levels improve CV disease risk stratification (21). Recently, an improved algorithm to estimate CV risk in women, the Reynolds risk score, was developed by incorporating the level of C-reactive protein into the traditional Framingham risk score, which may improve the prediction of the 10 yr risk of cardiac death or myocardial infarction (26, 32). Additionally, the Cardiovascular Inflammation Reduction Trial is currently testing the inflammation hypothesis of atherothrombosis by evaluating whether or not low-dose methotrexate will reduce rates of adverse CV outcomes among a cohort of stable CAD patients with diabetes or metabolic syndrome, conditions associated with an enhanced proinflammatory response (8). Our discovery that variation in rs2301753 in RNF39 is associated with risk for nonobstructive CAD is consistent with previous findings in WISE that inflammatory processes play a key role and further highlight the critical role of inflammatory factors in the development of nonobstructive CAD in women.
However, inflammation is not the only factor contributing to nonobstructive CAD. Other cardiac risk factors, such as hypertension, diabetes, and high levels of LDL cholesterol, contribute to nonobstructive CAD, dependently or independently. ATP2B1 codes for an ATP-driven calcium channel on the cell membrane, and SNPs in ATP2B1 have been associated with hypertension and CAD in previous GWAS (23, 36). This calcium channel functions to pump Ca2+ out of the cell and maintain cellular Ca2+ homeostasis. Downregulation of ATP2B1 abundance causes increased Ca2+ level in vascular smooth muscle cells, resulting in vasoconstriction and elevated BP (28). Previous findings from WISE indicate that an intronic variant in ATP2B1 (rs12817819) is associated with increased risk for resistant hypertension (9). Even though the top five SNPs in ATP2B1 locus (Table 2) are in either introns or intergenic regions, a query with RegulomeDB (http://regulomedb.org/) indicates that rs10506975, which is in high LD with our lead signal rs12818945, is predicted to be highly functional (RegulomeDB score 1f). A meta-analysis of two GWAS of CAD in a sample including ∼33,000 Chinese with Han ancestry identified the ATP2B1 locus to be associated with CAD with genome-wide significance (24), although the GWAS did not evaluate the effect of sex.
It is reasonable to hypothesize a synergistic mechanism between inflammation and BP, with increasing levels of both contributing to nonobstructive CAD. In the setting of hypertension, the altered mechanical force across the arterial walls likely impairs the endothelium. However, the causal relationship between calcium regulation at the level of the microvasculature and immunological processes is not well described.
When diastolic BP and waist-hip ratio were respectively included in the final regression models, we found that, while the further adjustment with diastolic BP didn't affect much of the significance of the top signals (e.g., rs2301753 and rs12818945), the additional adjustment of waist-hip ratio attenuated the contribution of rs2301753 toward the risk of nonobstructive CAD, with the level of significance decreasing from 8.9 × 10−6 to 4.0 × 10−4. This attenuated significance suggests that the metabolic milieu identified by the waist-hip ratio contributes to the development of nonobstructive CAD in this cohort of women. Previous studies have described an association of waist-hip ratio and endothelial function, CAD and CV death. (3, 13, 27, 39).
Several of the SNPs identified among the top signals (Table 2) in the present study have been implicated previously to be associated with CAD or other CV diseases. Duan et al. in 2013 (5) published their identification of AGPAT4, a gene encoding a member of the 1-acylglycerol-3-phosphate O-acyltransferase family and involved in de novo phospholipid biosynthesis with CAD susceptibility via integrative network analysis. Westaway et al. in 2011 (40) reported that common variants in NOS1AP, a gene encoding a cytosolic protein that binds to the signaling molecule, neuronal nitric oxide synthase, were significantly associated with the risk of sudden death in CAD patients. Ellinor et al. in 2012 (6) demonstrated through meta-analysis that genetic variants in SYNE2, a gene encoding a nuclear outer membrane protein that binds cytoplasmic F-actin and is involved in the maintenance of the structural integrity of the nucleus, were significantly associated with atrial fibrillation in European descendants. The association of these loci with CAD or CV diseases in the literature was not studied specifically related to men or women. However, they greatly decrease the likelihood that the observed signals in the studied cohort of women are spurious. Further investigation is warranted to assess the potential for these genetic markers to aid in the prediction of increased or decreased risk for nonobstructive CAD in other groups of women.
There are several limitations to the present study. The sample size is relatively small (332 cases and 1,003 controls) with limited power to detect significant associations, particularly for rare genetic variants. Additionally, this study was conducted in Caucasian women from two cohorts with some differing baseline characteristics, which may introduce some confounding with regard to risk factor associations. Results from our study cannot be generalized to other race groups or populations. Lastly, use of the Cardio-MetaboChip, which was developed in 2012, only includes GWAS signals related to metabolic and CV diseases identified prior to that time. Newer genetic variants associated with metabolic or CV diseases were not included in this study. Despite these limitations, we did identify biologically plausible genetic markers for nonobstructive CAD that warrant further investigation and confirmation in an independent population.
In conclusion, this study identified suggestive evidence that genetic variants in RNF39 and ATP2B1 are associated with nonobstructive CAD in women. These findings further underscore the importance of inflammation and BP variation and reactivity in the development of nonobstructive CAD and warrant further investigation and replication in other cohorts. If replicated, incorporation of these genetic variants into predictive models may improve the prediction of nonobstructive CAD in Caucasian women.
GRANTS
This work was supported by grants from the Society for Women's Health Research, Washington, DC (to R. M. Cooper-DeHoff); the Gerlach Translational Award provided by the Gerlach Family from Columbus, OH; the Women & Philanthropy Grant from the Ohio State University, Columbus, OH (to Martha Gulati); and the Bill and Sarah Soter family from Palm Beach, FL; National Institutes of Health contracts and grants as follows: U01 GM-074492, N01-HV-68161, N01-HV-68162, N01-HV-68163, N01-HV-68164, U0164829, U01 HL-649141, U01 HL-649241, T32HL-69751, R01 HL-090957, 1R03AG-032631, MO1-RR-00425, UL1TR-000124, P30 DK-063491; and grants from the Gustavus and Louis Pfeiffer Research Foundation, Danville, NJ; The Women's Guild of Cedars-Sinai Medical Center, Los Angeles, CA; The Ladies Hospital Aid Society of Western Pennsylvania, Pittsburgh, PA; and QMED, Inc., Laurence Harbor, NJ; the Edythe L. Broad and the Constance Austin Women's Heart Research Fellowships, Cedars-Sinai Medical Center, Los Angeles, CA; the Barbra Streisand Women's Cardiovascular Research and Education Program, Cedars-Sinai Medical Center, Los Angeles, CA; The Linda Joy Pollin Women's Heart Health Program and the Erika Glazer Women's Heart Health Project, Cedars-Sinai Medical Center, Los Angeles, CA.
This work is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: L.W. and R.M.C.-D. analyzed data; L.W., K.D.T., and R.M.C.-D. interpreted results of experiments; L.W. and R.M.C.-D. prepared figures; L.W. and R.M.C.-D. drafted manuscript; L.W., K.D.T., Y.-D.I.C., G.S., S.F.K., C.N.B.M., C.J.P., V.M.M., J.I.R., M.G., M.O.G., and R.M.C.-D. edited and revised manuscript; L.W., K.D.T., Y.-D.I.C., G.S., S.F.K., C.N.B.M., C.J.P., V.M.M., J.I.R., M.G., M.O.G., and R.M.C.-D. approved final version of manuscript; K.D.T., Y.-D.I.C., V.M.M., J.I.R., M.G., M.O.G., and R.M.C.-D. conception and design of research; K.D.T. performed experiments.
REFERENCES
- 1.Al Suwaidi J, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation 101: 948–954, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Bellasi A, Raggi P, Merz CNB, Shaw LJ. New insights into ischemic heart disease in women. Cleve Clin J Med 74: 585–594, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Brook RD, Bard RL, Rubenfire M, Ridker PM, Rajagopalan S. Usefulness of visceral obesity (waist/hip ratio) in predicting vascular endothelial function in healthy overweight adults. Am J Cardiol 88: 1264–1269, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med 356: 830–840, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Duan S, Luo X, Dong C. Identification of susceptibility modules for coronary artery disease using a genome wide integrated network analysis. Gene 531: 347–354, 2013. [DOI] [PubMed] [Google Scholar]
- 6.Ellinor PT, Lunetta KL, Albert CM, Glazer NL, Ritchie MD, Smith AV, Arking DE, Müller-Nurasyid M, Krijthe BP, Lubitz SA. Meta-analysis identifies six new susceptibility loci for atrial fibrillation. Nat Genet 44: 670–675, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Epstein FH, Ross R. Atherosclerosis-an inflammatory disease. N Engl J Med 340: 115–126, 1999. [DOI] [PubMed] [Google Scholar]
- 8.Everett BM, Pradhan AD, Solomon DH, Paynter N, Macfadyen J, Zaharris E, Gupta M, Clearfield M, Libby P, Hasan AA, Glynn RJ, Ridker PM. Rationale and design of the Cardiovascular Inflammation Reduction Trial: a test of the inflammatory hypothesis of atherothrombosis. Am Heart J 166: 199–207. e115, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fontana V, McDonough CW, Gong Y, El Rouby NM, Sa AC, Taylor KD, Chen YD, Gums JG, Chapman AB, Turner ST, Pepine CJ, Johnson JA, Cooper-DeHoff RM. Large-scale gene-centric analysis identifies polymorphisms for resistant hypertension. J Am Heart Assoc 3: e001398, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gulati M, Cooper-DeHoff RM, McClure C, Johnson BD, Shaw LJ, Handberg EM, Zineh I, Kelsey SF, Arnsdorf MF, Black HR, Pepine CJ, Merz CN. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: a report from the Women's Ischemia Syndrome Evaluation Study and the St James Women Take Heart Project. Arch Intern Med 169: 843–850, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gulati M, Pandey DK, Arnsdorf MF, Lauderdale DS, Thisted RA, Wicklund RH, Al-Hani AJ, Black HR. Exercise capacity and the risk of death in women: the St James Women Take Heart Project. Circulation 108: 1554–1559, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Halcox JP, Schenke WH, Zalos G, Mincemoyer R, Prasad A, Waclawiw MA, Nour KR, Quyyumi AA. Prognostic value of coronary vascular endothelial dysfunction. Circulation 106: 653–658, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Hartz A, Grubb B, Wild R, Van Nort J, Kuhn E, Freedman D, Rimm A. The association of waist hip ratio and angiographically determined coronary artery disease. Int J Obes 14: 657–665, 1990. [PubMed] [Google Scholar]
- 14.Hoyert DL, Xu J. Deaths: preliminary data for 2011. Natl Vital Stat Rep 61: 1–51, 2012. [PubMed] [Google Scholar]
- 15.Humphries KH, Pu A, Gao M, Carere RG, Pilote L. Angina with “normal” coronary arteries: sex differences in outcomes. Am Heart J 155: 375–381, 2008. [DOI] [PubMed] [Google Scholar]
- 16.Jespersen L, Hvelplund A, Abildstrøm SZ, Pedersen F, Galatius S, Madsen JK, Jørgensen E, Kelbæk H, Prescott E. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J 33: 734–744, 2012. [DOI] [PubMed] [Google Scholar]
- 17.Johnson BD, Kip KE, Marroquin OC, Ridker PM, Kelsey SF, Shaw LJ, Pepine CJ, Sharaf B, Bairey Merz CN, Sopko G, Olson MB, Reis SE. Serum amyloid A as a predictor of coronary artery disease and cardiovascular outcome in women: the National Heart, Lung, and Blood Institute-Sponsored Women's Ischemia Syndrome Evaluation (WISE). Circulation 109: 726–732, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Johnson BD, Shaw LJ, Buchthal SD, Bairey Merz CN, Kim HW, Scott KN, Doyle M, Olson MB, Pepine CJ, den Hollander J, Sharaf B, Rogers WJ, Mankad S, Forder JR, Kelsey SF, Pohost GM. Prognosis in women with myocardial ischemia in the absence of obstructive coronary disease: results from the National Institutes of Health-National Heart, Lung, and Blood Institute-Sponsored Women's Ischemia Syndrome Evaluation (WISE). Circulation 109: 2993–2999, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Johnson BD, Shaw LJ, Pepine CJ, Reis SE, Kelsey SF, Sopko G, Rogers WJ, Mankad S, Sharaf BL, Bittner V. Persistent chest pain predicts cardiovascular events in women without obstructive coronary artery disease: results from the NIH-NHLBI-sponsored Women's Ischaemia Syndrome Evaluation (WISE) study. Eur Heart J 27: 1408–1415, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Kip KE, Marroquin OC, Kelley DE, Johnson BD, Kelsey SF, Shaw LJ, Rogers WJ, Reis SE. Clinical importance of obesity versus the metabolic syndrome in cardiovascular risk in women a report from the Women's Ischemia Syndrome Evaluation (WISE) study. Circulation 109: 706–713, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Kip KE, Marroquin OC, Shaw LJ, Arant CB, Wessel TR, Olson MB, Johnson BD, Mulukutla S, Sopko G, Merz CN, Reis SE. Global inflammation predicts cardiovascular risk in women: a report from the Women's Ischemia Syndrome Evaluation (WISE) study. Am Heart J 150: 900–906, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Kurata R, Nakaoka H, Tajima A, Hosomichi K, Shiina T, Meguro A, Mizuki N, Ohono S, Inoue I, Inoko H. TRIM39 and RNF39 are associated with Behcet's disease independently of HLA-B *51 and -A *26. Biochem Biophys Res Commun 401: 533–537, 2010. [DOI] [PubMed] [Google Scholar]
- 23.Levy D, Ehret GB, Rice K, Verwoert GC, Launer LJ, Dehghan A, Glazer NL, Morrison AC, Johnson AD, Aspelund T. Genome-wide association study of blood pressure and hypertension. Nat Genet 41: 677–687, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu X, Wang L, Chen S, He L, Yang X, Shi Y, Cheng J, Zhang L, Gu CC, Huang J. Genome-wide association study in Han Chinese identifies four new susceptibility loci for coronary artery disease. Nat Genet 44: 890–894, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merz CN, Kelsey SF, Pepine CJ, Reichek N, Reis SE, Rogers WJ, Sharaf BL, Sopko G. The Women's Ischemia Syndrome Evaluation (WISE) study: protocol design, methodology and feasibility report. J Am Coll Cardiol 33: 1453–1461, 1999. [DOI] [PubMed] [Google Scholar]
- 26.Michos ED, Nasir K, Braunstein JB, Rumberger JA, Budoff MJ, Post WS, Blumenthal RS. Framingham risk equation underestimates subclinical atherosclerosis risk in asymptomatic women. Atherosclerosis 184: 201–206, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Nishtar S, Wierzbicki AS, Lumb PJ, Lambert-Hammill M, Turner CN, Crook MA, Mattu MA, Shahab S, Badar A, Ehsan A. Waist-hip ratio and low HDL predict the risk of coronary artery disease in Pakistanis. Curr Med Res Opin 20: 55–62, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Olson S, Wang MG, Carafoli E, Strehler EE, McBride OW. Localization of two genes encoding plasma membrane Ca2+-transporting ATPases to human chromosomes 1q25-32 and 12q21-23. Genomics 9: 629–641, 1991. [DOI] [PubMed] [Google Scholar]
- 29.Proudfit WL, Bruschke AV, Sones FM. Natural history of obstructive coronary artery disease: ten-year study of 601 nonsurgical cases. Prog Cardiovasc Dis 21: 53–78, 1978. [DOI] [PubMed] [Google Scholar]
- 30.Quyyumi AA. Women and ischemic heart disease: pathophysiologic implications from the Women's Ischemia Syndrome Evaluation (WISE) Study and future research steps. J Am Coll Cardiol 47: S66–S71, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14 719 initially healthy American women. Circulation 107: 391–397, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA 297: 611–619, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Sharaf BL, Williams DO, Miele NJ, McMahon RP, Stone PH, Bjerregaard P, Davies R, Goldberg AD, Parks M, Pepine CJ, Sopko G, Conti CR. A detailed angiographic analysis of patients with ambulatory electrocardiographic ischemia: results from the Asymptomatic Cardiac Ischemia Pilot (ACIP) study angiographic core laboratory. J Am Coll Cardiol 29: 78–84, 1997. [DOI] [PubMed] [Google Scholar]
- 34.Shaw LJ, Bugiardini R, Merz CN. Women and ischemic heart disease: evolving knowledge. J Am Coll Cardiol 54: 1561–1575, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sheifer SE, Canos MR, Weinfurt KP, Arora UK, Mendelsohn FO, Gersh BJ, Weissman NJ. Sex differences in coronary artery size assessed by intravascular ultrasound. Am Heart J 139: 649–653, 2000. [DOI] [PubMed] [Google Scholar]
- 36.The International Consortium for Blood Pressure Genome-wide Association Studies. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature 478: 103–109, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Voight BF, Kang HM, Ding J, Palmer CD, Sidore C, Chines PS, Burtt NP, Fuchsberger C, Li Y, Erdmann J, Frayling TM, Heid IM, Jackson AU, Johnson T, Kilpelainen TO, Lindgren CM, Morris AP, Prokopenko I, Randall JC, Saxena R, Soranzo N, Speliotes EK, Teslovich TM, Wheeler E, Maguire J, Parkin M, Potter S, Rayner NW, Robertson N, Stirrups K, Winckler W, Sanna S, Mulas A, Nagaraja R, Cucca F, Barroso I, Deloukas P, Loos RJ, Kathiresan S, Munroe PB, Newton-Cheh C, Pfeufer A, Samani NJ, Schunkert H, Hirschhorn JN, Altshuler D, McCarthy MI, Abecasis GR, Boehnke M. The metabochip, a custom genotyping array for genetic studies of metabolic, cardiovascular, and anthropometric traits. PLoS Genet 8: e1002793, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Volpato S, Guralnik JM, Ferrucci L, Balfour J, Chaves P, Fried LP, Harris TB. Cardiovascular disease, interleukin-6, and risk of mortality in older women: the women's health and aging study. Circulation 103: 947–953, 2001. [DOI] [PubMed] [Google Scholar]
- 39.Welborn TA, Dhaliwal SS, Bennett SA. Waist-hip ratio is the dominant risk factor predicting cardiovascular death in Australia. Med J Aust 179: 580–585, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Westaway SK, Reinier K, Huertas-Vazquez A, Evanado A, Teodorescu C, Navarro J, Sinner MF, Gunson K, Jui J, Spooner P. Common variants in CASQ2, GPD1L and NOS1AP are significantly associated with risk of sudden death in patients with coronary artery disease. Circ Cardiovasc Genet 4: 397–402, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]