Abstract
Prior to the initiation of menopausal hormone treatment (MHT), genetic variations in the innate immunity pathway were found to be associated with carotid artery intima-medial thickness (CIMT) and coronary arterial calcification (CAC) in women (n = 606) enrolled in the Kronos Early Estrogen Prevention Study (KEEPS). Whether MHT might affect these associations is unknown. The association of treatment outcomes with variation in the same 764 candidate genes was evaluated in the same KEEPS participants 4 yr after randomization to either oral conjugated equine estrogens (0.45 mg/day), transdermal 17β-estradiol (50 μg/day), each with progesterone (200 mg/day) for 12 days each month, or placebo pills and patch. Twenty SNPs within the innate immunity pathway most related with CIMT after 4 yr were not among those associated with CIMT prior to MHT. In 403 women who completed the study in their assigned treatment group, single nucleotide polymorphisms (SNPs) within the innate immunity pathway were found to alter the treatment effect on 4 yr change in CIMT (i.e., significant interaction between treatment and genetic variation in the innate immunity pathway; P < 0.001). No SNPs by treatment effects were observed with changes of CAC >5 Agatston units after 4 yr. Results of this study suggest that hormonal status may interact with genetic variants to influence cardiovascular phenotypes, specifically, the pharmacogenomic effects within the innate immunity pathway for CIMT.
Keywords: atherosclerosis, candidate genes, estrogen, innate immunity, thrombosis
controversy surrounding whether or not menopausal hormone treatment (MHT) slows progression of cardiovascular disease reflects, in part, differences among studies in experimental design (timing of initiation of hormone treatment, doses and formulations) and coexisting cardiovascular risk factors of the population or study participants (4, 6, 16, 22, 24, 30, 33). In addition, the potential benefit of MHT against development of atherosclerosis is offset by the potential risk of venous thrombosis especially in women with genetic variation in factors associated with coagulation, which may be more pronounced with the use of oral compared with transdermal estrogen products and the impact of the oral products on liver metabolism (8, 9, 12, 35). In addition, several studies have implicated genetic variants in estrogen receptors with progression of cardiovascular disease and venous thrombosis in men and women (1, 23, 34). However, estrogen receptor polymorphisms were not associated with adverse cardiovascular outcomes in women randomized to oral conjugated equine estrogens in the Women's Health Initiative (31). Therefore, much remains to be learned about phenotypic expression of polymorphisms in genes associated with estrogen responsiveness and thrombotic capacity in evaluating potential benefits and risk of MHT in postmenopausal women.
The Kronos Early Estrogen Prevention Study (KEEPS) was designed to evaluate the effects of MHT on progression of cardiovascular disease defined by quantitative changes in carotid artery intima-medial thickness (CIMT) and coronary artery calcification (CAC), accepted measures of subclinical atherosclerosis. A targeted candidate genetic analysis demonstrated no association of genetic polymorphisms in estrogen receptor-α or -β with the absolute value of either CIMT or CAC in women enrolled in KEEPS prior to randomization to treatment. Of the polymorphisms of genes within the anticoagulant, procoagulant, fibrinolytic, or innate immunity pathways, only polymorphisms of genes within the innate and humoral immunity pathways were associated with the baseline absolute value of CIMT or CAC, respectively (26). However, at the time of that study, women had transitioned into menopause and were estrogen depleted (serum 17β-estradiol <40 pg/ml). Thus, it remains to be determined whether estrogen treatments alter these associations. Therefore, associations of genetic variants within the same targeted candidate genes were evaluated with progression (i.e., change in measures) of subclinical atherosclerosis following randomization to either active estrogen treatments or placebo for 4 yr. Two hypotheses were considered in these analyses: 1) genetic markers would be associated with change in CIMT or CAC after the 4 yr independent of treatment, and 2) there would be pharmacogenomic effects of genes with respect to change in CIMT or CAC after application of treatment or placebo (i.e., genetic markers will modify the effects of the treatment/placebo on CIMT and CAC progression).
METHODS
Participants
Women meeting inclusion criteria for the KEEPS (NCT00154180) and who gave informed consent to have their DNA used for research purposes were included in this study. There were nine centers participating in KEEPS: Brigham and Women's Hospital; Columbia University College of Physicians and Surgeons; the Kronos Longevity Research Institute; Mayo Clinic, Rochester, MN; Montefiore Medical Center; University of California at San Francisco; University of Utah; University of Washington; and Yale University. Each institutional review board of these participating institutions approved the study.
KEEPS inclusion/exclusion criteria are detailed elsewhere (15). In brief, women were excluded from KEEPS if they had a history of, or were symptomatic for, cardiovascular disease; smoked >10 cigarettes/day; had coronary artery calcification [i.e., ≥50 Agatston units (AU)], body mass index >35 kg/m2, dyslipidemia (low-density lipoprotein cholesterol >190 mg/dl), hypertriglyceridemia (triglycerides, >400 mg/dl), 17β-estradiol >40 pg/ml; uncontrolled hypertension (systolic blood pressure >150 mmHg and/or diastolic blood pressure >95 mmHg) or fasting blood glucose >126 mg/dl; or used lipid-lowering drugs (15, 25). Women meeting inclusion criteria were randomized to treatment: oral conjugated equine estrogens (Premarin, 0.45 mg/day), transdermal 17β-estradiol (via skin patch, Climara, 50 μg/day) both with progesterone (oral Prometrium, 200 mg/day) for the first 12 days of the month, or placebo group (inactive pills/patch) for 4 yr. Of women randomized to treatment, 684 consented to allow analysis of their DNA. Of these, 606 had clinical data, CIMT, and CAC measurements available for analysis prior to treatment (baseline). Follow-up data were available for 565 women at 1 yr, 539 women at 2 yr, 519 women at 3 yr, and 512 women at 4 yr.
Clinical Methodology and Genotype Quality Control
All blood samples were collected after an overnight fast, frozen at −70°C on site until they were either processed locally or sent to the Kronos Science Laboratory (Phoenix, AZ) for storage or assays. Genomic DNA was extracted from whole blood with the QIAamp DNA Blood Midi Kit (Qiagen), and the DNA concentration was measured by the PicoGreen technique (Invitrogen). The genotyping panel for identification of the single nucleotide polymorphisms (SNPs) for the custom 16,720 bead Illumina Infinium [13,229 SNPs including 492 ancestry informative markers (AIMs) (32)] are described in detail elsewhere (17).
Clinical phenotypic characteristics, genotyping, and quality control were performed as previously described (26). CIMT measured by B-mode ultrasound and CAC measured from nonenhanced cardiac computed tomography scans were quantified at Core reading centers for KEEPS (14, 15, 26). All CIMT scans were obtained with high-resolution ultrasonographic equipment using standardized methods for reproducing transducer angulation and cardiac gating (18, 19) by personnel at each site who were trained at the central reading center. The scans were read by trained personnel blinded to treatment assignment at the central reading center. The intima-media thickness of the far wall of the distal common carotid artery was determined as the average of 70–100 standardized measurements between the intima-lumen and media-adventitia interfaces by automated computerized edge detection software (patents obtained in 2005, 2006, and 2011). To determine variability of the readings, two scans were obtained at separate visits (from 3 days to 6 wk apart) prior to randomization. As reported previously, the mean coefficient of variation between these two readings was 0.6% [SD, 0.7 (range, 0.0% to 7.7%)] (14).
Statistical Analysis
Clinical characteristics were summarized at baseline and 4 yr separately, changes in clinical characteristics between baseline and the 4 yr measurement were tested by Wilcoxon signed rank tests. The analysis consisted of two outcomes, 4 yr change in CIMT as a continuous measure and 4 yr change in CAC as a binary measure (change >5 vs. ≤5 AU). CIMT was measured at yearly intervals for 4 yr, and modeling all 4 yr of measurements yielded similar conclusions (data not shown). CAC was measured at baseline and at year 4. First, the relationships of the two outcomes (CIMT and CAC) and conventional cardiovascular risk variables were tested by linear regression and logistic regression, respectively. None of these variables were significantly correlated with the outcomes after multiple testing correction (data not shown) and were not adjusted for in subsequent genetic analyses. However, percentage of European ethnicity was adjusted for in subsequent genetic analyses to address possible population stratification. Previously, using AIMs, we had established that most KEEPS participants were Caucasian, and we used percentage CEU ancestry from the STRUCTURE program to estimate the proportion of European ancestry within each individual (26). STRUCTURE allows for population admixture and assigns individuals in the sample of interest (the KEEPS sample) population probabilities. The technique assumes the loci are unlinked and it assumes Hardy-Weinberg equilibrium within the populations.
Two genetic analyses were considered for each outcome, first testing for SNP effects on the outcomes and second testing for SNP*treatment interactions (i.e., SNP affecting the relationship of the treatment and outcome, a pharmacogenomic effect). In both analyses, SNPs (as count of minor allele) were used to model change in CIMT and change in CAC, via linear and logistic regression, respectively. In the case of the pharmacogenomic analysis, a treatment main effect and treatment*SNP interaction were also modeled with the treatment*SNP interaction being tested with a likelihood ratio test. To correct for multiple testing we estimated the effective number of independent tests, and using the Bonferroni method we set our threshold of significance at P < 7.73E-06 (11).
To test the overall association of SNPs in each of four genetic pathways [anticoagulant, procoagulant, fibrinolysis, and innate immunity; a complete list of SNPs was published previously (26)], we conducted a global test of the genetic variation in each pathway, modeling 4 yr change in CIMT or CAC by pathway SNPs using random effects models and testing all SNPs in a pathway simultaneously with a likelihood ratio test (13).
To the test the overall pharmacogenomic effect of pathways of SNPs on change in CAC and CIMT, we used the principal components (PC)-gamma method (3). PCs were formed from SNPs within each gene, and enough PCs were retained for each gene to explain 80% of the variation in SNPs within that gene. Then we conducted gene level tests for each gene using F-tests of all treatment interactions with PCs retained in a regression model for the continuous change in CIMT and likelihood ratio test for all treatment interactions with PCs retained in a logistic regression model of dichotomized change in CAC. Fisher's P value combination method was then used to generate a test statistic for the pathway. Since genes in a pathway may be correlated to some degree, an asymptotic test was not used; instead, a parametric bootstrap approach was applied (7) to obtain a pathway level P value. In brief, we fit a null model without the PC*treatment interactions and for each individual calculated their fitted value. Next, a bootstrap sample of participants was taken with replacement, and a set of new CIMT or CAC values was simulated for the individuals in the sample. This process was repeated to generate 1,000 bootstrap samples under the null hypothesis on no treatment-gene interactions. Each of these 1,000 datasets was analyzed by the PC-gamma method to obtain an empirical distribution of test statistics for the gene-treatment interaction at the pathway level under the null hypothesis. The observed pathway test statistic was then compared with this empirical null distribution, with the P value being the fraction of empirical test-statistic values that were greater than or equal to the observed test statistic. All analyses were performed in R v2.14.0.
RESULTS
Clinical phenotypic characteristics of women for whom SNP analyses were performed over the 4 yr of treatment are shown in Table 1.
Table 1.
Phenotypic characteristics of KEEPS participants in the genetic association analysis
| Baseline (pretreatment) | 4 yr Posttreatment | P Value | |
|---|---|---|---|
| Treatment, participants, n | |||
| A = premarin | 188 | 157 | |
| B = patch | 186 | 161 | |
| C = placebo | 232 | 194 | |
| Weight, kg | 70.6(11.8) | 71.1(12.5) | <0.01 |
| Body mass index, kg/m2 | 26.3(4.3) | 26.6(4.62) | <0.01 |
| Waist circumference, cm | 84.9(11.7) | 85.1(11.5) | 0.20 |
| Systolic blood pressure, mmHg | 119(15.1) | 118(13.9) | 0.95 |
| Diastolic blood pressure, mmHg | 75.1(9.26) | 73.7(9.1) | 0.02 |
| C-reactive protein, pg/ml | 2.18(3.38) | 3.05(4.38) | <0.01 |
| Fasting blood glucose, mg/dl | 79.7(9.57) | 81.3(9.28) | <0.01 |
| Total cholesterol, mg/dl | 208(34.5) | 210(35.7) | 0.11 |
| High-density lipoprotein cholesterol, mg/dl | 72.1(14.6) | 73.1(15.1) | 0.08 |
| Low-density lipoprotein cholesterol, mg/dl | 111(28.4) | 111(30.7) | 0.65 |
| Triglycerides, mg/dl | 86.3(54.9) | 90.8(52.3) | <0.01 |
| Insulin, pmol/l | 6.01(7.65) | 5.23(6.38) | 0.01 |
| HOMA-IR score | 1.23(1.74) | 1.07(1.28) | 0.09 |
Data are shown as means (SD); P values depict difference between baseline and year 4 independent of treatment by Wilcoxon signed ranks test.
KEEPS, Kronos Early Estrogen Prevention Study; HOMA-IR, homeostasis model assessment of insulin resistance.
CIMT
Independent of treatment.
Mean CIMT increased over the 4 yr of the study with variability increasing with time (Fig. 1). To evaluate these increases two analyses were performed: one examining only the change in CIMT from baseline to 4 yr and the second considering simultaneously all measures of CIMT over 4 yr that included data of women with readings at multiple time points (longitudinal analysis). Results of these two analyses were similar, so for clarity, only changes from baseline to 4 yr are presented here. None of the conventional cardiovascular risk factors (Table 1) measured at baseline or their change over the 4 yr was found to be associated with changes in CIMT at 4 yr (data not shown). The relationship between SNPs and the change in CIMT, after we adjusted for percentage European ancestry for an individual, did not identify any significant associations after we corrected for the number of SNPs tested (Table 2, Table 3). None of the SNPs previously reported to be associated with the absolute value of CIMT at baseline (i.e., prior to hormone treatment) (21) were here found to be associated with the change in CIMT after treatment. Furthermore, adjusting for baseline CIMT did not affect the outcomes.
Fig. 1.
Median, standard deviation, and 95% confidence intervals of changes in carotid artery intima-medial thickness from baseline (prior to randomization, n = 606), at 1 yr (n = 565), 2 yr (n = 539), 3 yr (n = 519), and 4 yr (n = 512) after randomization but independent of treatment assignment in women enrolled in the Kronos Early Estrogen Prevention Study (KEEPS).
Table 2.
20 SNPs with the smallest P values of longitudinal association with changes in CIMT at 4 yr of treatment in women enrolled in KEEPS
| SNP | Gene | Chr. | Position, bp | Common Allele | Minor Allele | MAF | SNP Call Rate | Estimated Effect on Change in CIMT, difference in 1 minor allele | SE of Estimate | P Value |
|---|---|---|---|---|---|---|---|---|---|---|
| rs6884061 | TNFAIP8 | 5 | 118711330 | G | A | 0.204 | 1.000 | 1.184E-02 | 3.177E-03 | 2.164E-04 |
| rs12848910 | CYBB | 23 | 37551189 | A | G | 0.065 | 0.990 | −1.992E-02 | 5.432E-03 | 2.711E-04 |
| rs4896243 | LOC100131120//IFNGR1 | 6 | 137556483 | A | G | 0.447 | 1.000 | −9.243E-03 | 2.545E-03 | 3.098E-04 |
| rs1860545 | TNFRSF1A//SCNN1A//PLEKHG6 | 12 | 6317038 | G | A | 0.352 | 0.979 | −1.003E-02 | 2.834E-03 | 4.402E-04 |
| rs4850994 | IL1R2 | 2 | 102020660 | G | A | 0.139 | 0.995 | −1.234E-02 | 3.578E-03 | 6.088E-04 |
| rs11954573 | F2R | 5 | 76070823 | G | A | 0.288 | 0.985 | 9.789E-03 | 2.894E-03 | 7.743E-04 |
| rs17027013 | IMMT | 2 | 86263580 | T | A | 0.458 | 0.998 | −8.620E-03 | 2.587E-03 | 9.270E-04 |
| rs2341746 | COLEC12 | 18 | 495472 | A | C | 0.232 | 0.998 | 1.019E-02 | 3.063E-03 | 9.465E-04 |
| rs17037397 | MTHFR//CLCN6 | 1 | 11784750 | C | A | 0.045 | 0.998 | 1.985E-02 | 5.969E-03 | 9.474E-04 |
| rs2274976 | MTHFR//C1orf167 | 1 | 11773514 | G | A | 0.046 | 0.995 | 1.964E-02 | 5.915E-03 | 9.623E-04 |
| rs1027702 | DUSP12 | 1 | 159979481 | G | A | 0.409 | 1.000 | 9.138E-03 | 2.768E-03 | 1.031E-03 |
| rs264846 | DOCK2 | 5 | 169059316 | A | T | 0.369 | 0.998 | 9.133E-03 | 2.768E-03 | 1.039E-03 |
| rs6707029 | IMMT | 2 | 86253595 | A | G | 0.459 | 0.990 | −8.448E-03 | 2.587E-03 | 1.167E-03 |
| rs1801131 | MTHFR//C1orf167 | 1 | 11777063 | A | C | 0.296 | 0.998 | 9.029E-03 | 2.781E-03 | 1.245E-03 |
| rs12649582 | ANXA5 | 4 | 122832341 | A | G | 0.482 | 0.998 | 8.473E-03 | 2.618E-03 | 1.290E-03 |
| rs2296135 | IL15RA | 10 | 6034700 | C | A | 0.481 | 1.000 | 8.092E-03 | 2.516E-03 | 1.385E-03 |
| rs2153875 | ITGB1 | 10 | 33230573 | A | C | 0.289 | 1.000 | −9.190E-03 | 2.866E-03 | 1.429E-03 |
| rs4951771 | KIAA1522//YARS | 1 | 33005810 | A | G | 0.311 | 0.998 | −8.893E-03 | 2.813E-03 | 1.667E-03 |
| rs1360151 | C8A | 1 | 57136629 | G | A | 0.141 | 1.000 | 1.154E-02 | 3.683E-03 | 1.831E-03 |
| rs2871444 | IL1R2 | 2 | 101979282 | A | G | 0.315 | 0.997 | −9.049E-03 | 2.888E-03 | 1.832E-03 |
SNP, single nucleotide polymorphism; CIMT, carotid intima-medial thickness; MAF, mean allele frequency; Chr., chromosome.
Table 3.
P values from Pathway analysis of SNPs in 4 pathways for association with CIMT or CAC for direct genetic or pharmacogenetic effects
| Phenotype | Type of Effect | Anticoagulant | Fibrinolysis | Innate Immunity | Procoagulant |
|---|---|---|---|---|---|
| CIMT | genetic only | 0.381 | 0.849 | 0.316 | 0.051 |
| CIMT | pharmacogenetic | 0.299 | 0.220 | <0.001 | 0.062 |
| CAC >5 | genetic only | 0.015 | 0.808 | 0.251 | 0.516 |
| CAC >5 | pharmacogenetic | 0.446 | 0.835 | 0.303 | 0.941 |
CAC, coronary artery calcification.
Pharmacogenomic effect of SNPs on the relationship of treatment and changes in CIMT at 4 yr.
Of women with DNA for analysis, 403 completed the study in their assigned treatment group. Based on the P value cutoff (see methods), there were no statistically significant signals for any particular SNP with a pharmacogenomic effect (data not shown); however, SNPs in the innate immunity pathway had an overall pharmacogenomic effect on 4 yr change in CIMT (interaction of SNPs in the genetic pathway and treatment) in these women (P < 0.001, Table 3). We also observed that the SNPs effects on the 4 yr change in CIMT varied by treatments (i.e., presence of common or rare allele within transdermal 17β-estradiol or conjugated equine estrogens groups) compared with placebo (Table 4, Fig. 2).
Table 4.
20 SNPs in the Innate Immune Pathway with the smallest P values of pharmacogenomic association (interaction of SNP and treatment with changes in CIMT at 4 yr of treatment in women enrolled in KEEPS who completed the study in their assigned treatment group
| Estimate (SE) for Treatment vs.
Placebo (given genotype) Adjusted for Ethnicity |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SNP | Chr., bp | Common/Minor Allele | MAF | Gene | E2/CC | E2/CR | E2/RR | CCE/CC | CCE/CR | CCE/RR | P Value |
| rs11466536 | 3/30710160 | G/A | 0.060 | TGFBR2 | −0.002(0.005) | 0.012(0.013) | 0.026(0.026) | 0.006(0.005) | −0.042(0.014) | −0.090(0.029) | 1.59E-04 |
| rs1569723 | 20/44175471 | A/C | 0.249 | CD40 | −0.005(0.006) | 0.006(0.006) | 0.016(0.013) | 0.012(0.006) | −0.013(0.007) | −0.039(0.014) | 1.81E-04 |
| rs261060 | 5/169258640 | G/A | 0.143 | DOCK2* | 0.0104(0.006) | −0.031(0.009) | −0.072(0.018) | 0.004(0.006) | −0.019(0.008) | −0.042(0.017) | 2.26E-04 |
| rs776514 | 3/10250475 | G/A | 0.431 | IRAK2 | 0.019(0.008) | −0.002(0.005) | −0.022(0.009) | 0.024(0.008) | −0.002(0.005) | −0.027(0.009) | 2.47E-04 |
| rs7768807 | 6/353246 | A/G | 0.267 | IRF4 | 0.0103(0.007) | −0.008(0.006) | −0.025(0.012) | −0.009(0.007) | 0.009(0.006) | 0.028(0.013) | 2.66E-04 |
| rs4073829 | 16/80527689 | G/C | 0.359 | PLCG2 | 0.007(0.007) | −0.001(0.005) | −0.008(0.011) | 0.022(0.007) | −0.008(0.005) | −0.037(0.011) | 3.11E-04 |
| rs138981 | 22/41927759 | G/A | 0.151 | SCUBE1 | −0.003(0.006) | 0.008(0.007) | 0.018(0.015) | −0.011(0.006) | 0.030(0.009) | 0.071(0.018) | 3.80E-04 |
| rs4791035 | 17/62237690 | G/C | 0.457 | PRKCA | −0.0003(0.008) | 0.001(0.005) | 0.002(0.009) | 0.023(0.008) | −0.001(0.005) | −0.026(0.009) | 4.19E-04 |
| rs261054 | 5/169261062 | G/A | 0.137 | DOCK2* | 0.009(0.006) | −0.031(0.009) | −0.071(0.019) | 0.004(0.006) | −0.019(0.009) | −0.043(0.017) | 4.44E-04 |
| rs9378805 | 6/362727 | A/C | 0.443 | IRF4 | −0.003(0.008) | 0.0002(0.006) | 0.003(0.009) | 0.021(0.008) | −0.002(0.005) | −0.026(0.009) | 5.49E-04 |
| rs261072 | 5/169248202 | A/G | 0.089 | DOCK2* | 0.009(0.005) | −0.038(0.011) | −0.085(0.023) | 0.003(0.005) | −0.017(0.011) | −0.035(0.022) | 6.12E-04 |
| rs8056564 | 16/80537520 | A/G | 0.489 | PLCG2 | 0.007(0.008) | 0.0005(0.005) | −0.006(0.008) | 0.025(0.008) | −0.0002(0.005) | −0.025(0.008) | 7.88E-04 |
| rs2243191 | 1/205082580 | G/A | 0.239 | IL19 | 0.0122(0.006) | −0.012(0.007) | −0.037(0.013) | 0.013(0.006) | −0.015(0.007) | −0.042(0.014) | 7.91E-04 |
| rs3774934 | 4/103646506 | G/A | 0.089 | NFKB1 | 0.004(0.005) | −0.014(0.011) | −0.032(0.022) | 0.007(0.005) | −0.042(0.012) | −0.091(0.024) | 8.05E-04 |
| rs12598402 | 16/80526349 | A/G | 0.442 | PLCG2 | 0.003(0.008) | 0.001(0.005) | −0.002(0.009) | 0.022(0.008) | −0.002(0.005) | −0.027(0.009) | 8.18E-04 |
| rs8056122 | 16/31335179 | A/G | 0.413 | ITGAD | 0.006(0.008) | −0.001(0.005) | −0.008(0.009) | −0.015(0.007) | 0.004(0.005) | 0.026(0.010) | 8.42E-04 |
| rs261071 | 5/169249624 | G/A | 0.130 | DOCK2* | 0.010(0.006) | −0.029(0.009) | −0.069(0.019) | 0.003(0.006) | −0.013(0.009) | −0.029(0.017) | 9.14E-04 |
| rs7736549 | 5/79415294 | C/A | 0.149 | THBS4† | −0.005(0.006) | 0.016(0.008) | 0.036(0.017) | 0.005(0.006) | −0.014(0.008) | −0.032(0.017) | 9.16E-04 |
| rs518162 | 11100505711/ | G/A | 0.104 | PGR | 0.009(0.005) | −0.033(0.011) | −0.074(0.023) | 0.001(0.005) | 0.001(0.0104 | 0.0011(0.021) | 9.36E-04 |
| rs264827 | 5/169054785 | A/G | 0.332 | DOCK2 | 0.019(0.007) | −0.009(0.006) | −0.037(0.011) | 0.006(0.007) | −0.003(0.005) | −0.013(0.011) | 9.63E-04 |
The SNP call rate for the SNPs was >0.98.
Next to LOC100131897;
next to LOC100129870.
E2, transdermal 17β-estradiol; CEE, oral conjugated equine estrogens.
Fig. 2.
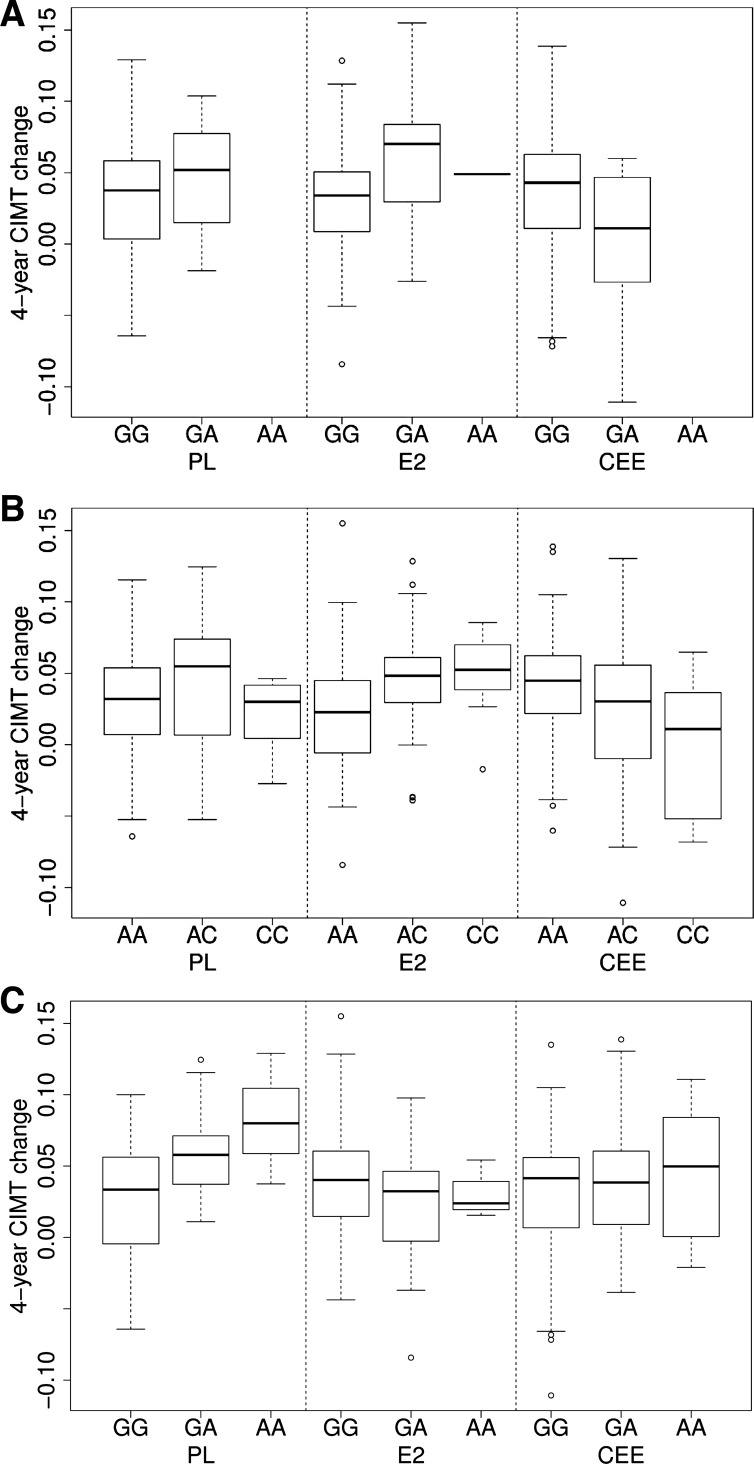
Depiction of single nucleotide polymorphisms (SNPs) on 3 genes with the lowest P values of pharmacogenomic association (interaction of SNP and treatment).with respect to change in carotid artery intima-medial thickness (CIMT) from baseline to 4 yr in women who completed the study in each treatment randomized treatment assignment or placebo (n = 160) transdermal 17β-estradiol (E2, n = 119) or oral conjugated equine estrogen (CEE, n = 123). Data are shown as median. The box is the 25th and 75th percentile range; vertical lines represent the 1.5 interquartile range (IQR); points outside the IQR are plotted as is (outliers). Top: r11466536 for gene TGFBR2 (transforming growth factor beta receptor 2). The complex phosphorylates proteins and acts as a transcription factor regulating cell proliferation. Middle: r1569723 for gene CD40, a protein of the tumor necrotic factor superfamily of receptors involved in triggering immunological activation. Bottom: r261060 for gene DOCK2, dictator of cytokinesis 2, encodes a protein involved with small G protein-coupled intracellular signaling. Although these individual SNPs within the innate immunity pathway did not reach statistical significance, the overall pathway analysis that represents the collective analysis of all of the genes/SNPS included in this study did reach statistical significance.
CAC
Independent of treatment.
Change in CAC between baseline and 4 yr of treatment was dichotomized as change >5 AU (set by the sensitivity of the measurement) due to the large number of women with a CAC score of zero at baseline and after 4 yr of treatment. Baseline clinical parameters showing significant association with the change in CAC were baseline fasting blood glucose, triglycerides, and diastolic blood pressure (Table 5). However, after we corrected for all of the generally accepted risk factors, only baseline CAC appeared to be associated with change in CAC > 5 AU (P < 0.001) and was included as an adjustment factor in subsequent genetic analyses.
Table 5.
Clinical variables prior to randomization (baseline) and change in CAC >5 AU after 4 yr of randomization in women of KEEPS
| Clinical Parameter at Baseline | n | Odds Ratio | P Value |
|---|---|---|---|
| Age | 495 | 1.016 | 0.76 |
| Months past menopause | 495 | 1.063 | 0.283 |
| Body mass index | 495 | 1.011 | 0.74 |
| Systolic blood pressure | 495 | 1.015 | 0.11 |
| Diastolic blood pressure | 495 | 1.038 | 0.01 |
| Pulse pressure | 495 | 1.001 | 0.91 |
| Fasting blood glucose | 495 | 1.029 | 0.03 |
| Insulin | 495 | 1.006 | 0.71 |
| Total cholesterol | 495 | 1.002 | 0.54 |
| High-density lipoprotein cholesterol | 495 | 0.990 | 0.29 |
| Low-density lipoprotein cholesterol | 495 | 1.004 | 0.36 |
| Triglycerides | 495 | 1.005 | 0.02 |
| Interleukin-6 | 495 | 0.982 | 0.29 |
| High sensitivity C-reactive protein | 494 | 0.927 | 0.20 |
| European ancestry | 495 | 0.681 | 0.45 |
| Baseline CAC | 495 | 1.471 | <0.001 |
AU, Agatston units.
Using a stepwise algorithm of clinical variable effects in multivariable logistic models, we found that the “best” predictive model for change in CAC included baseline CAC > 0 AU (P < 2e-16), baseline triglycerides (P = 0.009), baseline weight (P = 0.03), and change in CIMT (4 yr baseline, P = 0.04).
The relationship between SNPs and change in CAC was modeled first by multiple logistic regression, with adjustment for percentage European ancestry and baseline CAC > 0 (data not shown). None of the SNPs associated with changes of CAC > 5 AU after 4 yr (Table 6) were among the SNPs of interest based on association with the absolute AU score for CAC prior to treatment (21).
Table 6.
20 SNPs with the smallest P values of association with change in CAC >5 AU after 4 yr of treatment in women enrolled in KEEPS
| SNP | Gene | Chr. | Position, bp | Common Allele | Minor Allele | MAF | SNP Call Rate | OR CAC >5 for 1 Minor Allele Difference | SE log OR | P Value |
|---|---|---|---|---|---|---|---|---|---|---|
| rs762484 | F3 | 1 | 94776998 | A | G | 0.242 | 0.998 | 3.169 | 0.281 | 4.05E-05 |
| rs7761846 | ESR1 | 6 | 152254201 | A | G | 0.120 | 1.000 | 3.612 | 0.353 | 2.73E-04 |
| rs854541 | PPP1R9A//PON1 | 7 | 94758416 | G | A | 0.443 | 1.000 | 0.379 | 0.273 | 3.78E-04 |
| rs3753019 | COL18A1//SLC19A1 | 21 | 45749213 | G | A | 0.295 | 1.000 | 2.478 | 0.264 | 5.93E-04 |
| rs11159198 | ESRRB | 14 | 75937134 | G | A | 0.411 | 1.000 | 0.352 | 0.304 | 6.03E-04 |
| rs7115100 | CADM1 | 11 | 114673869 | A | C | 0.114 | 0.995 | 2.732 | 0.296 | 6.77E-04 |
| rs7944529 | CADM1 | 11 | 114657017 | A | T | 0.123 | 0.998 | 2.746 | 0.298 | 7.11E-04 |
| rs17686640 | PRKCA | 17 | 62048816 | G | A | 0.061 | 1.000 | 4.105 | 0.422 | 8.10E-04 |
| rs9623806 | SCUBE1 | 22 | 42015152 | G | A | 0.141 | 0.997 | 0.187 | 0.503 | 8.60E-04 |
| rs2854946 | SERPINA5 | 14 | 94118132 | G | C | 0.230 | 1.000 | 0.325 | 0.342 | 1.00E-03 |
| rs3814415 | EDNRA | 4 | 148632039 | A | G | 0.160 | 0.998 | 2.918 | 0.326 | 1.01E-03 |
| rs2017424 | TNFRSF21 | 6 | 47376942 | G | C | 0.470 | 0.997 | 2.439 | 0.272 | 1.05E-03 |
| rs2072474 | IL1R2 | 2 | 102005641 | A | G | 0.200 | 1.000 | 2.553 | 0.290 | 1.21E-03 |
| rs3759333 | LTBR//SCNN1A | 12 | 6362208 | G | A | 0.257 | 0.998 | 2.340 | 0.263 | 1.22E-03 |
| rs6055955 | PLCB1 | 20 | 8552181 | A | G | 0.479 | 0.997 | 2.364 | 0.266 | 1.23E-03 |
| rs11567699 | IL7R | 5 | 35894768 | G | C | 0.284 | 1.000 | 2.387 | 0.271 | 1.33E-03 |
| rs11719243 | IL1RAP | 3 | 191719795 | A | G | 0.254 | 1.000 | 2.338 | 0.269 | 1.60E-03 |
| rs3194051 | IL7R | 5 | 35912031 | A | G | 0.283 | 0.998 | 2.357 | 0.272 | 1.65E-03 |
| rs4876435 | COLEC10 | 8 | 120156387 | G | A | 0.231 | 1.000 | 2.387 | 0.281 | 1.93E-03 |
| rs1885550 | SFTPD | 10 | 81702420 | G | A | 0.187 | 1.000 | 2.551 | 0.302 | 1.95E-03 |
Pharmacogenomic effect of SNPs on the relationship of treatment to changes in CAC at 4 yr.
In those women who completed the trial in their assigned treatment group or placebo over the 4 yr (n = 403), and after we adjusted for ethnicity and CAC > 0 AU, there was little evidence for a SNP by treatment interaction effect (Table 7). Genes not within the innate immunity pathway that showed nominal significance for a pharmacogenomics effect of treatment on CAC were SERAPINE 2 and beta adrenergic receptor 2 (ADRB2), respectively (Table 7).
Table 7.
20 SNPs with smallest P values of pharmacogenetic association (interaction of SNP and treatment) with change in CAC (change in CAC >5 AU) after 4 yr of treatment in women enrolled in KEEPS who completed the study in their treatment group
| OR (95% CI) for Treatment
vs. Placebo (given genotype) Adjusted for Ethnicity and
Baseline CAC >0 AU |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SNP | Gene | Chr., bp | C/M allele | MAF | E2/CC | E2/CR | E2/RR | CCE/CC | CCE/CR | CCE/RR | P Value |
| rs3802857 | CADM1 | 11/114583828 | C/G | 0.357 | 0.105(0.021,0.513) | 2.01(0.703,5.77) | 38.7(4.73,317) | 0.19(0.038,0.947) | 1.18(0.383,3.61) | 7.27(0.683,77.3) | 2.42E-04 |
| rs2250889 | MMP9* | 20/44075813 | G/C | 0.080 | 0.461(0.156,1.36) | NA | NA | 0.573(0.208,1.58) | NA | NA | 2.87E-04 |
| rs669607 | C3orf68 | 3/28046448 | A/C | 0.439 | 3.21(0.768,13.4) | 0.757(0.283,2.03) | 0.178(0.026,1.24) | 0.026(0.002,0.447) | 0.272(0.072,1.04) | 2.84(0.59,13.7) | 3.54E-04 |
| rs10738763 | TEK | 9/82710576 | A/G | 0.226 | 0.391(0.123,1.25) | 6.44(1.15,36.2) | 106(2.99,3770) | 0.131(0.029,0.596) | 3.82(0.702,20.8) | 111(3.67,3370) | 3.57E-04 |
| rs615375 | TEK | 9/27102311 | A/C | 0.269 | 0.337(0.099,1.14) | 7.86(1.31,47.3) | 184(4.26,7910) | 0.129(0.027,0.622) | 4.56(0.773,26.9) | 161(4.27,6040) | 4.16E-04 |
| rs8083599 | COLEC12 | 18/362837 | C/A | 0.291 | 3.84(1.01,14.6) | 0.456(0.142,1.46) | 0.054(0.005,0.547) | 3.94(0.96,16.2) | 0.224(0.062,0.819) | 0.013(0.001,0.195) | 4.22E-04 |
| rs343321 | PLSCR1 | 3/147716959 | G/A | 0.125 | 0.802(0.287,2.24) | 6.07(0.527,70) | 46(0.327,6470) | 0.191(0.052,0.708) | 19.7(1.71,226) | 2020(12.7,321000) | 6.36E-04 |
| rs11583394 | IL19 | 1/205035516 | A/G | 0.225 | 1.02(0.317,3.25) | 1.16(0.309,4.35) | 1.32(0.0844,20.7) | 1.88(0.593,5.93) | NA | NA | 6.75E-04 |
| rs9276976 | HLA-DOA | 6/33081772 | G/A | 0.148 | 0.274(0.08,0.944) | 10.7(1.97,58.2) | 417(11.4,15200) | 0.413(0.128,1.34) | 2.57(0.36,18.4) | 16(0.275,927) | 6.83E-04 |
| rs1983357 | LOC730057 | 3/64969335 | A/C | 0.223 | 2.24(0.66,7.62) | 0.39(0.099,1.53) | 0.068(0.004,1.15) | 2.6(0.755,8.92) | 0.043(0.004,0.422) | 7.0e-4(6.0e-6,0.082) | 7.84E-04 |
| rs2292483 | TRAF5 | 1/209599650 | A/G | 0.265 | 0.243(0.068,0.87) | 2.95(0.873,9.96) | 35.8(3.07,418) | 0.621(0.189,2.04) | 0.702(0.151,3.25) | 0.792(0.035,18) | 8.31E-04 |
| rs9323910 | SERPINA3† | 14/94158379 | G/C | 0.247 | 4.75(1.35,16.8) | 0.268(0.078,0.917) | 0.015(0.001,0.206) | 1.61(0.394,6.57) | 0.272(0.068,1.08) | 0.046(0.002,0.933) | 8.61E-04 |
| rs4252287 | IL10RA | 11/117373848 | G/A | 0.095 | 1.83(0.672,4.97) | NA | NA | 1.09(0.377,3.17) | NA | NA | 8.63E-04 |
| rs12654778 | ADRB2 | 5/148185934 | G/A | 0.374 | 0.257(0.051,1.29) | 1.15(0.427,3.09) | 5.12(0.84,31.4) | 1.5(0.404,5.58) | 0.307(0.077,1.23) | 0.063(0.004,1.13) | 9.19E-04 |
| rs3794660 | IRF8 | 16/84500669 | C/G | 0.034 | 0.972(0.387,2.45) | 1.42(0,Inf) | NA | 0.35(0.112,1.09) | NA | NA | 1.03E-03 |
| rs10406069 | CD22‡// | 19/40528370 | G/A | 0.157 | 0.507(0.177,1.45) | NA | NA | 0.344(0.104,1.14) | NA | NA | 1.09E-03 |
| rs10191694 | SERPINE22§ | 2/224565510 | C/A | 0.380 | 2.08(0.544,7.92) | 0.805(0.299,2.17) | 0.312(0.046,2.11) | 3.52(0.91,13.6) | 0.083(0.009,0.736) | 0.002(2.2e-05,0.174) | 1.13E-03 |
| rs1042713 | ADRB2 | 5/148186633 | G/A | 0.399 | 0.238(0.048,1.19) | 1.13(0.422,3.03) | 5.36(0.93,30.8) | 1.3(0.351,4.78) | 0.312(0.078,1.25) | 0.075(0.004,1.33) | 1.15E-03 |
| rs13068939 | ITPR1 | 3/4563300 | G/A | 0.257 | 1.42(0.42,4.79) | 0.716(0.199,2.58) | 0.361(0.024,5.51) | 0.115(0.022,0.601) | 1.44(0.467,4.42) | 18(1.85,175) | 1.15E-03 |
| rs894685 | C1QL1 | 17/40410565 | A/G | 0.301 | 0.369(0.101,1.34) | 1.8(0.578,5.61) | 8.8(0.992,78) | 1.35(0.424,4.28) | 0.288(0.052,1.61) | 0.061(0.002,2.03) | 1.18E-03 |
SNP call rate >0.99 for all SNPS.
Next to LOC100128028;
next to LOC390503;
next to FFAR1;
next to LOC100129171.
C/M, common/minor; NA, no results due to small sample size.
DISCUSSION
Changes in CIMT and CAC, the main outcomes of KEEPS, were not significantly altered by either oCEE or tE2 compared with placebo over the 4 yr of the study (14). In the present study, a targeted candidate gene analysis provides insight into processes contributing to these main outcomes by identifying significant gene-by-treatment interactions in the progression of CIMT but not CAC.
CIMT increases with age and time past menopause (38). The absence of association of increases in CIMT with conventional risk factors most likely reflects the narrow range for most of these variables (Table 1) and, in particular, mean systolic blood pressure did not differ significantly from baseline to year 4 and was within what would be considered clinically a “normative” range. Variability in progression of CIMT reflects in part natural aging processes reflected by chronological and menopausal age, treatment assignment, and gene-treatment interactions.
Genes of the innate immunity pathway associated with the absolute value of CIMT at baseline prior to randomization to treatment (26). Using pathway analysis, we found the innate immunity pathway associated with pharmacogenomic effects of the hormone treatments on changes in CIMT. Although individual SNPs did not achieve statistical significance with the change in CIMT, the SNPs altered the treatment effect (Fig. 2). Depending upon the mean allele frequency in the population, the penetrance of this pharmacogenomic effect would manifest as increased variance in the phenotype (Fig. 1) and reduce the ability to differentiate among groups, a result consistent with the cumulative analysis of the KEEPS cohort (14).
Other studies have identified associations of genes related to the immunity pathway with increases in CIMT (37). However, some genome-wide association studies fail to identify genes associated with increases in CIMT, which may reflect that these studies do not perform hormone stratified analysis nor account for sex as a dichotomous variable in the analysis. (28). Apolipoprotein E 4 (ApoE4) was shown to be associated with CIMT in several studies (21), but unfortunately, this gene/SNP was not tested in the present study. Examining ApoE4 might be interesting as this gene/SNP has greater critical risk factor for Alzheimer's disease in women compared with men (29), and a sex differential of this gene/SNP in association with other phenotypes would be interesting to explore.
Although fasting blood glucose, triglycerides, and diastolic blood pressure associated with CAC in univariate analysis, these conventional cardiovascular risk factors did not remain significant after correction for multiple testing, which may reflect that the KEEPS participants were relatively healthy women with conventional cardiovascular risk factors within normative ranges. The first two variables relate to energy utilization, while diastolic blood pressure may reflect general arterial stiffening/reduced compliance in postmenopausal women (10, 27, 36) and may become more relevant with aging.
One gene not within the innate immunity pathway that showed nominal association with CAC, SERPINE2, may affect the coagulation cascade as this gene encodes a protein that inhibits thrombin and urokinase plasminogen activator type 2. In addition, the adrenergic system may affect CAC as changes in beta adrenergic receptors are associated with changes in total peripheral resistance in women after menopause and variants in the receptor are implicated in response to beta adrenergic blockers used in the treatment of hypertension (2, 20). Conventional risk factors such as hypertension, hypercholesterolemia, and Type 2 diabetes (T2D) exacerbate accumulation of calcium in the coronary arteries. In expression of complex traits and despite the weak genetic effects, certain genetic variants in metabolic or immune pathways may become more important mechanisms of disease progression. For example, in persons with T2D, CAC negatively correlated with variants in CD40 (5). Also, in women screened for KEEPS, 14% were excluded based on CAC without other risk factors (25), suggesting that in women, at least, coronary calcification may have several etiologies.
This study has several limitations. The study included a small number of mostly Caucasian women who were at low risk for cardiovascular disease. It would be interesting to observe these women over a longer time-course, even in the absence of hormone treatment, to determine whether the same or different SNPs associate with disease progression with aging. Serum levels of hormones were not consistently above that defining menopause for women randomized to treatment, and different associations might be found if serum levels of estrogen were increased further. As in the previous analysis, polymorphisms in estrogen receptors did not associate with either phenotype. Estrogen response elements reside in the promoter regions of many genes and affect gene expression, which was not measured in the present study. Influences of MHT on gene expression would be expected to differ among formulations given that the metabolites of estrogen have differing binding affinity for estrogen receptors. In the present study, it was not possible to determine estrogen receptor response elements in all of the genes of interest and thus interactions of estrogen receptor polymorphisms with other specific SNPs of interest. Additional work is needed to understand how polymorphisms in estrogen receptors contribute to development of cardiovascular disease in women.
The magnitude of the pharmacogenomics effects differed by type of MHT. It is unlikely that methodological variability in measurement of CIMT contributed to the pharmacogenomic effects as methods to obtain the ultrasound scans were standardized and performed by trained personnel and coefficient of variations between two scans obtained prior to randomization was <1%. Power to detect pharmacogenomic effects on change in CAC was limited by the small number of women with changes of CAC > 5 AU, as the approach that was used to assess significance of treatment-by-gene interactions at the pathway level (PC-gamma method with the parametric bootstrap) is expected to have low power for evaluating association with dichotomous outcomes when the number of cases is small. Finally, the study used a candidate gene approach specifically to investigate genes related to coagulation and immunity to evaluate the genetic components of MHT and thrombotic risk. Other pathways may have been discovered with a genome wide association approach.
Despite these limitations, the results of this study emphasize that, in genetic association studies of complex disease traits, it is critical to account for sex and hormonal status of the study participants. In addition, this analysis provides value to the scientific community as it is the first study to identify pharmacogenomic effects of MHT on vascular remodeling and, thus, provides insight into why there is variability in cardiovascular effects of MHT in women.
GRANTS
KEEPS is funded by grants from the Aurora Foundation to the Kronos Longevity Research Institute, the Mayo Foundation; the National Institutes of Health (NIH) HL-90639 to V. M. Miller, Mayo CTSA1 UL1 RR-024150, Brigham and Women's Hospital/Harvard Medical School CTSA UL1 RR-024139, and UCSF CTSA UL1 RR-024131 from the National Center for Advancing Translational Sciences (NCATS), a component of the NIH and NIH Roadmap for Medical Research.
The manuscript's contents are solely the responsibility of the authors and do not necessarily represent the official view of NCATS or NIH. Information on the National Center for Research Resources is available at http://www.ncrr.nih.gov. Study medications were supplied in part by Bayer Health Care and by Abbott Pharmaceuticals.
Role of the Sponsors: The Aurora Foundation, Bayer Health Care, and Abbott Pharmaceuticals did not have input into the design or conduct of the study or the review or approval of this article.
DISCLOSURES
M. J. Budoff received research grants from General Electric Company. Otherwise, no conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: V.M.M., J.A.H., J.E.M., F.N., S.M.H., and M.d.A. conception and design of research; V.M.M., G.D.J., J.M.B., H.N.H., D.M.B., and M.d.A. analyzed data; V.M.M., G.D.J., J.A.H., and M.d.A. drafted manuscript; V.M.M., G.D.J., J.M.B., J.A.H., G.S.H., H.N.H., M.J.B., R.A.L., H.S.T., J.E.M., D.M.B., F.N., S.M.H., and M.d.A. edited and revised manuscript; V.M.M., G.D.J., J.M.B., J.A.H., G.S.H., H.N.H., M.J.B., R.A.L., H.S.T., J.E.M., D.M.B., F.N., S.M.H., and M.d.A. approved final version of manuscript; G.D.J. and M.d.A. prepared figures; J.A.H., H.S.T., and M.d.A. interpreted results of experiments; G.S.H. and M.J.B. performed experiments.
ACKNOWLEDGMENTS
KEEPS would not have been possible without the dedicated volunteers participating in this study and collaborators and coworkers at each study center who include: Albert Einstein College of Medicine: Genevieve Neal-Perry, Ruth Freeman, Hussein Amin, Barbara Isaac, Maureen Magnani, Rachel Wildman; Brigham and Women's Hospital/Harvard Medical School: JoAnn Manson, Maria Bueche, Marie Gerhard-Herman, Kate Kalan, Jan Lieson, Kathryn M. Rexrode, Barbara Richmond, Frank Rybicki, Brian Walsh; Columbia College of Physicians and Surgeons: Rogerio Lobo, Luz Sanabria, Jolene Lalas, Michelle Warren; Kronos Longevity Research Institute: S. Mitchell Harman, Mary Dunn, Panayiotis D. Tsitouras, Viola Zepeda; Mayo Clinic: Philip A. Araoz, Rebecca Beck, Dalene Bott-Kitslaar, Sharon L. Mulvagh, Lynne T. Shuster, Teresa G. Zais; University of California, Los Angeles, CAC Reading Center: Matthew Budoff, Chris Dailing, Yanlin Gao, Angel Solano; University of California, San Francisco Medical Center: Marcelle I. Cedars, Nancy Jancar, Jean Perry, Rebecca S. Wong, Robyn Pearl, Judy Yee, Brett Elicker, Gretchen A. W. Gooding; UCSF Statistical Reading Center: Dennis Black, Lisa Palermo; University of Southern California, Los Angeles Atherosclerosis Research Unit, Core Imaging and Reading Center: Howard N. Hodis, Yanjie Li, Mingzhu Yan; University of Utah School of Medicine: Eliot Brinton, Paul N. Hopkins, M. Nazeem Nanjee, Kirtly Jones, Timothy Beals, Stacey Larrinaga-Shum; VA Puget Sound Health Care System and University of Washington School of Medicine: George Merriam (deceased), Pamela Asberry, SueAnn Brickle, Colleen Carney, Molly Carr, Monica Kletke, Lynna C. Smith; Yale University, School of Medicine: Hugh Taylor, Kathryn Czarkowski, Lubna Pal, Linda McDonald, Mary Jane Minkin, Diane Wall, Erin Wolff (now at NIH/NICHD); Others: Frederick Naftolin (New York University), Nanette Santoro (University of Colorado).
Institutional Review number for KEEPS Centers: The central KEEPS and Phoenix KEEPS (IRB protocol by the Western IRB): STUDY NUM: 1058663 and WIRB PRO NUM: 20040792; Brigham and Women's Hospital (Partners): #2004-P-002144 BWH; Mayo Clinic: 2241-04; Columbia: AAAA-8062; Yale: 0409027022; University of Utah: 13257; Einstein/Montefiore: 04-08-213; University of Wisconsin: H-2005-0059; UCSF: KEEPS (main study & cognitive substudy) #10-02980; University of Washington IRB #26702; VAPSHCS IRB #01048.
REFERENCES
- 1.Alessio AM, Hoehr NF, Siqueira LH, Ozelo MC, de Padua Mansur A, Annichino-Bizzacchi JM. Association between estrogen receptor alpha and beta gene polymorphisms and deep vein thrombosis. Thromb Res 120: 639–645, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Bairey Merz CN, Mark S, Boyan BD, Jacobs AK, Shah PK, Shaw LJ, Taylor D, Marban E. Proceedings from the scientific symposium: sex differences in cardiovascular disease and implications for therapies. J Womens Health (Larchmt) 19: 1059–1072, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biernacka JM, Jenkins GD, Wang L, Moyer AM, Fridley BL. Use of the gamma method for self-contained gene-set analysis of SNP data. Eur J Hum Genet 20: 565–571, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bray PF, Larson JC, Lacroix AZ, Manson J, Limacher MC, Rossouw JE, Lasser NL, Lawson WE, Stefanick ML, Langer RD, Margolis KL. Usefulness of baseline lipids and C-reactive protein in women receiving menopausal hormone therapy as predictors of treatment-related coronary events. Am J Cardiol 101: 1599–1605, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burdon KP, Langefeld CD, Beck SR, Wagenknecht LE, Carr JJ, Rich SS, Freedman BI, Herrington D, Bowden DW. Variants of the CD40 gene but not of the CD40L gene are associated with coronary artery calcification in the Diabetes Heart Study (DHS). Am Heart J 151: 706–711, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Bush TL, Barrett-Connor E. Noncontraceptive estrogen use and cardiovascular disease. Epidemiol Rev 7: 89–104, 1985. [PubMed] [Google Scholar]
- 7.Buzkova P, Lumley T, Rice K. Permutation and parametric bootstrap tests for gene-gene and gene-environment interactions. Ann Hum Genet 75: 36–45, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Canonico M, Oger E, Plu-Bureau G, Conard J, Meyer G, Levesque H, Trillot N, Barrellier MT, Wahl D, Emmerich J, Scarabin PV. Hormone therapy and venous thromboembolism among postmenopausal women. Circulation 115: 840–845, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Canonico M, Oger E, Plu-Bureau G, Conard J, Meyer G, Levesque H, Trillot N, Barrellier MT, Wahl D, Emmerich J, Scarabin PY. Estrogen and Thromboembolism Risk (ESTHER) Study Group. Hormone therapy and venous thromboembolism among postmenopausal women: impact of the route of estrogen administration and progestogens: the ESTHER study. Circulation 115: 840–845, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Cecelja M, Jiang B, Bevan L, Frost ML, Spector TD, Chowienczyk PJ. Arterial stiffening relates to arterial calcification but not to noncalcified atheroma in women. A twin study. J Am Coll Cardiol 57: 1480–1486, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao X, Starmer J, Martin ER. A multiple testing correction method for genetic association studies using correlated single nucleotide polymorphisms. Genet Epidemiol 32: 361–369, 2008. [DOI] [PubMed] [Google Scholar]
- 12.Glueck CJ, Ping W, Fontaine RN, Trent T, Sieve-Smith L, Lang JE. Effect of exogenous estrogen on atherothrombotic vascular disease risk related to the presence or absence of the Factor V Leiden mutation (resistance to activated protein C). Am J Cardiol 84: 549–554, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Goeman JJ, van de Geer SA, de Kort F, van Houwelingen HC. A global test for groups of genes: testing association with a clinical outcome. Bioinformatics 20: 93–99, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Harman SM, Black DM, Naftolin F, Brinton EA, Budoff MJ, Cedars MI, Hopkins PN, Lobo RA, Manson JEM, R G, Miller VM, Neal-Perry G, Santoro N, Taylor HS, Vittinghoff E, Yan M, Hodis HN. Arterial imaging outcomes and cardiovascular risk factors in recently menopausal women: A randomized trial. Ann Intern Med 161: 249–260, 2014. [DOI] [PubMed] [Google Scholar]
- 15.Harman SM, Brinton EA, Cedars M, Lobo R, Manson JE, Merriam GR, Miller VM, Naftolin F, Santoro N. KEEPS: The Kronos Early Estrogen Prevention Study. Climacteric 8: 3–12, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Harman SM, Vittinghoff E, Brinton EA, Budoff MJ, Cedars MI, Lobo RA, Merriam GR, Miller VM, Naftolin F, Pal L, Santoro N, Taylor HS, Black DM. Timing and duration of menopausal hormone treatment may affect cardiovascular outcomes. Am J Med 124: 199–205, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heit JA, Cunningham JM, Petterson TM, Armasu SM, Rider DN, De Andrade M. Genetic variation within the anticoagulant, procoagulant, fibrinolytic and innate immunity pathways as risk factors for venous thromboembolism. J Thromb Haemost 9: 113–1142, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hodis H, Mack W, Lobo R, Shoupe D, Sevanian A, Mahrer P, Selzer R, Liu C, Liu C, Azen S. Estrogen in the prevention of atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 135: 939–953, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Hodis HN, Mack WJ, LaBree L, Selzer RH, Liu CR, Liu CH, Azen SP. The role of carotid arterial intima-media thickness in predicting clinical coronary events. Ann Intern Med 128: 262–269, 1998. [DOI] [PubMed] [Google Scholar]
- 20.Joyner MJ, Barnes JN, Hart EC, Wallin BG, Charkoudian N. Neural control of the circulation: how sex and age differences interact in humans. Compr Physiol 5: 193–215, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Juo SH. Genetics of carotid atherosclerosis. Front Biosci 14: 4525–4534, 2009. [DOI] [PubMed] [Google Scholar]
- 22.Karim R, Hodis HN, Stanczyk FZ, Lobo RA, Mack WJ. Relationship between serum levels of sex hormones and progression of subclinical atherosclerosis in postmenopausal women. J Clin Endocrinol Metab 93: 131–138, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kunnas TA, Laippala P, Penttilä A, Lehtimäki T, Karhunen PJ. Association of polymorphism of human alpha oestrogen receptor gene with coronary artery disease in men: a necropsy study. BMJ 321: 273–274, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manson JE, Hsia J, Johnson KC, Rossouw JE, Assaf AR, Lasser NL, Trevisan M, Black HR, Heckbert SR, Detrano R, Strickland OL, Wong ND, Crouse JR, Stein E, Cushman M. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med 349: 523–534, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Miller VM, Black DM, Brinton EA, Budoff MJ, Cedars MI, Hodis HN, Lobo RA, Manson JE, Merriam GR, Naftolin F, Santoro N, Taylor HS, Harman SM. Using basic science to design a clinical trial: baseline characteristics of women enrolled in the Kronos Early Estrogen Prevention Study (KEEPS). J Cardiovasc Transl Res 2: 228–239, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller VM, Petterson TM, Jeavons EN, Lnu AS, Rider DN, Heit JA, Cunningham JM, Huggins GS, Hodis HN, Budoff MJ, Santoro N, Hopkins PN, Lobo RA, Manson JE, Naftolin F, Taylor HS, Harman SM, de Andrade M. Genetic polymorphisms associated carotid artery intima-media thickness and coronary artery calcification in women of the Kronos Early Estrogen Prevention Study. Physiol Genomics 45: 79–88, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moreau KL, Donato AJ, Seals DR, DeSouza CA, Tanaka H. Regular exercise, hormone replacement therapy and the age-related decline in carotid arterial compliance in healthy women. Cardiovasc Res 57: 861–868, 2003. [DOI] [PubMed] [Google Scholar]
- 28.O'Donnell CJ, Cupples LA, D'Agostino RB, Fox CS, Hoffmann U, Hwang SJ, Ingellson E, Liu C, Murabito JM, Polak JF, Wolf PA, Demissie S. Genome-wide association study for subclinical atherosclerosis in major arterial territories in the NHLBI's Framingham Heart Study. BMC Med Genet 8, Suppl 1: S4, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Payami H, Zareparsi S, Montee KR, Sexton GJ, Kaye JA, Bird TD, Yu CE, Wijsman EM, Heston LL, Litt M, Schellenberg GD. Gender difference in apolipoprotein E-associated risk for familial Alzheimer disease: a possible clue to the higher incidence of Alzheimer disease in women. Am J Hum Genet 58: 803–811, 1996. [PMC free article] [PubMed] [Google Scholar]
- 30.Rexrode KM, Manson JE, Lee IM, Ridker PM, Sluss PM, Cook NR, Buring JE. Sex hormone levels and risk of cardiovascular events in postmenopausal women. Circulation 108: 1688–1693, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Rossouw J, Bray P, Liu J, Kooperberg C, Hsia J, Lewis C, Cushman M, Bonds D, Hendrix S, Papanicolaou G, Howard T, Herrington D. Estrogen receptor polymorphisms and the vascular effects of hormone therapy. Arterioscler Thromb Vac Biol 31: 464–469, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seldin MF, Shigeta R, Villoslada P, Selmi C, Tuomilehto J, Silva G, Belmont JW, Klareskog L, Gregersen PK. European population substructure: clustering of northern and southern populations. PLoS Genet 2: e143, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shufelt CL, Merz CN, Prentice RL, Pettinger MB, Rossouw JE, Aroda VR, Kaunitz AM, Lakshminarayan K, Martin LW, Phillips LS, Manson JE. Hormone therapy dose, formulation, route of delivery, and risk of cardiovascular events in women: findings from the Women's Health Initiative Observational Study. Menopause 21: 260–266, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sudhir K, Chou TM, Chatterjee K, Smith EP, Williams TC, Kane JP, Malloy MJ, Korach KS, Rubanyi GM. Premature coronary artery disease associated with a disruptive mutation in the estrogen receptor gene in a man. Circulation 96: 3774–3777, 1997. [DOI] [PubMed] [Google Scholar]
- 35.Tempfer CB, Riener EK, Hefler LA, Huber JC, Muendlein A. DNA microarray-based analysis of single nucleotide polymorphisms may be useful for assessing the risks and benefits of hormone therapy. Fertil Steril 82: 132–137, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Waddell TK, Rajkumar C, Cameron JD, Jennings GL, Dart AM, Kingwell BA. Withdrawal of hormonal therapy for 4 weeks decreases arterial compliance in postmenopausal women. J Hypertens 17: 413–418, 1999. [DOI] [PubMed] [Google Scholar]
- 37.Wang L, Beecham A, Zhuo D, Dong C, Blanton SH, Rundek T, Sacco RL. Fine mapping study reveals novel candidate genes for carotid intima-media thickness in Dominican Republican families. Circ Cardiovasc Genet 5: 234–241, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wharton W, Gleason CE, Dowling NM, Carlsson CM, Brinton EA, Santoro MN, Neal-Perry G, Taylor H, Naftolin F, Lobo RA, Merriam G, Manson JE, Cedars MI, Miller VM, Black DM, Budoff M, Hodis HN, Harman SM, Asthana S. The KEEPS-Cognitive and Affective Study: baseline associations between vascular risk factors and cognition. J Alzheimers Dis 40: 331–341, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]