Abstract
Background:
We investigated the effects of Withania somnifera root (WS) on insulin resistance, tumor necrosis factor α (TNF-α), and interleukin-6 (IL-6) in fructose-fed rats.
Methods:
Forty-eight Wistar-Albino male rats were randomly divided into four groups (n=12); Group I as control, Group II as sham-treated with WS by 62.5mg/g per diet, Group III fructose-fed rats received 10%W/V fructose, and Group IV fructose- and WS-fed rats. After eight weeks blood samples were collected to measure glucose, insulin, IL-6, and TNF-α levels in sera.
Results:
Blood glucose, insulin, homeostasis model assessment for insulin resistance (HOMA-R), IL-6, and TNF-α levels were all significantly greater in the fructose-fed rats than in the controls. Treatment with WS significantly (P < 0.05) inhibited the fructose-induced increases in glucose, insulin, HOMA-R, IL-6, and TNF-α.
Conclusion:
Our data suggest that WS normalizes hyperglycemia in fructose-fed rats by reducing inflammatory markers and improving insulin sensitivity.
Key Words: Withania somnifera, Insulin resistance, IL-6, TNF- α
Introduction
Chronic fructose feeding leads to glucose intolerance, hyperinsulinemia, and loss of normal insulin sensitivity (1). Insulin resistance is defined as a state of decreased responsiveness of target tissues to normal circulating insulin levels, and plays a major role in the development of type 2 diabetes mellitus. Insulin resistance is a feature of a number of disorders, including glucose intolerance, obesity, dyslipidemia, and hypertension clustering in the so-called metabolic syndrome (2). In this regard, studies have demonstrated that insulin-stimulated glucose uptake was decreased in adipose tissue from fructose-fed rats (3). It is well known that inflammatory cytokines, such as interleukin 6 (IL‐6) and tumor necrosis factor‐α (TNF‐α), are elevated in insulin resistance (3). More studies suggested that TNF-α, IL-6, and C-reactive protein (CRP) play central roles in the development of insulin resistance (1). TNF-α, by impairing insulin receptor signaling and inhibiting lipoprotein lipase, causes insulin resistance (1-3). The level of TNF-α correlates positively with hyperinsulinemia and obesity (3, 4). Also, the level of TNF-α correlates negatively with lipoprotein lipase activity in adipose tissue (3, 4). The reduced tyrosine kinase activity of insulin receptors in animals with insulin resistance is fully reversed after treatment with a TNF‐α antagonist (5). Others studies have shown that the adipose tissue secretes pro-inflammatory cytokines such as IL-6 (6). Levels of IL-6 correlate most strongly with adiposity and type 2 diabetes and furthermore have been found to be inversely related to insulin sensitivity (6). It has been proposed that TNF‐α acts locally in adipose tissue through autocrine/paracrine mechanisms causing insulin resistance and inducing IL-6, whereas it appears to be released systemically by the adipose tissue acting as an endocrine signal that induces the hepatic acute‐phase response and insulin resistance in the liver in vitro and in vivo (7). Many oral hypoglycemic agents are available along with insulin for the treatment of hyperglycemia and diabetes, but these synthetic drugs can produce serious side effects and are not suitable for long-term use. This has led to use of natural products with fewer side effects than synthetic drugs; these are also relatively economical compared to synthetic oral hypoglycemic agents. It is assumed that herbal medicine can be effective as an alternative to oral hypoglycemic agents (8, 9). W. somnifera (WS) is an important medicinal plant that has been used in Ayurvedic and indigenous medicine. W. somnifera has anti-inflammatory, antitumor, antioxidant, immunomodulatory, and antistress effects (10). The possible protective effects of WS on TNF-α, IL-6, and insulin resistance in fructose-fed male rats have not yet been reported. Therefore, this study was designed to investigate the effect of WS on TNF-α, IL-6, and insulin resistance in fructose-fed male rats.
Materials and Methods
Animals
Forty-eight male Wistar rats (200±20g) were obtained from an animal breeding center of Zahedan University of Medical Sciences. They were given one week to adapt to their new surroundings and maintained under 12-hr light: dark cycles and a temperature of 20–24 °C. They were housed under ideal laboratory conditions, and maintained on standard pellet diets and water ad libitum throughout the study. This study was approved by the Animal Ethics Committee of the Medical University of Zahedan according to the National Health and Medical Research Council guidelines.
Experimental design
The study period lasted eight weeks. The rats (n=48) were divided into four groups comprised of twelve animals each as follows:
• Group I: control rats, received standard pellet diet and water
• Group II: WS-fed rats, received 62.5 mg/g WS powder with standard pellet diet and water
• Group III: Fructose-fed rats, received 10% W/V fructose dissolved in water with standard pellet diet
• Group IV: WS + fructose-fed rats, received WS and fructose as above with standard pellet diet
Induction of insulin resistance
To induce insulin resistance, fructose (10% W/V) was dissolved in water ad libitum and given until the end of the 8-week study period to rats in Groups III and IV (11).
Withania somnifera
W. somnifera powder was mixed with standard rat chow at a weight ratio of 6.25% and administered daily to the rats in Groups II and IV for eight weeks. On the last day of the study, blood samples were collected by nicking the tips of their tails for biochemical estimations (12).
Blood sampling
Blood samples were drawn in the morning after an overnight fast into glass tubes and allowed to clot for 20 minutes at room temperature. The samples were then pelleted at 3000 rpm for 10 minutes in a bench top centrifuge and serum was collected and stored at -70 °C until analyses were performed.
Determination of blood glucose
Blood glucose was determined using a commercial kit (Olympus AU-600, Tokyo, Japan).
Determination of Insulin, TNF-α, and IL-6 levels
Enzyme-linked immunosorbent assay (ELISA) kits (DRG, USA) were used to determine plasma levels for insulin, TNF-α, and IL-6.
Determination of insulin resistance
Homeostasis model assessment of insulin resistance (HOMA-R) was calculated using fasting blood glucose (FBG) and fasting insulin (FI) levels (Answer et al. 2013). The insulin sensitivity was calculated using the following formula: HOMA-R = FI (μU/ml) × FBG (mg/dl)/405.
Statistical analysis
Data were given as the mean ± SD. For a statistical analysis of the data, group means were compared by one-way ANOVA followed by Tukey’s post-hoc test. P < 0.05 was considered to be statistically significant.
Results
Effect of WS on hyperglycemia in fructose-fed rats
Table 1 shows the effect of WS on blood glucose. Blood glucose was significantly higher in fructose-fed rats than in controls. Blood glucose was significantly lower in WS + fructose-fed rats than in fructose-only-fed rats. No significant difference in glucose levels was observed between WS-treated and control rats.
Table 1.
Blood glucose concentrations in control and experimental groups
| Group | Treatment |
Blood glucose (mg/dl)
mean ± SD |
|---|---|---|
| I | Control | 112±7.23* |
| II | WS-fed rats | 110±6.85* |
| III | Fructose-fed rats | 133±9.31 |
Effect of WS on hyperglycemia in fructose-fed rats
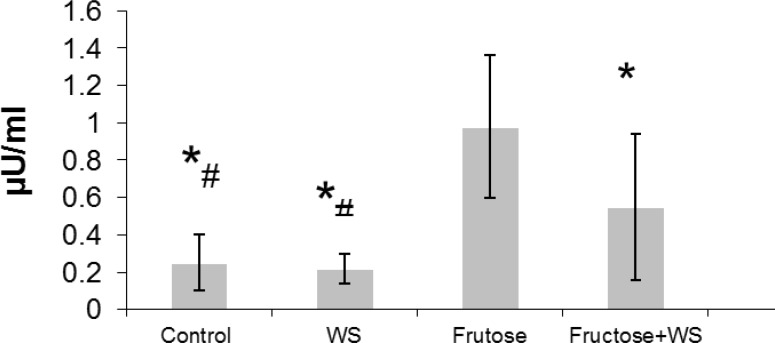
Fig. 1 shows the effect of WS on insulin level. Hyperinsulinemia was observed in fructose-fed rats when compared to control rats. WS treatment significantly (P < 0.001) reduces the elevated levels of insulin when compared to fructose-fed rats. The level of insulin in control, WS-fed rats and WS + fructose-fed rats were significantly (P < 0.001) lower than that of Fructose-fed rats. Also, the level of insulin in control and WS-fed rats were significantly (P < 0.001) lower than that of WS + fructose-fed rats.
Insulin concentrations in control and experimental groups. The data are expressed as mean ± S.D. (n = 12). *P < 0.001 as compared to fructose-fed rats. #P < 0.01 as compared to fructose-fed +WS rats
Effect of WS on insulin level in fructose-fed rats
Fig. 1 shows the effect of WS on insulin level. Hyperinsulinemia was observed in fructose-fed rats when compared to control rats. WS treatment significantly (P < 0.001) reduces the elevated levels of insulin when compared to fructose-fed rats. The level of insulin in control, WS-fed rats and WS + fructose-fed rats were significantly (P < 0.001) lower than that of Fructose-fed rats. Also, the level of insulin in control and WS-fed rats were significantly (P < 0.001) lower than that of WS + fructose-fed rats.
Effect of WS on insulin sensitivity in fructose-fed rats
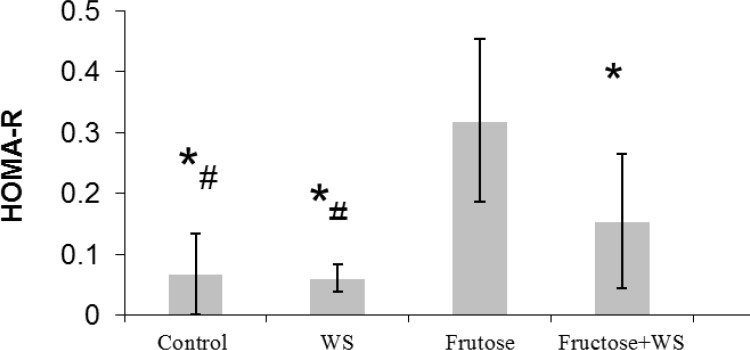
Fig. 2 shows the level of HOMA-R, an index of hepatic insulin resistance. Fructose-fed rats showed significant increase in HOMA-R level when compared to control rats. Treatment with WS significantly (P < 0.001) prevented increase in HOMA-R levels in fructose-fed rats when compared to control rats. The level of HOMA-R in control, WS-fed rats and WS + fructose-fed rats were significantly (P < 0.001) lower than that of Fructose-fed rats. Also, the level of HOMA-R in control and WS-fed rats were significantly (P < 0.001) lower than that of WS + fructose-fed rats.
Fig. 2.
HOMA-R levels in control and experimental groups. The data are expressed as mean ± S.E. (n = 12). *P < 0.001 as compared to fructose-fed rats. #P < 0.01 as compared to fructose-fed +WS rats
Effect of WS on IL-6 and TNF-α in fructose-fed rats
Table 2 illustrates the concentrations of TNF-α and IL-6. Fructose-fed rats produced significantly greater concentrations of TNF-α and IL-6 than control rats. Fructose + WS-fed rats produced significantly lower concentrations of them than fructose-only fed rats. No significant differences were observed in their level between WS-only-fed and control rats.
Table 2.
Serum concentrations of TNF-α and IL-6 in control and experimental
| Group | Treatment |
TNF-α (ng/L)
mean ± SD |
IL-6 (ng/L)
mean ± SD |
|---|---|---|---|
| I | Control | 19.29±2.76* | 79.50±14.22* |
| II | WS-fed rats | 20.86±1.23* | 75.62 ±13.02* |
| III | Fructose-fed rats | 25.14±2.93 | 92.37±10.36 |
| IV | WS+Fructose-fed rats | 21.37±1.44* | 72.25±11.29* |
The data are expressed in mean ± S.D.; n = 12 in each group.
P < 0.001 as compared to fructose-fed rats.
Discussion
Insulin resistance is a condition in which insulin is unable to produce its responses, such as decreasing gluconeogenesis and increasing glycogen synthesis. The fructose-fed rat is an animal model of insulin resistance that is associated with impaired glucose tolerance, hyperinsulinemia, hypertension, and hyperlipidemia (13). In the present study, fructose-fed rats had significantly greater blood glucose concentrations than control rats. Blood glucose was significantly less in WS + fructose-fed rats than in fructose-alone-fed rats, thereby showing its anti-hyperglycemic activity. The results of our study agree with other studies, hence showing that WS can decrease blood glucose (9). Hyperinsulinemia appears to be a compensatory mechanism that responds to increased levels of circulating glucose and is often associated with the progression to insulin resistance. Researchers have reported that rats fed high fructose diets develop significant elevations of basal serum glucose and insulin (14, 15). The present study demonstrated that insulin concentrations were greater in the fructose-fed than in the control rats. HOMA-R was measured to analyze insulin sensitivity. HOMA-R is a useful clinical index of hepatic insulin resistance. HOMA-R was significantly less in the fructose + WS-fed rats than in the fructose-only-fed rats. This result suggests that WS improves insulin sensitivity and agrees with a report from Answer, et al (2008), which also reported that WS lowered insulin and glucose in diabetes mellitus type II rats (9). A high fructose intake causes an increase in body weight due to the positive energy balance, which leads to obesity (16). Obesity represents an expansion of adipose tissue mass, and one explanation for obesity-related insulin resistance is the production of certain factors, including TNF-α and IL-6, by adipose tissue (17). Many studies showed that pro-inflammatory cytokines impair insulin sensitivity and glucose regulation in insulin resistance and diabetes (18). Klover and coworkers have reported that acute and chronic exposure to IL-6 inhibits insulin action in vivo (18). Tumor necrosis factor-α expression correlated positively with the degree of obesity and level of hyperinsulinemia and negatively with adipose tissue lipoprotein lipase activity (19, 20). The results of the present study indicate that increased dietary fructose leads to increases in TNF-α and IL-6 concentrations. Also, WS reduced TNF-α and Il-6 in fructose-fed rats. NF-κB, an important mediator of insulin resistance, activates TNF-α and IL-6 expression (21). Oh and Kwon (2009) showed that witaferin purified from WS inhibits NF-κB activity (22). Also, Anwer and coworkers showed that WS increased insulin sensitivity and has hypoglycemic and antidiabetic effects (23).
In the present study, fructose-fed rats had significantly greater blood glucose levels than control rats. Addition of WS to the fructose-fed rats reduced the elevated blood glucose levels to nearly those of the controls, thereby showing its anti-hyperglycemic activity. The results of our study agree with others showing that WS can reduce blood glucose, insulin, and the inflammatory factors TNF-α and IL-6. Therefore, WS might be useful in reducing the complications of various types of tissue damage seen in diabetes. Natural medicine therapy is an important treatment strategy for slowing diabetes progression and preventing diabetic complications such as hyperglycemia, inflammation, and insulin resistance (24). Although the mechanisms WS and much natural medicines cannot be fully explained by our results, several studies have explained some mechanisms of natural medicines and antioxidants. Natural medicine and WS antioxidant contents may directly eliminate free radicals such as lipid peroxyl, peroxyl and/or alkoxyl radicals in vitro and in vivo. Also, natural medicine and WS antioxidant contents are involved in inhibition of inflammation processes and increased insulin resistance. Therefore, WS as a natural medicine could be proposed as a supplement for diabetics care to prevent diabetic inflammation and insulin resistance.
This study showed that WS decreased the elevated serum glucose, TNF-α, IL-6, and insulin in fructose-fed rats. The inhibition of insulin resistance and reductions of glucose, TNF-α, and IL-6 may decrease diabetic complications in patients. Our data suggest that administration of WS may decrease hyperglycemia, inflammation, and insulin resistance; therefore, WS may be suitable supplement for the prevention of diabetic complications.
Acknowledgments
The authors thank the Research Deputy of Zahedan Medical University for grant supporting of this study.
References
- 1.Yeh TC, Liu CP, Cheng WH, Chen BR, Lu PJ, Cheng PW, Ho WY, Sun GC, Liou JC, Tseng CJ. Caffeine intake improves fructose-induced hypertension and insulin resistance by enhancing central insulin signaling. Hypertens. 2014;63(3):535–41. doi: 10.1161/HYPERTENSIONAHA.113.02272. [DOI] [PubMed] [Google Scholar]
- 2.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112(12):1821–30. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu L-Y, Juan C-C, Hwang L, Hsu Y-P, Ho P-H, Ho L-T. Green tea supplementation ameliorates insulin resistance and increases glucose transporter. Eur J Nutr. 2004;43(2):116–24. doi: 10.1007/s00394-004-0450-x. [DOI] [PubMed] [Google Scholar]
- 4.Bal Y Adas M, Helvaci A. Evaluation of the relationship between insulin resistance and plasma tumor necrosis factor-alpha, interleukin-6 and C reactive protein levels in obese women. Bratisl Lek Listy. 2010;111(4):200–4. [PubMed] [Google Scholar]
- 5.Kroder G, Bossenmaier B, Kellerer M, Capp E, Stoyanov B, Mühlhöfer A, Berti L, Horikoshi H, Ullrich A, Häring H. J Clin Invest. Tumor necrosis factor-alpha-and hyperglycemia-induced insulin resistance Evidence for different mechanisms and different effects on insulin signaling. J Clin Invest;97(6):1471–7. doi: 10.1172/JCI118569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freitas P, Carvalho D, Santos AC, Madureira AJ, Martinez E, Pereira J, Sarmento A, Medina JL. Adipokines, hormones related to body composition, and insulin resistance in HIV fat redistribution syndrome. BMC Infect Dis. 2014;14 doi: 10.1186/1471-2334-14-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iacobellis G. Epicardial adipose tissue in endocrine and metabolic diseases. Endocrine. 2014;46(1):8–15. doi: 10.1007/s12020-013-0099-4. [DOI] [PubMed] [Google Scholar]
- 8.Ahmadvand , Tavafi , Khosrowbeygi Amelioration of altered antioxidant enzymes activity and glomerulosclerosis by coenzyme Q10 in alloxan-induced diabetic rats. J Diabetes Complications. 2012;26(6):476–82. doi: 10.1016/j.jdiacomp.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Anwer T, Sharma M, Pillai KK, Iqbal M. Effect of Withania somnifera on Insulin Sensitivity in Non‐Insulin‐Dependent Diabetes Mellitus Rats. Basic Clin Pharmacol Toxicol. 2008;102(6):498–503. doi: 10.1111/j.1742-7843.2008.00223.x. [DOI] [PubMed] [Google Scholar]
- 10.Gupta GL, Rana A. Withania somnifera (Ashwagandha): a review. Pharmacogn Rev. 2013;1(1) [Google Scholar]
- 11.Sánchez-Lozada LG1, Tapia E, Jiménez A, Bautista P, Cristóbal M, Nepomuceno T, Soto V, Avila-Casado C, Nakagawa T, Johnson RJ, Herrera-Acosta J, Franco M. Fructose-induced metabolic syndrome is associated with glomerular hypertension and renal microvascular damage in rats. Am J Physiol Renal Physiol. 2007;292(1):F423–9. doi: 10.1152/ajprenal.00124.2006. [DOI] [PubMed] [Google Scholar]
- 12.Rashidi AA, Mirhashemi SM, Taghizadeh M, Sarkhail P. Iranian medicinal plants for diabetes mellitus: a systematic review. Pak J Biol Sci. 2013;16(9):401–11. doi: 10.3923/pjbs.2013.401.411. [DOI] [PubMed] [Google Scholar]
- 13.Tran LT, Yuen VG, McNeill JH. The fructose-fed rat: a review on the mechanisms of fructose-induced insulin resistance and hypertension. Mol Cell Biochem. 2009;332(1-2):145–59. doi: 10.1007/s11010-009-0184-4. [DOI] [PubMed] [Google Scholar]
- 14.Elliott SS, Keim NL, Stern JS, Teff K, Havel PJ. Fructose, weight gain, and the insulin resistance syndrome. Am J Clin Nutr. 2002;76(5):911–22. doi: 10.1093/ajcn/76.5.911. [DOI] [PubMed] [Google Scholar]
- 15.Qin B1, Anderson RA. An extract of chokeberry attenuates weight gain and modulates insulin, adipogenic and inflammatory signalling pathways in epididymal adipose tissue of rats fed a fructose-rich diet. Br J Nutr. 2012;108(4):581–7. doi: 10.1017/S000711451100599X. [DOI] [PubMed] [Google Scholar]
- 16.Jalal R1, Bagheri SM, Moghimi A, Rasuli MB. Hypoglycemic effect of aqueous shallot and garlic extracts in rats with fructose-induced insulin resistance. J Clin Biochem Nutr. 2007;41(3):218–23. doi: 10.3164/jcbn.2007031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bal Y Adas M, Helvaci A. Evaluation of the relationship between insulin resistance and plasma tumor necrosis factor-alpha, interleukin-6 and C-reactive protein levels in obese women. Bratisl Lek Listy. 2010;111(4):200–4. [PubMed] [Google Scholar]
- 18.Klover PJ, Zimmers TA, Koniaris LG, Mooney RA. Chronic exposure to interleukin-6 causes hepatic insulin resistance in mice. Diabetes. 2003;52(11):2784–9. doi: 10.2337/diabetes.52.11.2784. [DOI] [PubMed] [Google Scholar]
- 19.Kawamata Y, Imamura T, Babendure JL, Lu JC, Yoshizaki T, Olefsky JM. Tumor necrosis factor receptor-1 can function through a G alpha q/11-beta-arrestin-1 signaling complex. J Biol Chem. 2007;282(39):28549–56. doi: 10.1074/jbc.M705869200. [DOI] [PubMed] [Google Scholar]
- 20.Zhang L, Wheatley CM, Richards SM, Barrett EJ, Clark MG, Rattigan S. TNF-alpha acutely inhibits vascular effects of physiological but not high insulin or contraction. Am J Physiol Endocrinol Metab. 2003;285(3):E654–60. doi: 10.1152/ajpendo.00119.2003. [DOI] [PubMed] [Google Scholar]
- 21.Rutledge AC, Adeli K. Fructose and the metabolic syndrome: pathophysiology and molecular mechanisms. Nutr rev. 2007;65(s1):S13–3. doi: 10.1111/j.1753-4887.2007.tb00322.x. [DOI] [PubMed] [Google Scholar]
- 22.Oh JH, Kwon TK. Withaferin A inhibits tumor necrosis factor-α-induced expression of cell adhesion molecules by inactivation of Akt and NF-κB in human pulmonary epithelial cells. Int immunopharmacol. 2009;9(5):614–69. doi: 10.1016/j.intimp.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Anwer T, Sharma M, Pillai KK, Iqbal M. Effect of Withania somnifera on Insulin Sensitivity in Non‐Insulin‐Dependent Diabetes Mellitus Rats. Basic Clin Pharmacol Toxicol. 2008;102(6):498–503. doi: 10.1111/j.1742-7843.2008.00223.x. [DOI] [PubMed] [Google Scholar]
- 24.Ahmadvand , Tavafi , Khalatbary Hepatoprotective and Hypolipidemic Effects of Satureja Khuzestanica Essential Oil in Alloxan-induced Type 1 Diabetic Rats. Iran J Pharm Res. 2012;11(4):1219–226. [PMC free article] [PubMed] [Google Scholar]