Abstract
Background:
The multifunctional transforming growth factor beta (TGF-β) is a glycoprotein that exists in three isoforms. TGF-β3 expression increases in fetal wound healing and reduces fibronectin and collagen I and III deposition, and also improves the architecture of the neodermis which is a combination of blood vessels and connective tissue during wound healing. Fibroblasts are key cells in the wound healing process. TGF-β3 plays a critical role in scar-free wound healing and fibroblast actions in the wound healing process. The aim of this study was to express the TGF-β3 gene (tgf-b3) in human foreskin fibroblasts (HFF’s).
Methods:
We obtained HFF’s from a newborn and a primary fibroblast culture was prepared. The cells were transfected with TGF-β3-pCMV6-XL5 plasmid DNA by both lipofection and electroporation. Expression of TGF-β3 was measured by enzyme-linked immunosorbent assay (ELISA).
Results:
The highest TGF-β3 expression (8.3-fold greater than control) was obtained by lipofection after 72 hours using 3 µl of transfection reagent. Expression was 1.4-fold greater than control by electroporation.
Conclusions:
In this study, we successfully increased TGF-β3 expression in primary fibroblast cells. In the future, grafting these transfected fibroblasts onto wounds can help the healing process without scarring.
Key Words: Fibroblasts, Gene expression, TGF-β3
Introduction
Transforming growth factor-β (TGF-β) is a glycoprotein that acts as a cytokine. This multifunctional cytokine has a pivotal role in the regulation of many cellular activities, such as proliferation, differentiation, and other functions, and also plays a role in immunity, cancer, heart disease, diabetes, Marfan syndrome, Loeys–Dietz syndrome, Parkinson's disease, and AIDS (1).
Three TGF-β isoforms have been found in mammals: TGF-β1, 2, and 3, which are structurally and functionally similar (2). TGF-β is a chemo-absorbent for macrophages and can mediate collagen synthesis (3, 4). It can also increase granulation tissue formation (5). TGF-β3 has a role in embryogenesis and cell differentiation (6). Wound healing can be accompanied by keloid and hypertrophic scar formation. The healing process is the same and predictable for cuts, burns, and infarcted tissue (7).
Healing occurs in four phases: inflammatory, proliferative, granulation tissue formation, and remodelling. Multiple cell types cooperate in these phases (8). Inordinate collagen type III synthesis in the proliferative phase increases scar formation. Keratinocytes and fibroblasts migrate during wound healing. Keratinocytes migrate to the wound edge within hours, but fibroblast migration is undetectable until several days later.
TGF-β3 plays an important role in this process. TGF-β3 halts dermal migration and consequently causes wound re-epithelialization prior to wound remodeling (9). In fetal tissue, wound healing phases are less clear; however, the inflammatory phase increases and wounds heal without scarring. TGF-β3 expression increases during fetal wound healing. TGF-β3 reduces fibronectin, collagens type I and III deposition (10), and also improves architecture of the neodermis during wound healing. TGF-β3 also reduces scarring, in contrast to TGF-β1 and 2, which promote scar formation (6).
Fibroblasts are the key cells in the wound-healing process. Fibroblasts have an essential role in angiogenesis during wound healing via extracellular matrix (ECM) protein production. They produce collagen, therefore they form collagen scaffolds and also function as energy reservoirs in wound healing. Recently, these cells (11), and TGF-β3, due to its role in scar-free wound healing, have been extensively used for wound treatment. Growth factor production is essential during wound healing; however, the lifetime of growth factors in the wound environment is short (12). The aim of our study was to increase TGF-beta 3 expression in allogenic fibroblasts, which can be used to promote scar-free wound healing. Our long-term goal is to treat wounds with these fibroblasts without leaving scars.
Materials and Methods
Skin preparation
With parental consent, we obtained a foreskin sample from a newborn under sterile conditions. The sample was placed in Dulbecco's Modified Eagle Medium (DMEM) (Invitrogen, USA) supplemented with 10% fetal bovine serum (FBS) (Invitrogen), 1% L-glutamine (Sigma, USA), and 10% penicillin/streptomycin (Sigma , USA), and dermis separation was completed under a laminar hood.
Fibroblasts cultivation
The sample was washed with phosphate buffered saline (PBS) (Sigma, USA). Dermis was separated from epidermis in 0.25% Trypsin-EDTA (1X) (Gibco, USA). The dermis was cut into smaller than 1 mm pieces with sterile scissors and explanted into a 100-mm cell culture dish (Corning, USA) containing complete medium (DMEM supplemented with 10% FBS, 1% L-glutamine, 1% penicillin/streptomycin and incubated at 37 ˚C with 5% CO2. Each three days, the medium was replaced with the fresh complete medium. Confluent cells were harvested from the tissue culture dish using short-term trypsinization. Harvested cells were collected and pipetted into centrifuge tube. After centrifuge at 350×g for 5 minutes, supernatant was removed and collected fibroblasts were subcultured in 25 cm2 cell culture flasks (CELLSTAR, USA). Cells from the fifth subculture were stored at -80˚C.
Lipofection
The HFF’s were transfected with Fibroblast Transfection Reagent (Altogen Biosystems, USA) by both reverse and direct transfection. In reverse transfection, the cells were cultivated in dishes for 30 minutes, and then the transfection mixture was added. In the direct transfection method, the transfection mixture was added to the plates first, and then the cells were added.
To prepare the transfection mixtures, 40 µl of DMEM without serum and 750 ng TGF-β3-pCMV6-XL5 plasmid DNA (Origene Technologies, MD, USA) were combined and then 1.5, 2, 2.5, or 3 µl of transfection reagent were added to each sample. Mixtures were incubated at room temperature for 15-30 minutes. For reverse transfection, 2 x 104 - 3 x 104 fibroblasts were seeded in 24-well plates (Corning, USA) with 0.5 ml complete medium, and after 30 minutes the transfection mixtures were added. The cells were incubated at 37 ˚C and 5% CO2 and checked hourly. If cell viability was affected, the medium was changed. If the cell viability was unaffected after 16-24 h, the transfection enhancer reagent was added. Supernatants were assayed for TGF-β3 after 24, 48, 72, and 96 hours by ELISA (R&D Systems).
Electroporation
Confluent fibroblasts were dissociated from the wells using 0.25% trypsin (Invitrogen) and suspended in complete medium. The cell suspension was pelleted by centrifugation at 350×g for 5 minutes. OptiMEM (Invitrogen) was added to the cell pellet and the suspension was again centrifuged at 350×g for 5 minutes. Twenty µg of TGF-β3-pCMV6-XL5 plasmid DNA and OptiMEM were added to the cell pellet. The cell suspension was incubated for 10 minutes at room temperature and then added into an electroporation cuvette (Gene Pulser, 0.2 cm electrode, Bio-Rad). Electroporation was performed in a BioRad Gene Pulser™ (Bio-Rad Laboratories, CA, USA) with 350 V and 500 µF for 12.7-15.1 mS.
Assessing TGF-B3 production
Seventy-two hours post-transfection TGF-β3 production in transfected and control cells was assayed using a human TGF-beta 3 DuoSet ELISA Development kit (R&D Systems). Optical densities were read by a microplate reader (TECAN, SunRise) at 450 nm. Diagrams were drawn and concentrations were calculated using Magellan software (Tecan).
Statistics
Data are presented as means ± standard deviations (SD). Statistical significance for comparisons was determined by the Student’s two-tailed t-test. P value equal to or less than 0.05 was considered statistically significant.
Results
In this research fibroblasts were transfected with TGF-β3-pCMV6-XL5 plasmid DNA via lipofection and electroporation.
To determine the optimum the lipofection method, we tested different amounts of fibroblast transfection reagent by both reverse and direct methods.
The results showed that TGF-β3 expression was greater with reverse transfection than with direct transfection.
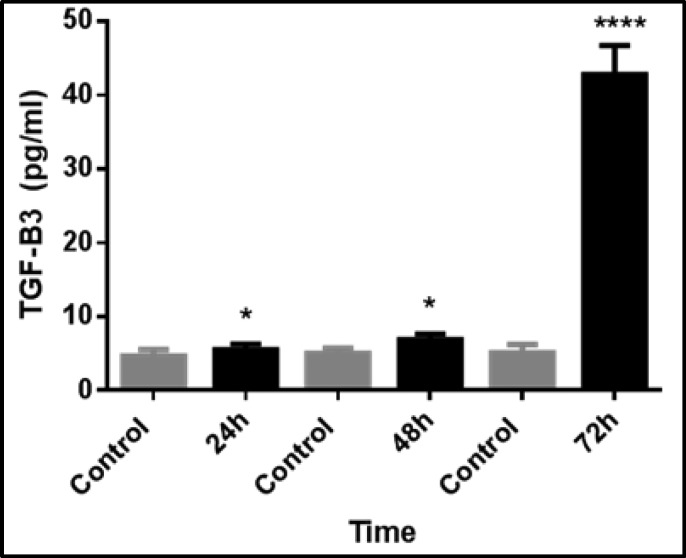
We found that TGF-β3 expression was greatest, at 8.3-fold over controls, in the reverse transfection method using 3 µl of transfection reagent after 72 hours of incubation (Fig. 1).
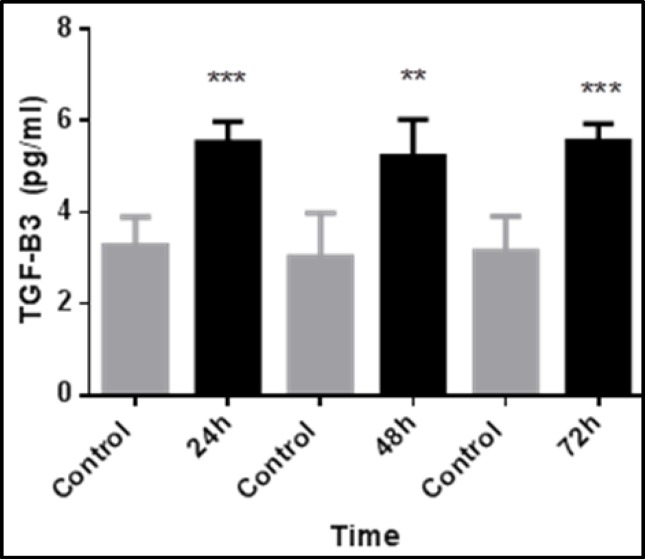
Fig. 1.
Time-dependent expression of TGF-β3 in fibroblasts after transfection with 3µL of transfection reagent via reverse transfection. TGF-β3 expression was significantly greater in transfected fibroblasts than control cells. The data represent the means ± SEMs of five independent experiments. (*p<0.05 and ****p<0.001).
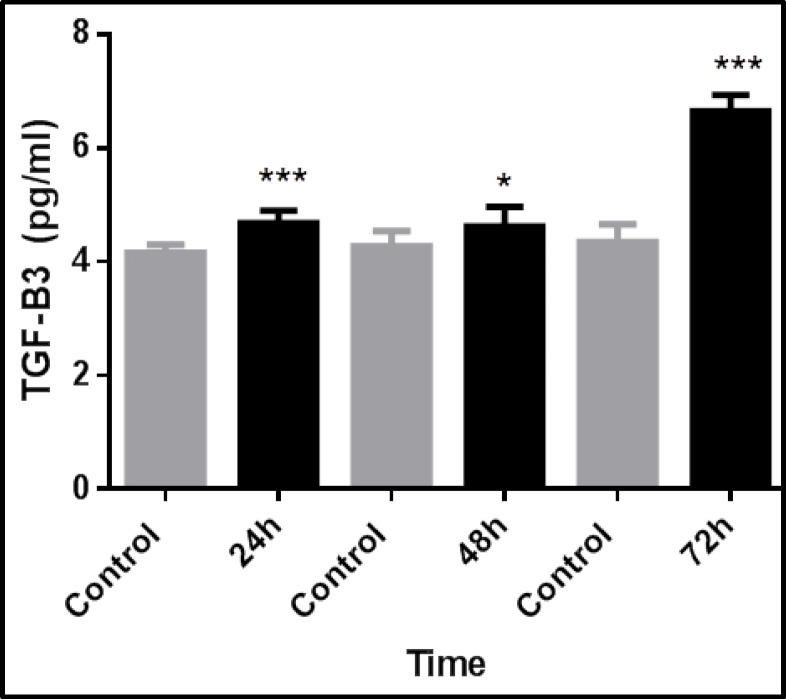
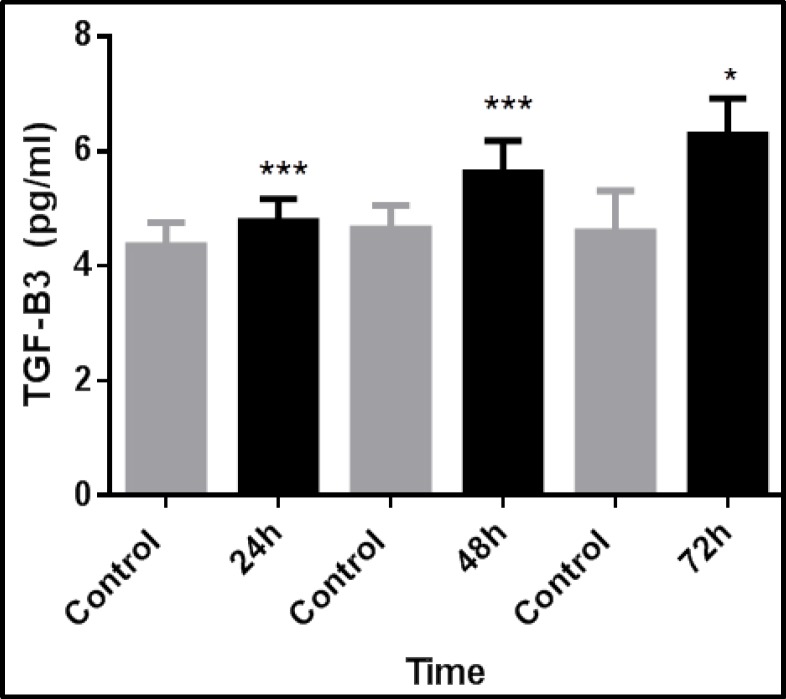
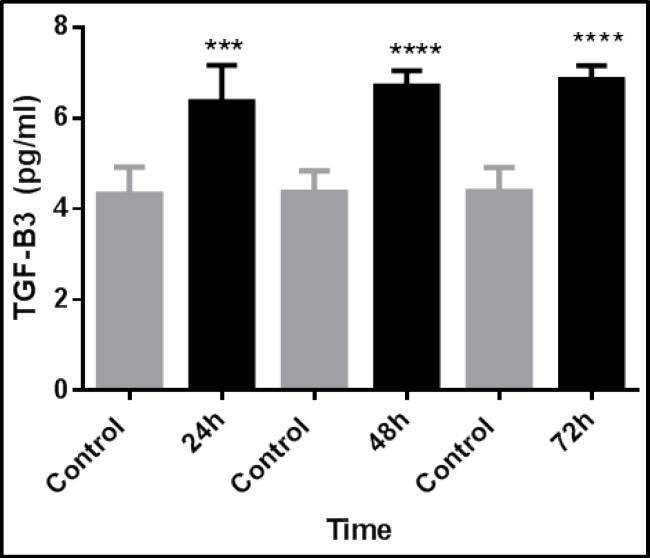
TGF-β3 expression increased with time and was greatest at 72 hours, but decreased after that. TGF-β3 expression was 1.5-fold greater than controls after 72 hours in the direct transfection method using 3 µl of transfection reagent (Fig. 2). Using 2.5 µl of transfection reagent in direct transfection method, after 72 hours, TGF-β3 expression in transfected cells was significantly greater, at 1.3-fold, than control cells (Fig. 3). Using 2.5 µl of transfection reagent in the reverse transfection method, after 72 hours TGF-β3 expression in transfected cells was significantly greater by more than 1.5-fold than control cells (Fig. 4). In the electroporation method, cell mortality was high and TGF-β3 expression in transfected cells after 72 hours was 1.4-fold greater than in control cells (Fig. 5).
Fig. 2.
Time-dependent expression of TGF-β3 in fibroblasts after transfection with 3µL of transfection reagent via direct transfection. TGF-β3 expression was significantly greater in transfected fibroblasts than control cells. The data represent the means ± SEMs of five independent experiments. (*p<0.05 and ***p<0.001
Fig. 3.
Time-dependent expression of TGF-β3 in fibroblasts after transfection with 2.5µL of transfection reagent via direct transfection. TGF-β3 expression was significantly greater in transfected fibroblasts than control cells. The data represent the means ± SEMs of five independent experiments. (*p<0.05 and ***p<0.001).
Fig. 4.
Time-dependent expression of TGF-β3 in fibroblasts after transfection with 2.5µL of transfection reagent via reverse transfection. TGF-β3 expression was significantly greater in transfected fibroblasts than control cells. The data represent the means ± SEMs of five independent experiments. (***p<0.001 and ****p<0.0001).
Fig. 5.
Time-dependent expression of TGF-β3 in fibroblasts after transfection with electroporation method. TGF-β3 expression was significantly greater in transfected fibroblasts than control cells. The data represent the means ± SEMs of five independent experiments. (**p<0.01 and ***p<0.001).
Discussion
Wound healing is a complex process in which different skin cell types, extracellular matrix, and soluble growth factors are all involved (8). Other methods, such as using recombinant growth factors and skin substitute products have been successful, but are not cost-effective (13). Scar-free wound healing is one of the most important goals of researchers and clinicians; however, inflammation inhibits fast wound healing and contributes to scar formation. Fetal wound healing is scar-free due to the lack of inflammation (6). Immediately after scar formation, remaining skin cells encounter serum, and when wounds heal, cells encounter plasma again. Human serum, unlike plasma, induces migration of keratinocytes via TGF-β3 (14). Replacement of plasma with serum regulates cell migration. TGF-β3 in serum regulates this migration and the TGF-β receptor II (TbRII) level on the cell surface works as a sensor for recognition of skin cell migration (15).
Recent research has focused on genetic and cell-based technologies to treat wounds. Tang et al. (16) transfected stable NIH3T3 fibroblasts using Lipofectamine 2000 to deliver pcDNA3.1(-)/TGF beta3 gene into the cells. Their results showed an increase of TGF-β3 production in 7 out of 11 transfected cell lines; however, MTT assays showed proliferation of the transfected cells was inefficiently slow. Stable transfection of TGF-β3 in fibroblasts by this method was costly, had undesirable side effects on the transfected cells, and the cells could not be used clinically.
Ferguson et al. reported that Avotermin (recombinant TGF-β3) can accelerate wound and scar healing (17). Although gene transfection in skin cells seems relatively difficult compared to other methods of tissue injury treatment, it is essential for good wound healing (18). In this research we attempted to optimize TGF-β3 gene transfection in HFF’s by transient transfection. Transient transfection is superior to recombinant protein production or stable transfection because the process is carried out in a single step, is easily scalable, achieves high expression quickly, and is safe and cost-effective (19). Many previous studies used viral vectors (20), such as adenovirus (21-22) or retrovirus (23) for gene transfection in skin cells, but viral vectors have some undesirable effects, such as such as harmful immune responses, toxicity, or tumor induction (24-27).
We used two safe methods: lipofection and electroporation. In the electroporation method, because of cell membrane sensitivity, cell mortality was high and TGF-β3 expression in the transfected cells was only 1.4 fold greater than in the control cells. However, by the lipofection method, 3 µL of the transfection reagent after 72 hours showed an 8.3-fold increase in TGF-β3 production in transfected cells compared to controls. As the lifetime of growth factors in the wound environment is short (8), we transfected allogenic fibroblasts with the TGF-β3 gene to use them in wound healing in future studies. In this way, we can heal wounds without scarring. Roland (28) reported differences between in vivo and in vitro gene expression. Therefore, grafting of these transfected allogenic fibroblasts on wounds can result in greater TGF-β3 concentrations than in vitro methods. Cell therapy is an effective method for wound healing.
Conflict of Interest
The authors state no conflict of interest.
Acknowledgements
The study was conducted by the fund provided by Skin Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran, for research affairs.
References
- 1.Hill JJ, Tremblay T-L, Cantin C, O'Connor-McCourt M, Kelly JF, Lenferink A. Glycoproteomic analysis of two mouse mammary cell lines during transforming growth factor (TGF)-beta induced epithelial to mesenchymal transition. Proteome Sci. 2009;7(2):1–17. doi: 10.1186/1477-5956-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shah M, Foreman D, Ferguson M. Control of scarring in adult wounds by neutralising antibody to transforming growth factor β. The Lancet. 1992;339(8787):213–4. doi: 10.1016/0140-6736(92)90009-r. [DOI] [PubMed] [Google Scholar]
- 3.Tuan T-L, Zhu JY, Sun B, Nichter LS, Nimni ME, Laug WE. Elevated levels of plasminogen activator inhibitor-1 may account for the altered fibrinolysis by keloid fibroblasts. Journal of investigative dermatology. 1996;106(5):1007–11. doi: 10.1111/1523-1747.ep12338552. [DOI] [PubMed] [Google Scholar]
- 4.Noble NA, Border WA, inventors. Methods for treating conditions associated with the accumulation of excess extracellular matrix. Google Patents. 2005
- 5.Sporn MB, Roberts AB, Shull JH, Smith JM, Ward JM, Sodek J. Polypeptide transforming growth factors isolated from bovine sources and used for wound healing in vivo. Science. 1983;219(4590):1329–31. doi: 10.1126/science.6572416. [DOI] [PubMed] [Google Scholar]
- 6.Ferguson MW, O'Kane S. Scar–free healing: from embryonic mechanisms to adult therapeutic intervention Philosophical Transactions of the Royal Society of London Series B. Biological Sciences. 2004;359(1445):839–50. doi: 10.1098/rstb.2004.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Enoch S, Leaper DJ. Basic science of wound healing. Surgery (Oxford) 2008;26(2):31–37. [Google Scholar]
- 8.Martin P. Wound healing--aiming for perfect skin regeneration. Science. 1997;276(5309):75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 9.Han A, Bandyopadhyay B, Jayaprakash P, Lua I, Sahu D, Chen M, et al. The anti-motility signaling mechanism of TGFβ3 that controls cell traffic during skin wound healing. Biology open. 2012;1(12):1169–77. doi: 10.1242/bio.20122246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah M, Foreman DM, Ferguson M. Neutralisation of TGF-beta 1 and TGF-beta 2 or exogenous addition of TGF-beta 3 to cutaneous rat wounds reduces scarring. Journal of cell science. 1995;108(3):985–1002. doi: 10.1242/jcs.108.3.985. [DOI] [PubMed] [Google Scholar]
- 11.Tettamanti G, Grimaldi A, Rinaldi L, Arnaboldi F, Congiu T, Valvassori R, et al. The multifunctional role of fibroblasts during wound healing in Hirudo medicinalis (Annelida, Hirudinea) Biology of the Cell. 2004;96(6):443–55. doi: 10.1016/j.biolcel.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Lynch SE, Nixon JC, Colvin RB, Antoniades HN. Role of platelet-derived growth factor in wound healing: synergistic effects with other growth factors. Proceedings of the National Academy of Sciences. 1987;84(21):7696–700. doi: 10.1073/pnas.84.21.7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyce ST, Goretsky MJ, Greenhalgh DG, Kagan RJ, Rieman MT, Warden GD. Comparative assessment of cultured skin substitutes and native skin autograft for treatment of full-thickness burns. Annals of surgery. 1995;222(6) doi: 10.1097/00000658-199512000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henry G, Li W, Garner W, Woodley DT. Migration of human keratinocytes in plasma and serum and wound re-epithelialisation. The Lancet. 2003;361(9357):574–6. doi: 10.1016/S0140-6736(03)12510-X. [DOI] [PubMed] [Google Scholar]
- 15.Bandyopadhyay B, Fan J, Guan S, Li Y, Chen M, Woodley DT, et al. A “traffic control” role for TGFβ3: orchestrating dermal and epidermal cell motility during wound healing. The Journal of cell biology. 2006;172(7):1093–105. doi: 10.1083/jcb.200507111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang S, Xie S, Hu S, Xiao Z, Shen J, Yi H. Construction of the stable expression system of NIH3T3 fibroblast with pcDNA3 1 (-) –hTGF beta3 and the study on the proliferation of the system fibroblasts. Zhonghua zheng xing wai ke za zhi Chinese journal of plastic surgery. 2006;22(2):109–12. [PubMed] [Google Scholar]
- 17.Ferguson MW, Duncan J, Bond J, Bush J, Durani P, So K, et al. Prophylactic administration of avotermin for improvement of skin scarring: three double-blind, placebo-controlled, phase I/II studies. The Lancet. 2009;373(9671):1264–74. doi: 10.1016/S0140-6736(09)60322-6. [DOI] [PubMed] [Google Scholar]
- 18.Hirsch T, Spielmann M, Yao F, Eriksson E. Gene therapy in cutaneous wound healing. Front Biosci. 2007;12:2507–18. doi: 10.2741/2251. [DOI] [PubMed] [Google Scholar]
- 19.Jeschke M, Richter G, Herndon D, Geissler E, Hartl M, Hofstätter F, et al. Therapeutic success and efficacy of nonviral liposomal cDNA gene transfer to the skin in vivo is dose dependent. Gene therapy. 2001;8(23):1777–84. doi: 10.1038/sj.gt.3301589. [DOI] [PubMed] [Google Scholar]
- 20.Zellmer S, Gaunitz F, Salvetter J, Surovoy A, Reissig D, Gebhardt R. Long-term expression of foreign genes in normal human epidermal keratinocytes after transfection with lipid/DNA complexes. Histochemistry and cell biology. 2001;115(1):41–7. doi: 10.1007/s004180000225. [DOI] [PubMed] [Google Scholar]
- 21.Jacobsen F, Mittler D, Hirsch T, Gerhards A, Lehnhardt M, Voss B, et al. Transient cutaneous adenoviral gene therapy with human host defense peptide hCAP-18/LL-37 is effective for the treatment of burn wound infections. Gene therapy. 2005;12(20):1494–502. doi: 10.1038/sj.gt.3302568. [DOI] [PubMed] [Google Scholar]
- 22.Galeano M, Deodato B, Altavilla D, Squadrito G, Seminara P, Marini H, et al. Effect of recombinant adeno-associated virus vector-mediated vascular endothelial growth factor gene transfer on wound healing after burn injury. Critical care medicine. 2003;31(4):1017–25. doi: 10.1097/01.CCM.0000059435.88283.C2. [DOI] [PubMed] [Google Scholar]
- 23.Lee JA, Conejero JA, Mason JM, Parrett BM, Wear-Maggitti KD, Grant RT, et al. Lentiviral transfection with the PDGF-B gene improves diabetic wound healing. Plastic and reconstructive surgery. 2005;116(2):532–8. doi: 10.1097/01.prs.0000172892.78964.49. [DOI] [PubMed] [Google Scholar]
- 24.Somia N, Verma IM. Gene therapy: trials and tribulations. Nature Reviews Genetics. 2000;1(2):91–9. doi: 10.1038/35038533. [DOI] [PubMed] [Google Scholar]
- 25.Thomas M, Klibanov A. Non-viral gene therapy: polycation-mediated DNA delivery. Applied Microbiology and Biotechnology. 2003;62(1):27–34. doi: 10.1007/s00253-003-1321-8. [DOI] [PubMed] [Google Scholar]
- 26.Mikkers H, Berns A. Retroviral insertional mutagenesis: tagging cancer pathways. Advances in cancer research. 2003;88:53–99. doi: 10.1016/s0065-230x(03)88304-5. [DOI] [PubMed] [Google Scholar]
- 27.Hacein-Bey-Abina S, von Kalle C, Schmidt M, Le Deist F, Wulffraat N, McIntyre E, et al. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. New England Journal of Medicine. 2003;348(3):255–6. doi: 10.1056/NEJM200301163480314. [DOI] [PubMed] [Google Scholar]
- 28.Rolland AP. From genes to gene medicines: recent advances in nonviral gene delivery. Critical Reviews™ in Therapeutic Drug Carrier Systems. 1998;15(2) doi: 10.1615/critrevtherdrugcarriersyst.v15.i2.20. [DOI] [PubMed] [Google Scholar]