Abstract
Background:
Although parental obesity is a well-established predisposing factor for the development of obesity, associations between regional body compositions, resting metabolic rates (RMR), and physical activity (PA) of parents and their pre-school children remain unknown. The objective of this study was to investigate parent-child correlations for total and regional body compositions, resting energy expenditures, and physical activity.
Methods:
Participants were 89 children aged 2-6 years and their parents, consisting of 61 families. Resting metabolic rate was assessed using indirect calorimetry. Total and regional body compositions were measured by both dual energy X-ray absorptiometry (DXA) and deuterium dilution. Physical activity was assessed by an accelerometer.
Results:
There was a significant parent-offspring regression for total fat free mass (FFM) between children and their mothers (P=0.02), fathers (P=0.02), and mid-parent (average of father and mother value) (P=0.002) when measured by DXA. The same was true for fat mass (FM) between children and mothers (P<0.01), fathers (P=0.02), and mid-parent (P=0.001). There was no significant association between children and parents for physical activity during the entire week, weekend, weekdays, and different parts of days, except for morning activity, which was positively related to the mothers’ morning activities (P<0.01) and mid-parent (P=0.009). No association was found between RMR of children and parents before and after correction for FFM and FM.
Conclusion:
These data suggest a familial resemblance for total body composition between children and their parents. Our data showed no familial resemblance for PA and RMR between children and their parents.
Key Words: Obesity, Familial resemblance, Children, Resting metabolic rate, Physical activity
Introduction
Obesity is a common disease caused by multiple factors, and families influence its development. Evidence suggests that both genetics and environment are important in the development of childhood obesity. Growing evidence points to heritability as a strong determining factor for obesity (1). Separating the family and environmental factors from genetic influences on childhood obesity is often difficult. However, it has been shown that genetics contributes 30-70% of heritability in body mass index (BMI) (2, 3). The remainder of the total BMI variation is attributed to the shared environment and mostly random variation. Parental obesity is associated with obesity in children as a result of interactions between shared genetic and environmental factors within families (4). Family and twin studies report heritability (h²) of BMI from 0.40 0.70 (5-8), while few data are available regarding the heritability of body composition. Available data regarding the heritability of specific body compartments, such as fat mass, indicate h² from 0.54 to 0.76% (9-11). Previous studies used a variety of tools to measure body composition, which might explain this variability in estimates of h². Only a few of these studies have used dual energy X-ray absorptiometry (DXA) to assess fat free mass (FFM) and fat mass (FM) (10, 11).
It is important to understand the physiological pathways involved in the development of common forms of childhood obesity. Because obesity is the end result of an imbalance between energy intake and expenditure, with the use of more precise measurement techniques for measuring physical activity (PA) and body composition than were previously available, a new look at the heritability of energy expenditure components is required. For instance, estimation of the heritability of energy expenditure and their components, such as PA and resting metabolic rate (RMR), may help to determine the susceptibility of individuals to obesity. Few studies have investigated the heritability of energy expenditure and its components; therefore, there has been considerable debate regarding the influence of parental energy expenditure on early manifestations of obesity. Although some studies have compared total energy expenditure, RMR, and activity between children with one obese and one non-obese parent (12,13), the heritability RMR and PA and their contribution to the aetiology of childhood obesity has not been well-characterised (14, 15). Moreover, few studies have employed techniques that are both objective and quantitative to measure PA in children.
There is currently considerable interest in the development of early obesity in pre-school children, and Franks et al. (1) proposed that genetic effects are potentially more identifiable in childhood than adulthood. Thus, looking for parent-offspring association of energy expenditure components and possible maternal effects at this stage might be important for future strategies of obesity prevention and identification of at-risk children. Although currently individuals have no means to control or change their genetic makeup, PA, diet, and environmental factors, which all influence body composition, can be controlled. The aim of this study was to examine the associations between measures of body composition, RMRs, and physical activities in parents and their pre-school children.
Materials and Methods
Subjects
Data reported in this study are selected from an observational study: The Rowett Assessment of Childhood Appetite and Metabolism (RASCAL). Eighty-nine healthy Caucasian children aged 2-6 years (42 boys and 47 girls) and 110 of their parents (49 fathers and 61 mothers) were recruited by advertisements from the Aberdeen, NE Scotland, UK using poster advertisements within local general practices and media. Sample size calculation was described in Chapter 4. 18.8% of the children were overweight (above the 85th centile), and 11.8% obese (above the 95th centile). Some families had more than one child less than 6 years old, and all of them took part in this study as sibling pairs (27 children). All parents provided written, informed consent while verbal consent was obtained from the children to participate in the study, which was approved by the Grampian Research Ethics Committee. All measurements were performed by trained persons at the Human Nutrition Unit, Rowett Research Institute, Scotland, UK. Due to the difficulty of measuring RMR and applying DXA in very young children, we could not measure RMR in 45 children, and body composition in 32 of them.
Resting metabolic rate
Resting energy expenditure was determined by indirect calorimetry (Deltatrac Metabolic Monitor, Datex Instruments, Helsinki, Finland) under standard conditions; early in the morning in the fasted state. Gas analysers were calibrated against standard mixed reference gases immediately before the measurements, and the correct air flow rates for the subjects’ sizes were selected. Subjects were asked to lie as still as possible, remain awake, and watch their favourite videos to minimize fidgeting. After subjects were supine, hoods were placed over their heads and their expired gases were measured each minute for 30 min in adults (16) and 20 min in children (17). The within-children coefficients of variation (CV) for RMR on three consecutive days using the same protocol was 6.8 (17). The RMR was calculated from data collected from the last 20 and 10 min from the adults and children, respectively. Children were monitored and all of their movements and aberrations such as sneezing, coughing, or talking were recorded and removed before RMR was calculated (17). Energy expenditure was calculated using the method of Weir (18). Forty-five of the children were unable to lie at rest or would not remain under the hood long enough to make accurate measurements; therefore, 44 children completed the study.
The potential physiological predictors of RMR (FFM, FM, age and sex) were considered in multiple regression analysis. Results showed that RMR is best calculated with an equation including FFM and FM measured by deuterium dilution. The percentage of the variance in RMR explained by the equation (RMR = 289 + 163 FFM + 812 FM) generated from the measured sample was 71.4%. Sex and age had no influence on RMR in this regression model. Then, this generated equation was used for predicting RMR in the children where RMR could not be measured.
The RMR in the remaining children was predicted using FFM and FM measured by deuterium dilution, which explained the 71.4% of RMR variance. Each individual’s body composition measurements were used to predict RMR using the following equation: RMR = 289 + 163 FFM + 812 FM, as described in Chapter 4.
Body Composition
Total body FFM and FM were measured using DXA (Norland XR-26, Mark II high-speed pencil beam scanner equipped with dynamic filtration, with version 2.5.2 of the Norland software; Norland Corporation, Fort Atkinson, WI). The principles, physical design, and operating procedures of DXA have been described elsewhere (19-21). The CVs for repeated measurements by this machine in a total-body scan have been reported previously (16). The CVs for the assessment of bone mineral density and body mineral content (BMC) were 0.94% and 1.52%, respectively. The CVs for the assessment of mineral determination and FM were reported as 1.4% and 2.6%, respectively (19).During the scan, each subject (parents and children) lay quietly on a bed and was scanned from head to foot, which took around 15 minutes. DXA allows the determination of FM, FFM, and BMC for the entire body and defined sub-regions, including head, trunk, abdomen, legs, and arms. A trained person performed all scan measurements. Body weights of the children and their parents were measured with a high-precision digital scale (OHAUS Corporation, Pine Brook, USA, Model: CD11) to the nearest 0.1 kg, and height was measured to the nearest 0.1 cm using a standard stadiometer (Holtain Ltd, Crymych, Dyfed, Wales). Body mass index was calculated as weight in kilograms (kg) divided by the square of the height in meters. Also, using the 1990 UK reference data, heights, weights, and BMIs of children were converted into age- and sex-corrected BMI standard deviation scores (SDS) (22, 23).
In addition, body composition was assessed by measurement of total body water (TBW). After fasting baseline urine samples were collected in the early morning, subjects received oral doses of deuterium-labelled water based on their weights. Urine samples were collected 2, 4, and 6 h after dosing with deuterium. Each sample was analyzed in duplicate and the mean value was used for analysis. Deuterium dilution space was calculated as described elsewhere (24). From TBW, the FFM, and hence FM, was calculated using age- and gender-specific constants for children ranging from 76.3 to 78.1 for boys and 77.5 to 78.2 for girls (25). In parents, FFM was assumed to have a hydration constant of 0.73 so that fat-free mass was calculated as TBW/0.73. Fat mass was calculated as body weight minus FFM.
PA: Accelerometry
Free-living PA of children and their parents were monitored for 7 days using the Actiwatch-L (Cambridge Neurotechnology Ltd). The Actiwatch data have been shown to be closely associated with the direct observation technique for measuring PA (26). The Actiwatch is a small uniaxial accelerometer that measures acceleration over 0.05 g of human motion. The monitor converts the detected accelerations in non-volatile memory to activity counts, after excluding the readings outside 3-11 Hz. Actiwatch data were downloaded using a reader unit connected to a computer and analysed using the Actiwatch Sleep Analysis software. In this study, each subject was asked to wear the monitor on the non-dominant wrist and remove it at any condition that might harm it (e.g., during swimming). The Actiwatch was programmed to sum the data over one-minute epochs or intervals before the commencement of data collection. A formula produced in a previous validation study of the Actiwatch was used to determine the amount of time the children engaged in PA of resting, light, moderate, or vigorous intensities (26). The data was averaged over pre-defined periods of one week, 5 consecutive weekdays, weekend, time awake, and different parts of the day, like morning, afternoon, etc.
Statistical analysis
Statistical analysis was performed with the MINITAB software (Version 14.12). Childrens’ and parents’ characteristics were expressed as means ± standard deviations (SD). If the data were not normally distributed on the raw scores, appropriate transformation was applied. Analysis of variance (ANOVA) was used to assess statistical differences between means. Associations between parameters of children and parents were examined for statistical significance by correlation and simple and multiple regressions.
In general, heritability is the fraction of total variation in a phenotypic trait that can be ascribed specifically to genetic variation. Phenotypic variations between individuals may be due to genetic or environmental factors, or a combination of the two. A key issue in statistical genetic analysis of quantitative traits is simply the determination of the extent to which a continuous variable is influenced by the genes encoding that trait, as opposed to environmental or chance effects. The total phenotypic variance for a trait (P) is a function of two main components, including genetic variance (G) and environmental variance (E 1). Vp = VG + VE (1)
Three components comprise the genetic value, so the simple model given by Equation 1 can be extended to Equation 2: Vp = (Va+ Vd + Ve) + VE (2)
Where Va, Vd, and Ve are additive, dominance, and epistatic variance, respectively. The sum of these three components is the total genetic variance, which has been described elsewhere (26).
Heritability can be referred to as the ratio of the genetic variance to the total phenotypic variance. This ratio is called the broad-sense heritability (H2), which includes all the components of genetic variance. However, the ratio of only the additive genetic variance over the total phenotypic variance is called the narrow-sense heritability (h²), also called the resemblance between relatives (27).
In this study, heritability (h²) estimates for total and regional body composition, PA, and RMR were obtained using parent-offspring regression (narrow-sense heritability). The slope of the regression line was used to estimate heritability for mid-parent versus offspring. Single-parent-offspringheritability was estimated by multiplying the slope of the line by two as the single-parent-offspring covariances estimatehalf of the additivegenetic variance (26). P values less than 0.05 were considered statistically significant.
Results
Descriptive Statistics of children
Descriptive characteristics of children are summarised in Table 1. When age was used as a covariate, no significant differences were found between boys and girls for log weight (F=0.45, P=0.51), height (F=0.19, P=0.67), BMI SDS (F=0.01, P=0.97), RMR (F=0.95, P=0.33) or total FFM and FM measured either by DXA or deuterium dilution. The regional body composition indices from DXA are shown in Table 2. There were no significant differences between boys and girls for head’ FFM (F=0.0, P=0.97), abdomen’ FFM (F=0.90, P=0.35), arms’ FFM (F=3.24, P=0.08), head’ FM (F=0.12, P=0.73), trunk’ FM (F=0.51, P=0.48), abdomen’ FM (F=0.06, P=0.81), legs’ FM (F=0.03, P=0.87) or arms’ FM (F=0.26, P=0.61). Boys had higher trunk (F=6.32, P=0.015) and leg FFMs (F=4.56, P=0.04) than girls. To eliminate potential artefacts among all regional traits of FFM and FM (correlations between traits in Tables 3 and 4), the value of each region was corrected for the rest of the other regional estimates and age in each sex before analysing for gender differences and subsequent analyses. Physical activity patterns measured objectively by Actiwatch were not significantly different between boys and girls during weekends (F=0.01; P=0.92), weekdays (F=3.04; P=0.09), entire weeks (F=1.78; P=0.19), afternoons (F=0.87; P=0.35), evenings (F=0.82; P=0.37), or nights (F=3.46; P=0.07). There were no gender differences between percentages of moderate (F=0.96; P=0.33) and sedentary (F=0.96; P=0.33) activities during the week. Morning PA was significantly higher in boys than in girls (F=5.31; P=0.02).
Table 1.
Physical characteristics of pre-school children in the study
|
|
Boys
|
Girls
|
Total
|
|||
|---|---|---|---|---|---|---|
| Mean | SD (n) | Mean | SD (n) | Mean | SD (n) | |
| Age (years) | 4.02 | 1.48 (42) | 4.1 | 1.20 (47) | 4.06 | 1.33 (89) |
| Weight (kg) | 18 | 5.14 (42) | 17.66 | 5.01 (46) | 17.82 | 5.04 (88) |
| Height (cm) | 1.05 | 0.11 (40) | 1.04 | 0.10 (45) | 1.04 | 0.11 (85) |
| BMI SDS | 0.15 | 1.36 (40) | 0.14 | 1.25 (45) | 0.15 | 1.30 (85) |
| FM (DXA) (kg) | 6.72 | 3.06 (25) | 7.47 | 3.13 (32) | 7.14 | 3.10 (57) |
| FFM (DXA) (kg) | 11.84 | 2.72 (25) | 10.68 | 2.80 (32) | 11.19 | 2.80(57) |
| FM (Deut) (kg) | 4.86 | 2.38 (33) | 5.14 | 2.76 (34) | 5 | 2.57 (67) |
| FFM (Deut) (kg) | 13.88 | 3.52 (33) | 12.52 | 2.84 (34) | 13.19 | 3.24 (67) |
| RMR (kJ) |
3040 |
681 (37) |
2883 |
697 (37) |
2962 |
689 (74) |
|
Actiwatch (Counts/min)
7 days |
458.1 | 97.8 (40) | 428.5 | 105.9 (45) | 442.4 | 102.6 (85) |
| Weekend | 444.6 | 109.6 (40) | 442.3 | 113.1 (45) | 443.4 | 110.8 (85) |
| Weekdays | 464.6 | 106.1 (40) | 423.1 | 112.4 (45) | 442.6 | 110.8 (85) |
| Morning | 765.6 * | 177.3 (40) | 674.4 * | 186.1 (45) | 717.3 | 186.6 (85) |
| Afternoon | 834.9 | 196.2 (40) | 794.2 | 205.5 (45) | 813.3 | 201 (85) |
| Evening | 790.8 | 193.7 (40) | 752.3 | 198 (45) | 770.4 | 195.8 (85) |
| Night | 149.4 | 108.3 (40) | 209.2 | 175.7 (45) | 181.1 | 150 (85) |
P<0.05, Total body composition of boys and girls was compared after correction for age effect.
Table 2.
Regional values of body composition in 57 children (Boys=25, Girls=32).
|
|
Boys
|
Girls
|
Total
|
|||
|---|---|---|---|---|---|---|
| Mean | SD (n) | Mean | SD (n) | Mean | SD (n) | |
| Fat Free Mass (kg) | ||||||
| Head | 1.9 | 0.51 (25) | 1.9 | 0.47 (32) | 1.88 | 0.48 (57) |
| Trunk | 5.30* | 1.23 (25) | 4.70* | 1.17 (32) | 4.95 | 1.23 (57) |
| Abdomen | 2.27 | 0.48 (25) | 2.11 | 0.64 (32) | 2.18 | 0.58 (57) |
| Arms | 1.29 | 0.38 (25) | 1.09 | 0.49 (32) | 1.18 | 0.45 (57) |
| Legs |
3.27*
|
0.24 (25) |
3.07*
|
1.12 (32) |
3.16 |
1.14 (57) |
| Fat Mass (kg) | ||||||
| Head | 0.89 | 0.34 (25) | 0.84 | 0.46 (32) | 0.87 | 0.41(57) |
| Trunk | 2.95 | 1.60 (25) | 3.42 | 1.59 (32) | 3.21 | 1.59 (57) |
| Abdomen | 1.13 | 0.62 (25) | 1.33 | 0.63 (32) | 1.24 | 0.62 (57) |
| Arms | 0.79 | 0.51 (25) | 0.84 | 0.59 (32) | 0.82 | 0.55 (57) |
| Legs | 2.21 | 1.01 (25) | 2.4 | 1.00 (32) | 2.32 | 1.00 (57) |
Table 3.
Correlation between regional FFM of children
| Variable |
FFM
|
||||
|---|---|---|---|---|---|
| Head | Trunk | Abdomen | Arms | ||
| FFM | Trunk | =0.415** | |||
| Abdomen | r=0.263* | r=0.798** | |||
| Arms | r=0.037 | r=0.546** | r=0.529** | r=0.627** | |
| Legs | r=0.409** | r=0.588** | r=0.700** | r=0.627** | |
P<0.05,
P<0.01
Table 4.
Correlation between regional FM of children
| Variable |
FFM
|
||||
|---|---|---|---|---|---|
| Head | Trunk | Abdomen | Arms | ||
| FFM | Trunk | r=0.003** | |||
| Abdomen | -r=0.012* | =0.946** | |||
| Arms | r=0.101 | r=0.800** | r=0.764** | r=0.837* | |
| Legs | -r=0.028** | r=0.933** | r=0.914** | ||
P<0.05,
P<0.01
Descriptive Statistics of parents
The physical characteristics and body compositions of the parents are shown in Table 5.
Table 5.
Physical characteristics of parents in the study
|
|
Fathers
|
Mothers
|
Total
|
|||
|---|---|---|---|---|---|---|
| Mean | SD (n) | Mean | SD (n) | Mean | SD (n) | |
| Age (years) | 36.84* | 5.75 (47) | 34.62* | 4.58 (61) | 35.59 | 5.21 (108) |
| Weight (kg) | 83.86* | 13.96 (47) | 69.86* | 16.36 (60) | 76.01 | 16.8 (107) |
| Height (cm) | 1.78* | 0.07(45) | 1.67* | 0.01 (58) | 1.72 | 0.09 (103) |
| BMI SDS | 26.41 | 3.65 (45) | 25.32 | 6.05 (58) | 25.80 | 5.15 (103) |
| FM (DXA) (kg) | 20.59* | 9.36 (45) | 25.81* | 12.01 (54) | 23.43 | 11.14 (99) |
| FFM (DXA) (kg) | 60.13 ** | 8.06 (45) | 41.44 ** | 6.82 (54) | 49.93 | 11.91(99) |
| FM (Deut) (kg) | 19.54 | 8.17 (36) | 21.65 | 10.01 (43) | 20.72 | 9.23 (79) |
| FFM (Deut) (kg) | 64.13 ** | 8.85 (36) | 48.09 ** | 7.72 (43) | 55.40 | 11.49 (79) |
| RMR (kJ) | 7085 | 982 (45) | 6263 | 931 (56) | 6629 | 1034 (101) |
|
Actiwatch (Counts/min)
7 days |
265.37* | 67.06 (48) | 297.5* | 63.56 (61) | 283.37 | 66.78 (109) |
| Weekend | 275.1 | 81.0 (48) | 292 | 79.1 (60) | 284.69 | 80.02 (108) |
| Weekdays | 262.4 ** | 74.1 (48) | 300 ** | 63.2 (61) | 283.03 | 70.34 (109) |
| Morning | 365 ** | 118 (48) | 466 ** | 139.4 (61) | 421.4 | 139.1 (109) |
| Afternoon | 404 ** | 115.8 (48) | 479 ** | 103.1 (61) | 445.9 | 114.5 (109) |
| Evening | 433.9 | 128.9 (48) | 465.9 | 105.5 (61) | 451.8 | 116.9 (109) |
| Night | 231.7 | 105.0 (48) | 250.1 | 88.2 (61) | 242.02 | 95.95(109) |
P<0.05,
P<0.01, RMRs of fathers and mothers was compared after correction for FFM and FM.
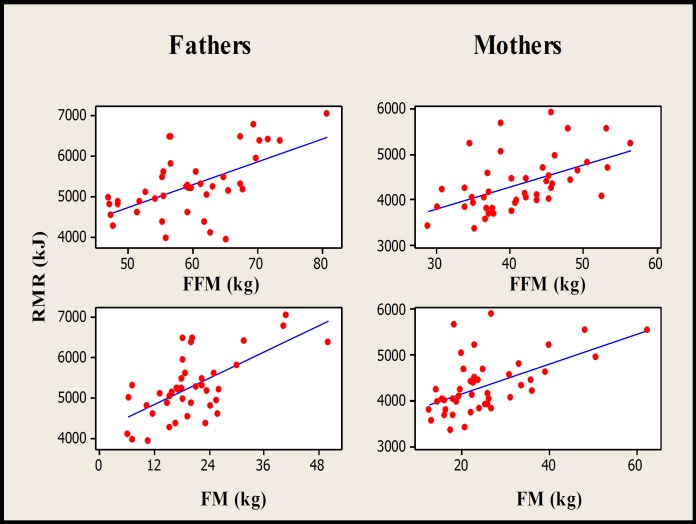
The fathers were significantly heavier (F=21.9, P<0.001), taller (F=70.4, P<0.001), and older (F=4.98, P=0.03) than the mothers. The fathers had significantly higher FFMs than the mothers measured by DXA (F=156.2, P<0.001) and deuterium dilution (F=74.9, P<0.001) and RMR (F=18.52, P<0.001). Fig. 1 presents the association between RMR and FFM in fathers (r²=40.4%, P<0.001) and mothers (r²=30.1%, P<0.001). After adjusting RMR for FFM and FM, the difference between mothers and fathers was not statistically significant (F=0.02, P=0.887). The FMs (F=5.64, P=0.02) measured by DXA and the level of PA during 7 days (F=6.55, P=0.012), weekdays (F=7.88, P=0.006), mornings (F=15.81, P<0.001), and afternoons (F=12.53, P<0.001) were significantly higher in mothers than fathers. The differences between mothers and fathers for total body FFM (F=57.35, P<0.001) and FM (F=23.17, P<0.001) measured by DXA, or FFM (F=24.08, P<0.001) and FM (F=9.9, P=0.002) measured by deuterium dilution, remained significant even when the FFM data was corrected for height, and the FM data was corrected for FFM values.
Fig. 1.
Scatterplot of associations between RMR of fathers (n=44) and mothers (n=52) with their FFMs and FMs measured by DXA (P<0.001
Table 6 presents the regional percentages of FFM and FM for mothers and fathers. It is clear that those subjects who have high body FM or FFM also have higher levels of regional FM or FFM. We observed strong correlations among regional traits for FM (up to 0.97) and FFM (up to 0.81) in each sex. To remove these potential artefacts, each region’s value was regressed onto the other region’s summed values and the calculated residuals were used for subsequent analyses. After this correction, our data showed that fathers had significantly higher FFMs for all traits and mothers had significantly higher FMs on all traits except ‘head’.
Table 6.
Regional values of body composition in parents
|
|
Fathers
|
Mothers
|
Total
|
|||
|---|---|---|---|---|---|---|
| Mean | SD (n) | Mean | SD (n) | Mean | SD (n) | |
| Fat Free Mass (kg) | ||||||
| Head | 4.04* | 0.54 (45) | 3.21* | 0.33 (54) | 3.59 | 0.60 (99) |
| Trunk | 28.63* | 3.64 (45) | 19.38* | 3.16 (54) | 23.58 | 5.72 (99) |
| Abdomen | 11.48* | 1.94 (45) | 8.46* | 1.49 (54) | 9.83 | 2.27 (99) |
| Arms | 7.18* | 1.64 (45) | 4.38* | 1.5 (54) | 5.65 | 2.09 (99) |
| Legs | 20.29* | 3.33 (45) | 14.51* | 2.81 (54) | 17.14 | 4.20 (99) |
| Fat Mass (kg) | ||||||
| Head | 1.02 | 0.41 (45) | 0.88 | 0.38 (54) | 0.94 | 0.40 (99) |
| Trunk | 10.97* | 5.22 (45) | 12.28* | 6.5 (54) | 11.68 | 5.96 (99) |
| Abdomen | 4.38* | 2.16 (45) | 4.95* | 2.76 (54) | 4.69 | 2.51 (99) |
| Arms | 2.54 * | 1.59 (45) | 3.77* | 2.21 (54) | 3.21 | 2.04 (99) |
| Legs | 6.01* | 2.71(45) | 8.90* | 3.75 (54) | 7.62 | 3.59 (99) |
P<0.001, Regional body composition of fathers and mothers was compared after correction for other trait values
Familial Associations for body composition
Associations between the children’ and parents’ body-composition measures, PAs, and RMRs are shown in Table 7. To give valuable indexes, total body FFM and FM were normalized for height and FFM, respectively. The children’s values for BMI SDS (Fig. 2) and FFMs measured by deuterium dilution were correlated to their mid-parents’ (P<0.01)
Table 7.
Association between the children and their parents for physical activity measured by accelerometry, RMR, anthropometry, and residual of body fat-free mass and fat mass measured with DXA and deuterium dilution corrected for height and FFM, respectively
| Mothers | Fathers | Mid-Parents | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r 2 | h 2 | p | n | r 2 | h 2 | p | n | r 2 | h 2 | p | n | |
| RMR | 0 | 0 | 0.93 | 69 | 4.1 | 0.1 | 0.12 | 61 | 2.9 | 0.18 | 0.2 | 59 |
| BMI (kg/m2) | 8 | 0.13 | 0.01 | 69 | 3.8 | 0.1 | 0.11 | 82 | 10.7 | 0.13 | 0.007 | 68 |
| FM (DXA) | 12.5 | 0.16 | 0.01 | 55 | 11.2 | 0.15 | 0.02 | 50 | 20.3 | 0.14 | 0.001 | 49 |
| FFM (DXA) | 10.3 | 0.16 | 0.02 | 55 | 11.2 | 0.15 | 0.02 | 50 | 19.1 | 0.13 | 0.002 | 49 |
| FM (Deuterium) | 1.4 | 0.04 | 0.42 | 50 | 14.2 | 0.11 | 0.01 | 43 | 10.1 | 0.06 | 0.06 | 37 |
| FFM (Deuterium) | 10.5 | 0.17 | 0.02 | 50 | 0.9 | 0.04 | 0.54 | 43 | 6 | 0.06 | 0.14 | 37 |
| Trunk-FM | 0.1 | 0.02 | 0.857 | 50 | 0 | 0.02 | 0.897 | 48 | 0 | 0.01 | 0.92 | 45 |
| Abdomen-FM | 1 | 0 | 0.498 | 50 | 0.1 | 0 | 0.82 | 48 | 0.1 | 0 | 0.88 | 45 |
| Arms-FM | 3.2 | 0.07 | 0.212 | 50 | 5.3 | 0.18 | 0.121 | 48 | 5.3 | 0.08 | 0.13 | 45 |
| Legs-FM | 0.5 | 0.01 | 0.634 | 51 | 6.3 | 0.07 | 0.081 | 49 | 3.9 | 0.03 | 0.19 | 46 |
| Trunk -FFM | 0.5 | 0.06 | 0.493 | 54 | 1.5 | 0.08 | 0.401 | 49 | 6.5 | 0.11 | 0.08 | 48 |
| Abdomen-FFM | 11.1 | 0.3 | 0.014 | 54 | 1 | 1.2 | 0.485 | 49 | 9.8 | 0.17 | 0.03 | 48 |
| Arms-FFM | 3.3 | 0.12 | 0.192 | 54 | 7.7 | 0.14 | 0.054 | 48 | 11.7 | 0.12 | 0.017 | 48 |
| Legs-FFM | 0.2 | 0.03 | 0.776 | 54 | 0.6 | 0.03 | 0.593 | 49 | 1 | 0.03 | 0.50 | 48 |
| 7 days PA | 0.1 | 0.52 | 0.734 | 69 | 2.1 | 0.44 | 0.234 | 82 | 1.3 | 0.26 | 0.362 | 68 |
| Weekend PA | 0.8 | 0.25 | 0.423 | 69 | 2.7 | 0.43 | 0.180 | 80 | 0.7 | 0.17 | 0.49 | 66 |
| Weekdays PA | 0 | 0.05 | 0.877 | 69 | 0.6 | 0.24 | 0.521 | 82 | 0.7 | 0.2 | 0.51 | 68 |
| Morning PA | 13.4 | 0.8 | 0.001 | 69 | 0.1 | 0.01 | 0.812 | 82 | 10 | 0.62 | 0.009 | 67 |
| Afternoon PA | 2.2 | 0.62 | 0.181 | 69 | 0.4 | 0.2 | 0.630 | 82 | 1.5 | 0.35 | 0.33 | 67 |
| Evening PA | 0.1 | 0.08 | 0.831 | 69 | 0.4 | 0.18 | 0.624 | 82 | 0 | 0.03 | 0.9 | 68 |
| Night PA | 1.3 | 0 | 0.304 | 69 | 0.4 | 0 | 0.584 | 82 | 4.9 | 0 | 0.07 | 69 |
r2 = squared correlation, h²= heredity, p= P value, sd= standard deviation, RMR was corrected for FFM and FM before analysing for familial resemblance.
Fig. 2.
Scatterplot of childrens’ BMI SDS vs. mid-parents’ (r2 =10.7%, P=0.007, n=68) and mothers’ BMIs (r2 =8%, P=0.01, n=69).
and mothers’ (P<0.05), but not fathers’,values. FM obtained with deuterium dilution was significantly associated between children and their fathers (P<0.05).
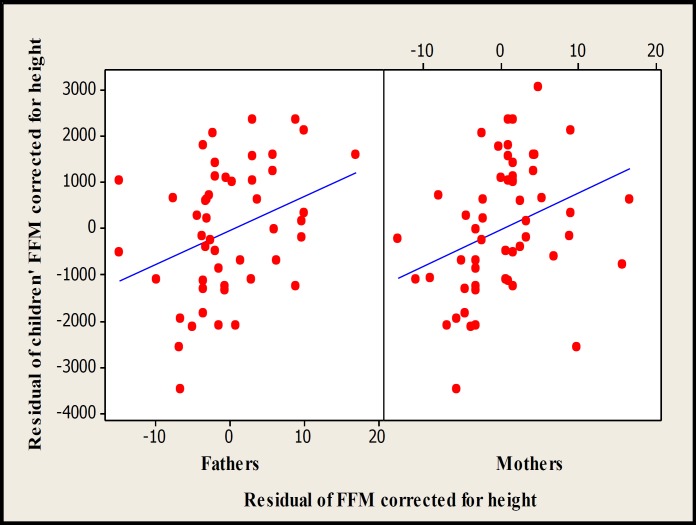
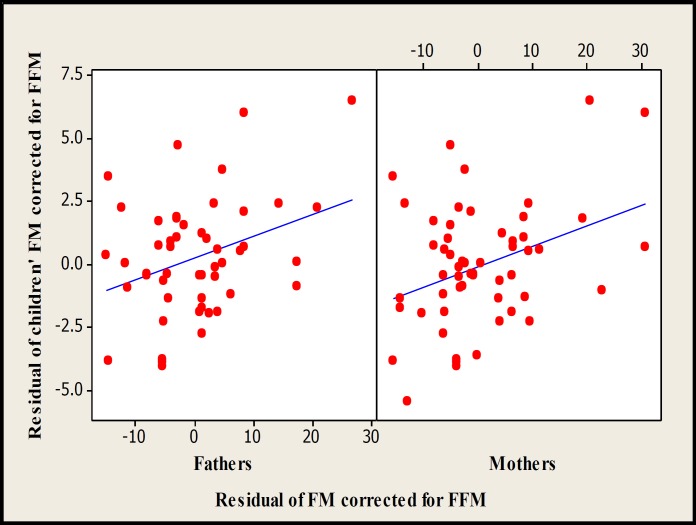
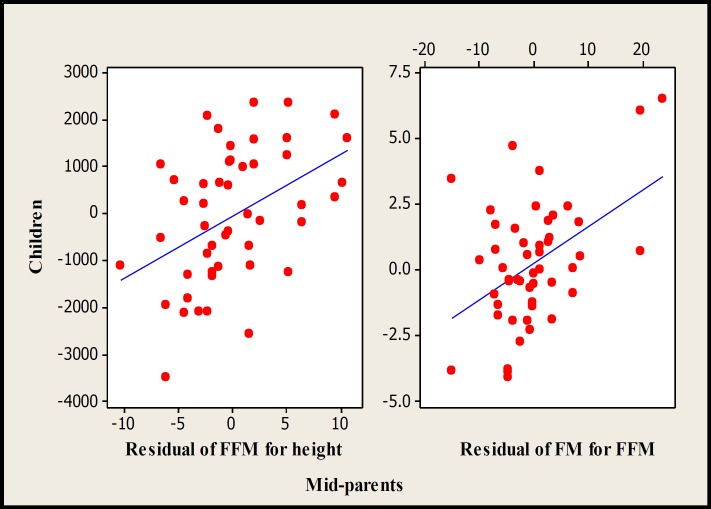
There were significant associations between children with mid-parents (P<0.01) and both the mothers (P<0.05) and fathers (P<0.05) for FM and FFM obtained with DXA (Figs. 3-5). In multiple regression analyses, the significant associations between children and their fathers for FM and FFM values obtained with DXA disappeared. The regression equations for total FFM corrected for height and FM corrected for FFM and sex measured by DXA were as follow:
Fig. 3.
Scatterplot of children vs fathers (r2 =11.1%, P=0.02, n=50) and mothers (r2 =12.5%, P=0.008, n=55) for resemblance of FM measured by DXA.
Fig. 5.
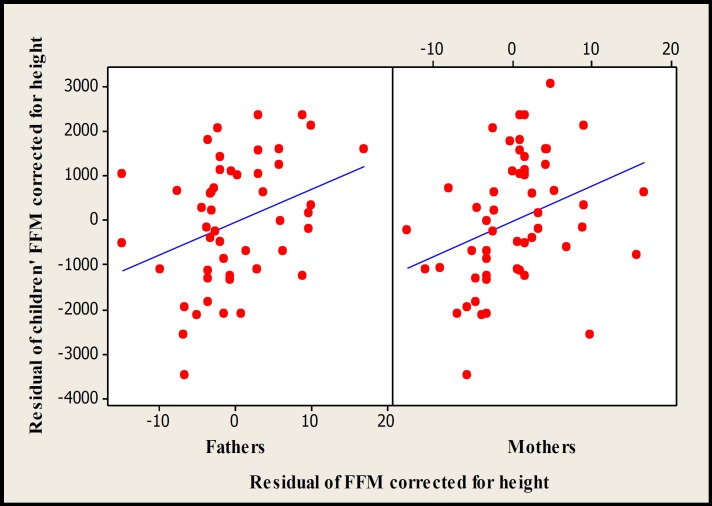
Scatterplot of children vs mid-parents for residual of FFM corrected for height (r2 =19.1%, P=0.002, n=49) and FM corrected for FFM (r2 =20.3%, P=0.001, n=49) measured by DXA.
FFM of children = - 70 + 51.3 fathers’FFMs + 86 mothers’ FFMs
FM of children = 0.246 + 0.0571 fathers’ FMs + 0.0789 mothers’FMs
Fig. 4.
Scatterplot of childrens’ FFMs vs fathers’ (r2 =11.2%, P=0.02, n=49) and mothers’ (r2 =10.3%, P=0.02, n=55) FFMs measured by DXA
No associations were observed between children and their parents for regional body composition except for children’ abdominal FFMs, which were significantly associated with their mid-parents’ (P<0.05) and mothers’ values (P<0.05) and also, children’ arms FFM, which was significantly associated with their mid-parents’ values (P<0.05).
Familial Associations for PA and RMR
Actiwatch activity counts were not significantly correlated between children and their parents (Table 7), except for children’ morning PA, which was significantly associated with their mid-parents’ (r²=1%, h²=0.62, P=0.009) and mothers’ values (r²=13.4%, h²=0.8, P=0.001). No significant association was found between the PA pattern of fathers and mothers except for morning PA (r=0.316, p=0.007). There was no association between parents and children in RMR before (Table 7) or after (r²=3%, h²=0.09, P=0.25) adjusting for body composition.
Discussion
The findings of this study support previous research, which indicated that familial resemblance has an important role in determining body composition. With regard to total body composition, associations between children’ and parents’ values for FFM and FM obtained with DXA were significant. When body composition results measured by deuterium dilution were used, only the relation between children’s FFM and the relative measure of their mothers, and children’s FM and similar measure in their fathers were significant. This discrepancy in our finding has shown that choosing different methods and tools for assessing body composition might be a reason for observing different heritability estimates in previous studies (8, 28-30). While the heritability of BMI has been investigated in a large number of twins, adoptions, and families (5, 6, 14, 28-33), few studies have been conducted in paediatric samples (32). The results from adults cannot necessarily be generalized to children. However, heritability estimates derived from twin studies (up to 90%) are greater than those reported in family studies (20-52%) (5-7, 14, 28-33).
Some studies showed correlations for BMIs between children and their fathers as well as their mothers (36, 37). The Framingham cohorts study showed that heritability of BMI increases with age (38), therefore, the lack of association between children and their fathers and the low heritability between children and their mothers for BMI in this study (0.13) may be due to the young age of our participants compared to those in previous studies. Several studies indicated that a large part of the variability in BMI as a frequently used indicator of obesity is attributable to inheritance (5-7, 14, 28-33); however, it should be noted that BMI is a relatively crude measure of “heaviness” rather than “fatness.” In addition, given the relationship between BMI and body fat, this relationship may vary significantly by age, gender, and ethnic group (39).
Fewer studies have investigated heritability estimates for FM than BMI, and most of those used skinfold thickness to assess body FM, which may inaccurate in children (42). One study reported significant heritability (up to 50%) between children with their mothers and fathers for percentage body fat measured by DXA, in contrast to our study (10). Another recent study in 554 sibling pairs of women and men reported the heritability estimates of 0.71 for whole body FM after adjusting for age, gender, ethnicity, and height (11). A study of 57 monozygotic and 55 dizygotic female twins showed similar heritability estimates (65%) (43). Although these three studies, as well as our own, used DXA to assess body fat, careful consideration is required when interpreting their results. It is difficult to compare our results directly with these three studies, because of differences in the studies designs and statistical approaches, participants’ ages, and methods of heritability estimation. Many studies have indicated that heritability has a greater effect on FFM than on FM (43, 44) and reported heritability estimates of FFM from 0.56 to 0.84. Although we observed significant familial resemblance (h²=0.16) of FFM in this study, which was in agreement with these studies, it was lower than their estimates. It should be noted that unlike our study, those three studies were conducted in twins and had different designs (43, 44).
The study of 554 twin females reported that after adjustment for height, 60% of the variance in FFM was accounted for by genetic factors (11). Higher heritability of FFM in twin populations than in non-twin siblings appears normal. To our knowledge, only one paediatric study measured whole body FM by DXA to assess the heritability of FM (10). However, the children who participated in that study were pre-pubertal girls and therefore older (11 years) than the children in our study. Another study in 3-17 year old twins attributed 75% to 80% of the variation in the percentage of body fat measured by bio-impedance analysis to genetic influences (45). Taken together, the results of this part of our study suggest that the quantity of FFM in children traces the FFMs of their parents. The same occurs for FM and this heritability might be age-specific. A longitudinal study indicated that the genetic and environmental architecture of body size changes from birth to adulthood. The authors of this study observed age-linked genetic and environmental influences on growth even in monozygotic twins who share their genetic background (46).
Although the heritability estimates for total FM and FFM have been discussed in several adult studies (11, 43, 44), little has been published on the heritability of regional FFM and FM between adults and children (47). The role of fat distribution as a risk factor for some health problems such as cardiovascular disease in both adults and children encourages further studies in this field. Therefore, better understanding of the association between genetic and specific patterning of fat distribution may be useful in the management of obesity and related disorders. With regard to regional FM distribution, the study in 554 twin females reported a heritability estimate of 55% for trunk FM after adjustment for whole body FM (11). The significant correlations between pre-pubertal girls and their parents for regional FM and FFM have been reported previously (10). In that study higher adjusted r2 values were observed for regional FFM (0.17–0.33) than those for regional FM (around 0.10). One study in 119 monozygotic and 97 dizygotic post-menopausal twins suggested that up to 60% of variation in central body fat, measured by DXA, can be attributed to genetic factors independent of heritability of total fat (48). Due to interrelations between regional traits of FM and FFM, data for heritability of regional body composition must be interpreted with caution. In our study, after normalizing each region trait of FFM and FM for other traits, no significant associations were found between children and their parents for regional body composition except for abdominal and arms FFM, where there was a significant association between children and mid-parents and their mothers for abdominal FFM. Also, another association for regional body composition was observed between mid-parents and children for arms FFM, while no association was found between children and their parents separately. A possible explanation for such an association might be the assortative mating (49) for regional body composition, although this has not been previously documented. Although it is difficult to compare the results of regional body composition in this study directly, our finding suggested that total body composition is more heritable than regional body composition, at least in pre-school age children. Similar to our findings, estimated heritability derived from 327 Caucasian 5–75 year-olds from 102 nuclear families suggested that heritability of fatness is greater than that for fat distribution (50). Although this study had a larger sample size than our study, the authors did not use direct measurements of total and regional body. Despite the number of studies dealing with the heritability of body composition, few used precise methods such as DXA to measure total and regional FFM and FM. A previous study that measured regional body composition by DXA suggested that among regional body composition, the legs’ FFMs and trunks’ FMs of children were highly associated with both their mothers' and fathers' values (10). However, direct comparison of our results with this previous study must be made with caution, as this study did not detect the inter-relationship between regional measures of body composition, and it only included comparisons of girls with their parents. In addition, the children’s age in that study was higher (11 years) than in ours, which might be another explanation for the discrepancy between our results and theirs. Another recent family study also observed higher heritability for anthropometric indexes ranging from 0.24 to 0.75, whereas the h2 for the body-composition traits ranged from 0.18 to 0.35 (51).
Although RMR is the major component of daily energy expenditure in individuals (16, 52), information regarding its heritability is limited. However, several earlier studies in parent-child and monozygotic and dizygotic twins showed that RMR has a strong heritability and this heritability remained significant even after adjusting RMR for age, gender, and body composition (14, 35). A recent study in 12 monozygotic and 8 same-sex dizygotic twin pairs with a mean age of 25 years showed that genetic factors play a significant role in variation of sleeping metabolic rate (53). In a study assessing heritability of RMR (14-58 years old) in 31 pairs of parent-child, 21 pairs of dizygotic twins, and 37 pairs of monozygotic twins, the genetic effect ranged from 25-40% for parent-child and around 70% for dizygotic twins (35). This contrasts another study in 14 pairs of MZ twins and 12 pairs of DZ twins of adult age that found no significant association for RMR in dizygotic twins, although a high correlation (0.80-0.85) was seen in monozygotic twins (54). Despite the strong heritability of RMR in twins, little is known about the contribution of genetic factors to RMR in parent-child relationships. A study in a Nigerian population reported heritability of RMR around 0.30 after adjustment for body size. Also, the genome scan results of this study detected a linkage signal on chromosome16 for heritability of RMR (55). In contrast with previous published data, we found no association between parents and children for RMR either before or after adjusting for body composition. To our knowledge, this study is the first to investigate the familial resemblance of RMR in pre-school-age children. Therefore, it is difficult to compare our finding with other published studies. A possible explanation for this result is that the expression of genes affecting RMR may vary with age. Results from a young adult twin study indicated that genetic influences on RMR were entirely explained by body weight (56). Another study suggested that the gene(s) affecting each of FFM and FM also influence the RMR (9). However, our findings did not support this suggestion, at least in pre-school age, as we found a significant association between children and their parent for body composition but not RMR.
Several previous investigations reported that the variations in human PA might be determined by genetic factors (29, 50, 57, 58). Although nearly all of them emphasised the potential contribution of the shared family environment in this broad-sense heritability estimate, most applied subjective methods to measure PA. A recent study, which used an objective method to measure activity-induced energy expenditure in 38 dizygotic and 62 monozygotic twin pairs aged 4-10 years, showed that shared environmental factors were the main indicator for the familial resemblance in PA and they found no effect of genetic factors (1). Another recent study that investigated genetic influence on PA and activity-related energy expenditure (AEE) with objective measurements was performed in 12 monozygotic and eight same-sex dizygotic twin pairs aged 18-39 years. Physical activity was measured by accelerometry and AEE by a combination of respiration chamber and DLW. That study revealed that genetic factors explained a large part of the variation only in daily life, but not in the restricted respiration chamber (53). To quantify genetic determinants of PA level, PA has been previously assessed in a parents-children study by standardized interview. Participants were from 111 Mexican-American and 95 Anglo families. This study suggested that families moderately influence PA and mothers had a greater influence than families on their children’ PA levels (59). In addition, a study in 1,610 subjects from 375 families indicated significant genetic influences on PA measured by a three-day activity record, with genes estimated to account for 29% of variation in children’s activity (29). In both of these two studies PA was measured by subjective methods, which are unreliable. In contrast with most previous reports, no significant familial resemblance was found in this study for PA pattern except that morning PA was highly associated between children and their mothers and mid-parents. Using different study designs and different methods to assess PA in previous works makes the direct comparison of their findings difficult. In addition, in contrast with our results, a previous study in 100 children aged 4-7 years indicated a familial resemblance between activity levels of parents and their children. Physical activity was measured with the Caltrac accelerometer for around 9 days in children and 8 days in their parents. This study observed that active children had active mothers or fathers and the children of two active parents were 5.8 times more likely to be active than those with two inactive parents (15).
Another study assessing familial resemblance of PA with the Caltrac accelerometer found 70% familial association for fathers-children and 66% for mothers-children (60). Although the Quebec Family Study revealed that shared familial environmental and genetic factors were important for the familial resemblance in PA level, the mean ages of offspring and parents were 27 and 53 years, respectively (57). Due to the mean age of offspring in that study, which was much higher than in ours, it is difficult to compare the two studies. The influences of genetics and environmental factors on PA may vary depending on subjects’ ages.
Therefore, the age of subjects might be an explanation for frequently reported genetic predisposition of PA in adults (1). Association between mothers and children for the values of morning PA in our study may relate to similar wake-up times of mothers and children. However, no previous studies reported familial resemblance of different parts of the day, thus we could not compare our findings with others. Although we found no association between children and their parent resemblance for PA, the possible familial resemblance of PA is supported by several lines of evidence. Unlike other components of total energy expenditure, the variation of PA may be prone to confounding factors. Lack of familial resemblance for PA in our study may be explained partly by small variation of PA in our children, who spent half of their time in sedentary, and no time in vigorous, activity. In addition, young children are more highly influenced by other young children than older children or adults. For example, school or nursery environments might influence children’s PA more than those of their parents, as they spend most of their waking time in such places. Most children now attend nurseries (>90% in Scotland), which seems to be higher than past decades (40). This is a major social change, which has had the most impact on the family. Therefore, children spend most of their time in inactive environments (40) and have less shared environments with their parents than in the past. This may be an additional potential explanation why, in previous studies, familial resemblance of PA has frequently been reported. However, activity is important in preventing metabolic diseases and, because it appears to track from childhood to adulthood (61), encouraging children to be active is a necessary objective. Given the disappointing results of controlled prevention trials to date to reduce childhood obesity by PA intervention (41), there is a need for more research in this area by promoting PA at home, in the community, and at school.
Study design is one of the important approaches to distinguish genetic factors from shared environmental factors. Also, the extent to which these two main determinants of phenotype variation can be separated from each other might vary depending on the subjects’ ages. Therefore, estimated narrow-sense heritability for phenotype variation between children and their parents in our study may overestimate the genetic component.
In general, this study is one of few studies in pre-school children and one of the first to use direct measures of each parameter, which probably explains the different results from other studies. This study agrees with previous reports that found that that body composition is strongly genetically influenced. Discrepancy between the results of DXA and deuterium dilution in showing familial resemblance of total body composition revealed that variability between previous studies in heritability estimates might be due to different methods used to assess body composition. Our study revealed that total body composition was more heritable than regional body composition. In contrast with most previous studies we found no heritability for RMR and PA patterns between children and their parents. This may be a clue to other factors playing a role in these parameters, e.g. peer groups and environment on PA, hence more studies are needed to test this.
Acknowledgments
We thank all the children and their families who took part in the study. The authors' responsibilities were as follows—DMJ was the principal investigator; DMJ and JRS devised the study concept; KD and JS designed the study protocols; DMJ, KD, and JS collected the data; and DMJ, KD, and JRS interpreted and drafted the paper. All authors were involved in the data analysis.
References
- 1.Franks PW, Ravussin E, Hanson RL, Harper IT, Allison DB, Knowler WC, Tataranni PA, Salbe AD. Habitual physical activity in children: The role of genes and the environment. Am J Clin Nutr. 2005 Oct;82(4):901–8. doi: 10.1093/ajcn/82.4.901. [DOI] [PubMed] [Google Scholar]
- 2.Bell CG, Walley AJ, Froguel P. The genetics of human obesity. Nat Rev Genet. 2005 Mar;6(3):221–34. doi: 10.1038/nrg1556. [DOI] [PubMed] [Google Scholar]
- 3.Farooqi IS, O'Rahilly S. New advances in the genetics of early onset obesity. Int J Obes (Lond) 2005 Oct;29(10):1149–52. doi: 10.1038/sj.ijo.0803056. [DOI] [PubMed] [Google Scholar]
- 4.Cummings DE, Schwartz MW. Genetics and pathophysiology of human obesity. Annu Rev Med . 2003 Dec;54:453–71. doi: 10.1146/annurev.med.54.101601.152403. [DOI] [PubMed] [Google Scholar]
- 5.Allison DB, Kaprio J, Korkeila M, Koskenvuo M, Neale MC, Hayakawa K. The heritability of body mass index among an international sample of monozygotic twins reared apart. Int J Obes Relat Metab Disord. 1996 Jun;20(6):501–6. [PubMed] [Google Scholar]
- 6.Stunkard AJ, Harris JR, Pedersen NL, McClearn GE. The body mass index of twins who have been reared apart. N Engl J Med. 1990 May 24;322(21):1483–7. doi: 10.1056/NEJM199005243222102. [DOI] [PubMed] [Google Scholar]
- 7.Maes HHM, Neale MC, Eaves LJ. Genetic and environmental factors in relative body weight and human adiposity. Behav Genet. 1997 Jul;27(4):325–51. doi: 10.1023/a:1025635913927. [DOI] [PubMed] [Google Scholar]
- 8.Elks CE, den Hoed M, Zhao JH, Sharp SJ, Wareham NJ, Loos RJ, Ong KK. Variability in the Heritability of Body Mass Index: A Systematic Review and Meta-Regression. Front Endocrinol (Lausanne) 2012 Feb;3(29):1–16. doi: 10.3389/fendo.2012.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rice T, Daw E, Gagnon J, Bouchard C, Leon A, Skinner J, Wilmore J, Rao D. Familial resemblance for body composition measures: The HERITAGE family study. Obes Res. 1997 Nov;5(6):557–62. [PubMed] [Google Scholar]
- 10.Treuth MS, Butte NF, Ellis KJ, Martin LJ, Comuzzie AG. Familial resemblance of body composition in prepubertal girls and their biological parents. Am J Clin Nutr. 2001 Oct;74(4):529–33. doi: 10.1093/ajcn/74.4.529. [DOI] [PubMed] [Google Scholar]
- 11.Hsu F-C, Lenchik L, Nicklas BJ, Lohman K, Register TC, Mychaleckyj J, Langefeld CD, Freedman BI, Bowden DW, Carr JJ. Heritability of body composition measured by DXA in the diabetes heart study. Obes Res. 2005 Feb;13(2):312–9. doi: 10.1038/oby.2005.42. [DOI] [PubMed] [Google Scholar]
- 12.Griffiths M, Payne PR. Energy expenditure in small children of obese and non obese parents. Nature. 1976 Apr;260(5553):698–700. doi: 10.1038/260698a0. [DOI] [PubMed] [Google Scholar]
- 13.Goran MI, Carpenter WH, McGloin A, Johnson R, Hardin JM, Weinsier RL. Energy expenditure in children of lean and obese parents. Am J Physiol. 1995 May;268(5 Pt 1):E917–24. doi: 10.1152/ajpendo.1995.268.5.E917. [DOI] [PubMed] [Google Scholar]
- 14.Fontaine E, Savard R, Tremblay A, Despres JP, Poehlman E, Bouchard C. Resting metabolic rate in monozygotic and dizygotic twins. Acta Genet Med Gemellol (Roma) 1985;34(1-2):41–7. doi: 10.1017/s0001566000004906. [DOI] [PubMed] [Google Scholar]
- 15.Moore LL, Lombardi DA, White MJ, Campbell JL, Oliveria SA, Ellison RC. Influence of Parents Physical activity levels on activity levels of young children. J Pediatr. 1991 Feb;118(2):215–9. doi: 10.1016/s0022-3476(05)80485-8. [DOI] [PubMed] [Google Scholar]
- 16.Johnstone AM, Murison SD, Duncan JS, Rance KA, Speakman JR. Factors influencing variation in basal metabolic rate include fat free mass, fat mass, age, and circulating thyroxine but not sex, circulating leptin, or tri-iodothyronine. Am J Clin Nutr. 2005 Nov;82(5):941–8. doi: 10.1093/ajcn/82.5.941. [DOI] [PubMed] [Google Scholar]
- 17.Jackson D, Pace L, Speakman JR. The measurement of resting metabolic rate in young children: Reproducibility and comparison with predicted values. Obes Res. 2012 Dec;10(1):56–68. [Google Scholar]
- 18.Weir JBD. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949 Aug;109(1-2):1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tothill P, Avenell A, Love J, Reid DM. Comparisons between Hologic, Lunar and Norland dual energy X-ray absorptiometers and other techniques used for whole body soft tissue measurements. Eur J Clin Nutr. 1994 Nov;48(11):781–94. [PubMed] [Google Scholar]
- 20.Tothill P, Avenell A, Reid D. Precision and accuracy of measurements of whole body bone mineral: Comparisons between Hologic, Lunar and Norland dual energy X-ray absorptiometers. Br J Radiol. 1994 Dec;67(804):1210–7. doi: 10.1259/0007-1285-67-804-1210. [DOI] [PubMed] [Google Scholar]
- 21.Ellis K, Abrams S, Wong W. Body composition of a young, multiethnic female population. Am J Clin Nutr. 1997 Mar;65(3):724–31. doi: 10.1093/ajcn/65.3.724. [DOI] [PubMed] [Google Scholar]
- 22.Cole TJ, Freeman JV, Preece MA. Body mass index reference curves for the UK, 1990. Arch Dis Child. 1995 Jul;73(1):25–9. doi: 10.1136/adc.73.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freeman JV, Cole TJ, Chinn S, Jones PRM, White EM, Preece MA. Cross sectional stature and weight reference curves for the Uk 1990. Arch Dis Child. 1995 Jul;73(1):17–24. doi: 10.1136/adc.73.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pullicino E, Coward WA, Stubbs RJ, Elia M. Bedside and field methods for assessing body composition: Comparison with the deuterium dilution technique. Eur J Clin Nutr. 1990 Oct;44(10):753–62. [PubMed] [Google Scholar]
- 25.Fomon SJ, Haschke F, Ziegler EE, Nelson SE. Body composition of reference children from birth to age 10 years. Am J Clin Nutr. 1982 May;35(5 Suppl):1169–75. doi: 10.1093/ajcn/35.5.1169. [DOI] [PubMed] [Google Scholar]
- 26.Djafarian K, Hession M, Speakman JR, Jackson DM. Comparison of Activity Levels Measured by a Wrist Worn Accelerometer and Direct Observation in Young Children. Iran J Public Health. 2005;34(Sup):69–70. [Google Scholar]
- 27.Lynch MBW. Genetics and analysis of quantitative traits. 1st ed. Massachusetts: Sinauer Associates; 1998 Jan. [Google Scholar]
- 28.Heller R, Garrison RJ, Havlik RJ, Feinleib M, Padgett S. Family resemblances in height and relative weight in the framingham heart study. Int J Obes. 8(5):399–405. [PubMed] [Google Scholar]
- 29.Perusse L, Leblanc C, Bouchard C. Inter- generation transmission of physical fitness in the Canadian population. Can J Sport Sci. 1988 Mar;13(1):8–14. [PubMed] [Google Scholar]
- 30.Schousboe K, Willemsen G, Kyvik KO, Mortensen J, Boomsma DI, Cornes BK, et al. Sex differences in heritability of BMI: A comparative study of results from twin studies in eight countries. Twin Res. 2003 Oct;6(5):409–21. doi: 10.1375/136905203770326411. [DOI] [PubMed] [Google Scholar]
- 31.Austin MA, King MC BR, Hulley SB, Friedman GD. Risk factors for coronary heart disease in adult female twins. Genetic heritability and shared environmental influences. Am J Epidemiol. 1987 Feb;125(2):308–18. doi: 10.1093/oxfordjournals.aje.a114531. [DOI] [PubMed] [Google Scholar]
- 32.Sorensen TIA, Holst C, Stunkard AJ. Childhood body mass index, genetic and familial environmental influences assessed in a longitudinal adoption study. Int J Obes Relat Metab Disord. 1992 Sep;16(9):705–14. [PubMed] [Google Scholar]
- 33.Price RA, Cadoret RJ, Stunkard AJ, Troughton E. Genetic contributions to human fatness: An adoption study. Am J Psychiatry. 1987 Aug;144(8):1003–8. doi: 10.1176/ajp.144.8.1003. [DOI] [PubMed] [Google Scholar]
- 34.Llewellyn CH, Trzaskowski M, Plomin R, Wardle J. Finding the missing heritability in pediatric obesity: the contribution of genome-wide complex trait analysis. 2013 doi: 10.1038/ijo.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bouchard C, Tremblay A, Nadeau A, Despres JP, Theirault G, Boulay MR, et al. Genetic effect in resting and exercise metabolic rates. Metabolism. 1989 Apr;38(4):364–70. doi: 10.1016/0026-0495(89)90126-1. [DOI] [PubMed] [Google Scholar]
- 36.Khoury P, Morrison JA, Laskarzewski PM, Glueck CJ. Parent offspring and sibling body mass index associations during and after sharing of common household environments: The Princeton School District Family Study. Metabolism. 1983 Jan;32(1):82–9. doi: 10.1016/0026-0495(83)90161-0. [DOI] [PubMed] [Google Scholar]
- 37.Zonta LA, Jayakar SD, Bosisio M, Galante A, Pennetti V. Genetic analysis of human obesity in an Italian sample. Hum Hered. 1987;37(3):129–39. doi: 10.1159/000153690. [DOI] [PubMed] [Google Scholar]
- 38.Atwood L, Heard-Costa N, Fox C, Jaquish C, Cupples LA. Sex and age specific effects of chromosomal regions linked to body mass index in the Framingham Study. BMC Genet. 2006 Jan 26;7:7. doi: 10.1186/1471-2156-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gallagher D, Heymsfield SB, Heo M, Jebb SA, Murgatroyd PR, Sakamoto Y. Healthy percentage body fat ranges: An approach for developing guidelines based on body mass index. Am J Clin Nutr. 2000 Sep;72(3):694–701. doi: 10.1093/ajcn/72.3.694. [DOI] [PubMed] [Google Scholar]
- 40.Reilly JJ, Jackson DM, Montgomery C, Kelly LA, Slater C, Grant S, Paton JY. Total energy expenditure and physical activity in young Scottish children: Mixed longitudinal study. Lancet. 2004 Jan 17;363(9404):211–2. doi: 10.1016/s0140-6736(03)15331-7. [DOI] [PubMed] [Google Scholar]
- 41.Reilly JJ, Kelly L, Montgomery C, Williamson A, Fisher A, McColl JH, Lo Conte R, Paton JY, Grant S. Physical activity to prevent obesity in young children: Cluster randomised controlled trial. BMJ. 2006;333:1041–43. doi: 10.1136/bmj.38979.623773.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reilly JJ, Wilson J, Durnin J. Determination of body composition from skinfold thickness: A validation study. Arch Dis Child. 1995 Oct;73(4):305–10. doi: 10.1136/adc.73.4.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nguyen TV, Howard GM, Kelly PJ, Eisman JA. Bone mass, lean mass, and fat mass: Same genes or same environments? Am J Epidemiol. 1998;147(1):3–16. doi: 10.1093/oxfordjournals.aje.a009362. [DOI] [PubMed] [Google Scholar]
- 44.Seeman E, Hopper JL, Young NR, Formica C, Goss P, Tsalamandris C. Do genetic factors explain associations between muscle strength, lean mass, and bone density? A twin study. Am J Physiol. 1996 Feb;270(2 Pt 1):E320–7. doi: 10.1152/ajpendo.1996.270.2.E320. [DOI] [PubMed] [Google Scholar]
- 45.Faith MS, Pietrobelli A, Nunez C, Heo M, Heymsfield SB, Allison DB. Evidence for independent genetic influences on fat mass and body mass index in a pediatric twin sample. Pediatrics. 1999 Jul;104(1 Pt 1):61–7. doi: 10.1542/peds.104.1.61. [DOI] [PubMed] [Google Scholar]
- 46.Pietilainen KH, Kaprio J, Rasanen M, Rissanen A, Rose RJ. Genetic and environmental influences on the tracking of body size from birth to early adulthood. Obes Res. 2002 Sep;10(9):875–84. doi: 10.1038/oby.2002.120. [DOI] [PubMed] [Google Scholar]
- 47.Bogl LH, Latvala A, Kaprio J, Sovijärvi O, Rissanen A, Pietiläinen KH. An investigation into the relationship between soft tissue body composition and bone mineral density in a young adult twin sample. J Bone Miner Res. 2011 Jan;26(1):79–87. doi: 10.1002/jbmr.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Samaras K, Spector TD, Nguyen TV, Baan K, Campbell LV, Kelly PJ. Independent genetic factors determine the amount and distribution of fat in women after the menopause. J Clin Endocrinol Metab. 1997 Mar;82(3):781–5. doi: 10.1210/jcem.82.3.3803. [DOI] [PubMed] [Google Scholar]
- 49.Speakman JR, Djafarian K, Stewart J, Jackson DM. Assortative mating for obesity. Am J Clin Nutr. 2007 Aug;86(2):316–23. doi: 10.1093/ajcn/86.2.316. [DOI] [PubMed] [Google Scholar]
- 50.Katzmarzyk PT, Malina RM, Perusse L, Rice T, Province MA, Rao DC, Bouchard C. Familial resemblance in fatness and fat distribution. Am J Hum Biol. 2000 May;12(3):395–404. doi: 10.1002/(SICI)1520-6300(200005/06)12:3<395::AID-AJHB10>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 51.Butte NF, Cai G, Cole SA, Comuzzie AG. Viva la Familia Study: Genetic and environmental contributions to childhood obesity and its comorbidities in the Hispanic population. Am J Clin Nutr. 2006 Sep;84(3):646–54. doi: 10.1093/ajcn/84.3.646. quiz 673-4. [DOI] [PubMed] [Google Scholar]
- 52.Vermorel M, Lazzer S, Bitar A, Ribeyre J, Montaurier C, Fellmann N, Coudert J, Meyer M, Boirie Y. Contributing factors and variability of energy expenditure in non obese, obese, and post obese adolescents. Reprod Nutr Dev. 2005 Mar-Apr;45(2):129–42. doi: 10.1051/rnd:2005014. [DOI] [PubMed] [Google Scholar]
- 53.Joosen AM, Gielen M, Vlietinck R, Westerterp KR. Genetic analysis of physical activity in twins. Reprod Nutr Dev. 2005 Mar-Apr;45(2):129–42. doi: 10.1093/ajcn/82.6.1253. [DOI] [PubMed] [Google Scholar]
- 54.Henry CJK, Piggott SM, Rees DG, Priestley L, Sykes B. Basal metabolic rate in monozygotic and dizygotic twins. Eur J Clin Nutr. 1990 Oct;44(10):717–23. [PubMed] [Google Scholar]
- 55.Wu XD, Luke A, Cooper RS, Zhu XF, Kan DH, Tayo BO, Adeyemo A. A genome scan among Nigerians linking resting energy expenditure to chromosome 16. Obes Res. 2004 Apr;12(4):577–81. doi: 10.1038/oby.2004.66. [DOI] [PubMed] [Google Scholar]
- 56.Hewitt JK, Stunkard AJ, Carroll D, Sims J, Turner JR. A twin study approach towards understanding genetic contributions to body size and metabolic rate. Acta Genet Med Gemellol. 1991;40(2):133–46. doi: 10.1017/s0001566000002567. [DOI] [PubMed] [Google Scholar]
- 57.Simonen RL, Perusse L, Rankinen T, Rice T, Rao DC, Bouchard C. Familial aggregation of physical activity levels in the Quebec family study. Med Sci Sports Exerc. 2002 Jul;34(7):1137–42. doi: 10.1097/00005768-200207000-00014. [DOI] [PubMed] [Google Scholar]
- 58.Mustelin L, Joutsi J, Latvala A, Pietiläinen KH, Rissanen A, Kaprio J. Genetic influences on physical activity in young adults: a twin study. Med Sci Sports Exerc. 2012 Jul;44(7):1293–301. doi: 10.1249/MSS.0b013e3182479747. [DOI] [PubMed] [Google Scholar]
- 59.Sallis JF, Patterson TL, Buono MJ, Atkins CJ, Nader PR. Aggregation of physical activity habits in Mexican American and Anglo families. J Behav Med. 1988 Feb;11(1):31–41. doi: 10.1007/BF00846167. [DOI] [PubMed] [Google Scholar]
- 60.Freedson PS, Evenson S. Familial aggregation in physical activity. Res Q Exerc Sport. 1991 Dec;62(4):384–9. doi: 10.1080/02701367.1991.10607538. [DOI] [PubMed] [Google Scholar]
- 61.Tammelin T, Nayha S, Laitinen J, Rintamaki H, Jarvelin MR. Physical activity and social status in adolescence as predictors of physical inactivity in adulthood. Prev Med. 2003 Oct;37(4):375–81. doi: 10.1016/s0091-7435(03)00162-2. [DOI] [PubMed] [Google Scholar]