Abstract
Background:
Despite many efforts, the etiology of autism remains unknown. Food allergy has been suggested as a pathogenic factor in Autism Spectrum Disorder (ASD). Our aim in this study was to determine whether food allergy could be considered as a risk factor for autistic children.
Methods:
Thirty-nine autistic children were examined by the skin prick test (SPT), and total serum IgE was evaluated by ELISA. SPTs were performed for egg whites, oranges, peanuts, tomatoes, tuna fish, walnuts, aubergines, melons, grapes, and cow milk. Parents and teachers were then asked to exclude these items from the childrens’ diets for six months. After the treatment period, the autistic children who tested positive for food allergies were re-assessed by a standard questionnaire to obtain further information about their medical histories.
Results:
Three of the study’s 39 autistic children (7.7%) tested positive on the SPT. Total serum IgE levels were elevated in 56.4% of the subjects (mean=164±24.5, cut-off >155 IU/ml). The results showed a decreased mean in the childrens’ autistic behaviors on the Children Autism Rating Scale (CARS) after both eight weeks and six months; however, this decrease was not statistically significant.
Conclusion:
Food allergy may play a role in the pathophysiology of autism. We conclude that avoidance of certain foods benefits the behavior of autistic children.
Key Words: Autism, Food allergy, Skin prick test
Introduction
From the etiological and phonotypical aspects, autism is a heterogeneous disorder (1). It is influenced by genetic, environmental, and immunological factors, as well as increased inflammatory mediators (2). Unfortunately, despite many investigations conducted to explore the biologic basis of autism, its etiology is still far from understood.
Food allergy is considered one of the most important immune function abnormalities; it is thought to be caused by IgE-mediated reactions that occur via the cross-linking of food allergens to specific IgE molecules on the surface of mast cells and basophils, followed by degranulation of these cells so as to cause early- and late-phase reactions (3). Allergic patients commonly suffer from gastrointestinal and skin diseases. Symptoms include vomiting, diarrhea, abdominal pain, urticaria, atopic dermatitis, angioedema, asthma, rhinitis, and anaphylaxis in severe cases.
Food allergies are the most common causes of severe allergic reactions in children. Recently, some theories have proposed allergies to food and environmental antigens as etiological factors in autistic children (4). Gastrointestinal problems are important causes of morbidity among autistic children. These problems are often referred to as food allergies, even though recent studies of IgE-mediated food allergies were ambiguous and their results controversial. Moreover, some reports indicate beneficial effects of a gluten/casein regimen on behaviour and cognition in many autistic children (5-6).
The prevalence of both autism and allergies has increased in the last two decades. The signs and symptoms of autism and food allergies occur in the first few years of life. The most common time of involvement is during the first year with up to 5% of infants affected. The main onset of autism generally occurs before the age of three(7).
Interestingly, epidemiological studies have shown that the risk of ASD was elevated in the presence of maternal asthma and allergy during pregnancy (8). Recently, it was reported that the maternal allergy or asthma recorded during the second trimester of pregnancy was associated with a two-fold increased risk for ASD (8); moreover, during the past several decades, the prevalence of autism has increased more than ten-fold. The estimated prevalence of autism in approximately three children per 10,000 in 1970 increased dramatically to one per 152 in 2007, as reported by the CDC (9).
Another recent study reported that the levels of all Th2 cytokines were significantly higher in children with autism spectrum disorder than in matched controls; however, no evidence indicated a skewing toward either Th2 or Th1 (10). Elevated serum IgE (11) and eosinophils in peripheral blood(12) have also been reported in children with ASD and are further evidences of an allergic response.
One of the simplest immunotherapeutic strategies is allergen avoidance. Behavioral symptoms in autistic children may be improved by excluding certain foods from their diets. This is due to potential immunologic reactivities to food allergens that lead to intestinal lesions in these patients (5).
Approximately 76% of autistic children had at least one gastrointestinal problem vs. 30% in healthy children, and64% of autistic children had more than two GI symptoms. 18% of autistic children older than four were not toilet trained, vs. 2% of their siblings(13).
Libbey reported that mothers of individuals with autism possess antibodies that react with brain proteins. When these antibodies were passively transferred to pregnant non-human primates or rodents, the offspring exhibited behavioral and nervous system changes (14).
Research on autistic children with food allergies has been limited. There is little empirical evidence regarding the possible role of foods or additives causing behavioural disorders in children with autism. We hypothesize that allergen avoidance may lead to a decrease in certain behavioural disorders in autistic children with food allergies.
In this study, food allergies in autistic children were evaluated by the SPT. The children’s parents and teachers were then asked to exclude the items that caused the positive SPTs from the children's diets for six months. At the end of the treatment period, the autistic children with food allergies were re-evaluated using a standardized questionnaire that obtained information about their medical histories.
Materials and Methods
Subjects
In this case-control study we examined 25 boys and 14 girls with autism, with a mean age of 8.5±1.6. All patients were Iranian and enrolled in the Noor Hedayat Center of Autism Spectrum Disorders. Each child was assessed in the child psychiatry and neurology departments of Aria Hospital (Islamic Azad University of Mashhad). The Childhood Autism Rating Scale (CARS) was used for scoring. This is an observational test comprised of 15 items. A CARS score of 30–36.5 indicates mild to moderate,, and 37–60 indicates severe, autism (15). The Institutional Ethics Committee of Islamic Azad University approved the study, and a written informed parental consent was obtained for all patients before they entered the study.
To screen for allergic symptoms, a standard questionnaire currently used in the allergy department of the same hospital was completed by a physician. An allergist examined the questionnaire and rated allergic features from 0 to 3 (0: no suggestive symptoms in the family or patient, 1: symptoms in one or more family members, 2: symptoms in the patient, and 3: symptoms in both the family and patient). No autistic child had received immunosuppressive drugs in the six months before entry into the study, nor had any taken antihistamines within 10 days of the SPT.
Serum samples were collected and stored at −70 °C until assayed. Total serum IgE levels were measured using a commercially available ELISA Kit according to the manufacturer's instructions (Radim, PomeziaTerme, Italy). These were compared to previously-established normal values (cut off ≥155 IU/ml).
Skin and in vitro testing
The SPT was performed with ten common food allergens in Iran (Stallergene, France) using a lancet to puncture the skin. These included egg whites, oranges, peanuts, tomatoes, tuna fish, walnuts, aubergines, melons, grapes, and cow milk, and positive (histamine) and negative (saline-glycerine) controls. Reactions were evaluated 15-20 minutes post-puncture, with local wheal and flare responses indicating the presence of food-specific IgE antibodies. A mean wheal diameter of at least 3 mm greater than the negative control was considered positive.
Behavior summarized evaluation (BSE) observation scores (parental and teacher observations)
A single-blind design was used to obtain unbiased information from the parents and teachers. During the study, individual parent and teacher scores for each participant were obtained separately in questionnaire form in fifteen key behavioural areas (Table 1). Behavioural changes were scored using CARS. Such changes in three autistic children with positive SPTs were reported by the childrens’ psychiatrists and speech behaviour therapists. Autistic children without food allergies (negative control) were evaluated monthly, and this group’s mean CARS score after six months was not statistically significant.
Table 1.
Descriptive characteristics, and prevalence of gastrointestinal symptoms in children with autism.
| Children with autism ( n=39) | |
|---|---|
| Age | 8.54±1.68 |
| Gender (Boys/Girls) | 25/14 |
| Intensity Mild/Moderate Severe |
24 (61.5%) 15 (38.5%) |
| Atopy (on Parental questionnaire) bronchial asthma allergic rhinitis food allergy |
18 (46.1%) 1(3.3%) 12 (30.0%) 5 (13.3%) |
| Total IgE (IU/ml) | 164.5±152.8 |
| Skin Prick Test (Positive) | 3 (7.7%) |
| Gastrointestinal symptoms | |
| Diarrhea | 5(12.8%) |
| Constipation | 18(46.1%) |
| Gaseousness | 13(33.3%) |
| Bloating | 8(20.5%) |
| Abdominal pain | 0(0%) |
| Reflux | 5(12.8%) |
| Stool impaction | 10(25.6%) |
| Belching | 10(25.6%) |
| Number of gastrointestinal symptoms per child | |
| No symptom | 10(25.6%) |
| One symptom | 8(20.5%) |
| Two symptoms | 10(25.6%) |
| Three symptoms | 5(12.8%) |
| Four or more symptoms | 5(12.8%) |
Statistical analysis
Data concerning mean age, gender, family history, and clinical signs and symptoms of autistic children were also obtained and analyzed using SPSS Version 18.
Chi-square 2-way test and Student’s t-test were used to analyze qualitative and quantitative variables, respectively. For non-normal distributions, non-parametric procedures were applied. Linear models were used to perform multivariate analysis. AP value<0.05 was considered significant.
Results
Three of 39 (7.7%) SPTs were positive. Two of the three children who tested positive were reactive to peanuts and the other one to both oranges and egg whites. The characteristics of participants with and without reported histories of allergic disease are presented in Table 1.
Total serum IgE levels were elevated in 56.4% of all subjects (mean = 164±24.5; the cut-off was ≥155 IU/ml)). All patients with elevated serum IgE either had allergic symptoms or family members with allergic symptoms. In addition, the total serum IgE was elevated in the three SPT-positive children.
Based on the results of the questionnaires, 46.6% of the autistic children in the study's trial population had atopic allergies, with histories of bronchial asthma (3.3%), allergic rhinitis (30.0%), or food allergies (13.3%) (Table 1).
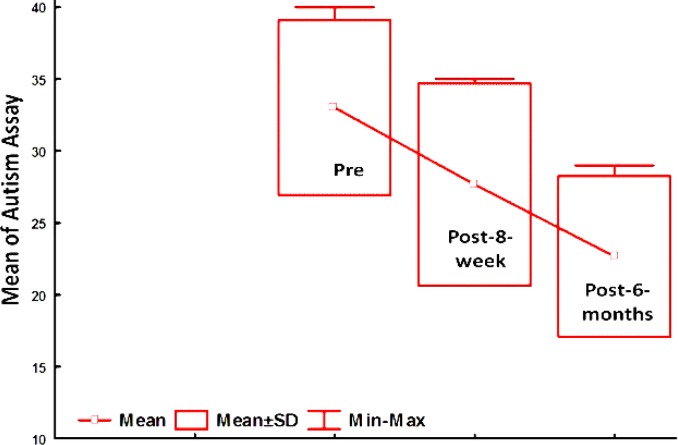
The mean score of the childrens’ autistic behaviors decreased on CARS after both eight weeks and six months; however, the differences were not statistically significant (Fig. 1).
Fig. 1.
The mean of childrens’ autistic behaviors on CARS criteria after both eight weeks and six months of avoidance to certain foods
Discussion
Recent reports indicate that the prevalence of food allergies in children with autism was 14.0% vs. 3.5% in children without autism (16). Also, the prevalence of respiratory allergies in children with autism was 26.4% vs. 14.9% in children without autism (16). The prevalence of positive SPT in our study was 7.7%; however, another study reported the prevalence of positive SPT in children with autism to be 14.9% vs. 9.8% in healthy and non-atopic controls matched for age, race, and gender.
Gupta et al. reported increased serum IgE levels in children with ASD(11). Our results confirmed this report. Although we did not determine food-specific IgE levels, total serum IgE was elevated in 56.4% of all subjects. Interestingly, all three children with IgE levels above the cut-off had clinical histories of atopy.
Because autism is a multi-factorial disorder, allergy cannot be the lone etiologic factor responsible for its increased prevalence in the past20 years. We believe that food allergies, as well as environmental factors, may play a role in the increased prevalence of autism. The “hygiene hypothesis” proposed that during the neonatal period and early infancy, the peak period of immune system sensitization, cleaner environmental conditions in westernized countries vs. developing countries play a role in the increased the prevalence of asthma and atopic disorders in western countries (17).
Based upon the above, we propose that improved hygiene should be considered as an etiologic factor of autism as well as asthma. Surprisingly, maternal asthma or allergy that occurs during the second trimester of pregnancy is associated with a two-fold increased risk for ASD (8).
The nervous and immune systems communicate with each other through multiple neuroanatomical and secretory routes, as well as via molecular mechanisms. Imbalance of the neuroimmune axis can lead to pathophysiological changes associated with gastrointestinal diseases such as food allergies.
Based on the results of this study, we suggest that food allergy is a probable risk factor in autistic children.
Crosstalk between the immune and the nervous systems may play a prominent role in the etiology of autism. Barbara et al. reported that mast cell mediators, including histamine and proteases, activate the sensitivity of neurons (18). In addition, arrays of released inflammatory mediators, such as histamine, serotonin, cytokines, and proteases, and newly-synthesized mediators, such as leukotrienes, thromboxanes, and prostaglandins, may interfere with the hyperactivity of functional neurons, leading to gut myoelectric activity alterations via effects on nerve endings. Furthermore, Ait-Belgnaoui et al. (19) demonstrated in animal models that stress increases intestinal permeability to macromolecules (19), and Casanova reported that leaky gut in humans is a possible culprit of neurologic stress-like migraine in autistic children via the same mechanism (20).
Here we report the results of a case-control study of evaluation of SPT in children with ASD. In this study, exclusion of certain foods resulted in positive effects on these childrens’ behaviors, but inherent methodological problems may affect interpretation of the results. First, parents and teachers were not blinded to the intervention, and this may have influenced their responses to the CARS criteria; thus, we suggest a double-blind study might give less biased results than those presented here.
In summary, we propose that food allergens may alter the behaviour of autistic children by deregulating immune system responses. The mechanism by which food allergens affect the nervous system is another important issue yet to be described. Thus, we believe that better understanding of cellular and molecular mechanisms between food allergies and other nervous system disorders such as autism will lead to new risk factor discoveries and therapeutic strategies.
Finally, we believe that food allergy plays an important role in the pathophysiology of autism because, in some cases, clinical hypersensitivity reactions resulted when certain foods were eaten by autistic children.This evidence suggests a role for neuroimmune mechanisms in the pathophysiology of autism.
It is not clear whether food allergies in children with autism actually play a role in the disease. It is our hope that the hypothesis proposed in this report will promote the study of the etiology of autism. Further research is needed to identify food allergens and determine how these could affect behavioural changes in autism.
Acknowledgements
This manuscript was prepared by six co-authors (M. R. Khakzad, M. Javanbakht, A. Soltanifar, M. Hojati, M. Delgosha, and M. Meshkat). Each co-author made substantial contributions to the paper’s conception and design, assisted in the analysis and interpretation of data, and provided critically important input in the creation of the final written manuscript.
References
- 1.Hughes JR. A review of recent reports on autism: 1000 studies published in 2007. Epilepsy Behav. 2008 Oct;13(3):425–37. doi: 10.1016/j.yebeh.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 2.Korvatska E, Van de Water J, Anders TF, Gershwin ME. Genetic and immunologic considerations in autism. Neurobiol Dis. 2002 Mar;9(2):107–25. doi: 10.1006/nbdi.2002.0479. [DOI] [PubMed] [Google Scholar]
- 3.Metcalfe DD. Immune mechanisms in food allergy. Clin Exp Allergy. 1991 Jan;21(Suppl 1):321–4. doi: 10.1111/j.1365-2222.1991.tb01748.x. [DOI] [PubMed] [Google Scholar]
- 4.Bakkaloglu B, Anlar B, Anlar FY, Oktem F, Pehlivanturk B, Unal F, et al. Atopic features in early childhood autism. Eur J Paediatr Neurol. 2008 Nov;12(6):476–9. doi: 10.1016/j.ejpn.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 5.Knivsberg AM, Reichelt KL, Hoien T, Nodland M. A randomised, controlled study of dietary intervention in autistic syndromes. Nutr Neurosci. 2002 Sep;5(4):251–61. doi: 10.1080/10284150290028945. [DOI] [PubMed] [Google Scholar]
- 6.Millward C, Ferriter M, Calver S, Connell-Jones G. Gluten- and casein-free diets for autistic spectrum disorder. Cochrane Database Syst Rev. 2004(2) doi: 10.1002/14651858.CD003498.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Lord C, Cook EH, Leventhal BL, Amaral DG. Autism spectrum disorders. Neuron. 2000 Nov;28(2):355–63. doi: 10.1016/s0896-6273(00)00115-x. [DOI] [PubMed] [Google Scholar]
- 8.Croen LA, Grether JK, Yoshida CK, Odouli R, Van de Water J. Maternal autoimmune diseases, asthma and allergies, and childhood autism spectrum disorders: a case-control study. Arch Pediatr Adolesc Med. 2005 Feb;159(2):151–7. doi: 10.1001/archpedi.159.2.151. [DOI] [PubMed] [Google Scholar]
- 9.Rice C. Prevalence of Autism Spectrum Disorders. 2007;SS01 [Google Scholar]
- 10.Molloy CA, Morrow AL, Meinzen-Derr J, Schleifer K, Dienger K, Manning-Courtney P, et al. Elevated cytokine levels in children with autism spectrum disorder. J Neuroimmunol. 2006 Mar;172(1-2):198–205. doi: 10.1016/j.jneuroim.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Gupta S, Aggarwal S, Heads C. Dysregulated immune system in children with autism: beneficial effects of intravenous immune globulin on autistic characteristics. J Autism Dev Disord. 1996 Aug;26(4):439–52. doi: 10.1007/BF02172828. [DOI] [PubMed] [Google Scholar]
- 12.Jyonouchi H, Sun S, Itokazu N. Innate immunity associated with inflammatory responses and cytokine production against common dietary proteins in patients with autism spectrum disorder. Neuropsychobiology. 2002;46(2):76–84. doi: 10.1159/000065416. [DOI] [PubMed] [Google Scholar]
- 13.Horvath K, Perman JA. Autistic disorder and gastrointestinal disease. Curr Opin Pediatr. 2002 Oct;14(5):583–7. doi: 10.1097/00008480-200210000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Libbey JE, Fujinami RS. Role for antibodies in altering behavior and movement. Autism Res. 2010 Aug;3(4):147–52. doi: 10.1002/aur.144. [DOI] [PubMed] [Google Scholar]
- 15.Schopler E, Reichler RJ, DeVellis RF, Daly K. Toward objective classification of childhood autism: Childhood Autism Rating Scale (CARS) J Autism Dev Disord. 1980 Mar;10(1):91–103. doi: 10.1007/BF02408436. [DOI] [PubMed] [Google Scholar]
- 16.Altarac M. Prevalence Of Allergies In Us Children With Autism. Annals of Epidemiology. 2008;8(9):708–41. [Google Scholar]
- 17.Martinez FD, Holt PG. Role of microbial burden in aetiology of allergy and asthma. Lancet. 1999 Sep;354(Suppl 2):SII12–5. doi: 10.1016/s0140-6736(99)90437-3. [DOI] [PubMed] [Google Scholar]
- 18.Barbara G, Wang B, Stanghellini V, de Giorgio R, Cremon C, Di Nardo G, et al. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology. 2007 Jan;132(1):26–37. doi: 10.1053/j.gastro.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 19.Ait-Belgnaoui A, Bradesi S, Fioramonti J, Theodorou V, Bueno L. Acute stress-induced hypersensitivity to colonic distension depends upon increase in paracellular permeability: role of myosin light chain kinase. Pain. 2005 Jan;113(1-2):141–7. doi: 10.1016/j.pain.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Casanova MF. The minicolumnopathy of autism: A link between migraine and gastrointestinal symptoms. Med Hypotheses. 2008;70(1):73–80. doi: 10.1016/j.mehy.2007.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]