Abstract
Background:
Holothuroids (sea cucumbers) are members of the phylum echinodermata, which produce saponins. Saponins exhibit a wide spectrum of pharmacological and biological activities. In this study, we isolated the crude saponins from the body wall of the dominant Iranian species of sea cucumber, Holothuria leucospilota (H. leucospilota). The purpose of this study was to confirm the presence of saponins in the Persian Gulf H. leucospilota and study the hemolytic and cytotoxic activities of these compounds.
Methods:
The body wall of sea cucumber was dried and powdered and the crude saponins were isolated using various solvents. The crude saponins were further purified by column chromatography using HP-20 resin. The foam test, Thin Layer Chromatography (TLC), hemolytic assay, and Fourier Transform Infrared Spectroscopy (FTIR) confirmed the presence of saponins. Cytotoxicity was analyzed using a 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay on A549 cells, a human lung cancer cell line.
Results:
The foam test, hemolytic assay, and TLC supported the presence of saponin compounds in the 80% ethanol fraction of H. leucospilota. The infrared (IR) spectrum of the extract showed hydroxyl (-OH), alkyl (C-H), ether (C-O) and ester (–C=O) absorption characteristic of teriterpenoid saponins. The C-O-C absorption indicated glycoside linkages to the sapogenins. The crude saponin extracted from sea cucumber was cytotoxic to A549 cells.
Conclusion:
The 80% ethanol fraction of saponin isolated from H. leucospilota exhibited hemolytic activity and offers promise as an anti-cancer candidate.
Key Words: Sea cucumber, Holothuria leucospilota, Saponin, Hemolytic assay, Cytotoxicity assay
Introduction
For many years natural products have played major roles in health and the prevention of disease. Since ancient times, a close relationship between natural products and medicine has existed (1). The marine environment, due to its physical and chemical characteristics, is a potential source of bioactive natural products (2). In the past decade, bioactive compounds have received much attention due to their pharmacological properties and therapeutic features (3). Many of these compounds are found in marine organisms. The sea cucumber is an important marine organism with commercial, pharmaceutical, and food value. Sea cucumbers have traditionally been used in China and Malaysia to treat hypertension, eczema, and cancer (4). The health benefits of these animals are associated with the presence of bioactive components such as saponins, glycosaminoglycans, sterols, cerberosides, peptides, sulfated polysaccharides, and essential fatty acids (3). Saponins are important bioactive compounds in sea cucumbers. Saponins are composed of triterpene or steroid aglycones (sapogenins) and sugar side chains, and because of their amphiphilic nature have the ability to generate stable foam and lyse blood cells (5). Numerous studies have shown that saponins have hemolytic (6), antiproliferative (7), antimicrobial (8), and antitumor (9) activities. Hu et al. (2010), showed that saponins isolated from the sea cucumber, Pearsonothuria graeffei can help to alleviate fatty liver (10). Tian et al. (2007), extracted a previously unknown sulfated saponin, philinopside E, from sea cucumber and investigated its role in angiogenesis and its effect on a breast cancer cell line (7). Many studies have investigated the cytotoxic effect of saponins extracted from sea cucumbers on different cell lines. The cytotoxic effects of five saponins: fuscocinerosides A, B, and C, pervicoside C, and holothurin A, extracted from Holothuria fuscocinerea Jaeger, on human leukemia HL-60 and human hepatoma BEL-7402 cells, were examined and the results demonstrated that all these saponins are strongly cytotoxic on both these cell lines (11). Despite numerous studies on the biological effects of secondary metabolites extracted from various species of sea cucumbers, there is a little information about the bioactive compounds isolated from sea cucumbers in Iran. The aim of this investigation was to extract saponins from the Persian Gulf sea cucumber H.leucospilota and evaluate their hemolytic and cytotoxic properties.
Materials and Methods
Sampling
Sea cucumbers )H. leucospilota( were collected from the coast of Qeshm, Bandar Abbas, Iran. After transfer to the laboratory, their body walls were isolated and stored at -70 °C for further analysis.
Chemicals and cells
HP-20 resin and 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT were purchased from Sigma-Aldrich (USA) Company. Thin-layer chromatography (TLC plates (silica-gel-60-F254), ethanol, n-butanol, dichloromethane, and acetic acid were purchased from Merck (Darmstadt, Germany). All cell culture reagents were obtained from Gibco (USA). A549 cells, a human lung cancer cell line, were purchased from the Pasteur Institute of Iran, Tehran.
Extraction of saponins from H.leucospilota
Saponins of H.leucospilota were extracted according to the method described by Hu et al (10). The body walls were air-dried, powdered by grinding, and stored in 70% ethanol at room temperature for two days to release the temperature-sensitive compounds. Then the saponins were refluxed in ethanol three times for six hours. In next step, obtained extract was filtered by watman paper 1 µm and evaporated on a rotary evaporator (Heidolph, Germany). The dry extract was diluted in dichloromethane/water for 24 hours. Then, the water phase was extracted using n-butanol. Finally, the organic layer was evaporated, dissolved in water, and loaded onto a Diaion HP-20 resin column. The column was washed with distilled water to remove inorganic salts and eluted, first with 80% and then 100% ethanol, to separate saponin compounds. The elutions were air-dried and lyophilized to obtain dried, crude saponin extracts (10).
Phytochemical screening
To analyze the n-butanol fraction for saponins, the foam test was used. For this purpose, 1 mg of extract was diluted in 10 ml of distilled water and shaken for 10 min.
Quantitative hemolytic activity assay
The hemolysis test was performed using the method described by Bondoc et al. (2013) (12). This test was performed using blood from an adult woman with O+ blood group. The blood was collected in tubes containing ethylenediaminetetraacetic acid as an anticoagulant and pelleted by centrifugation at 800 × g for 15 min to remove the plasma. The pellet containing erythrocytes was washed three times in phosphate-buffered saline (pH=7) (PBS) and suspended in PBS to a final concentration of 3% (12). Hemolysis assays were performed in triplicate in 96-well plates. For negative and positive controls we used PBS and standard saponin (Quillaja saponin), respectively. Initially, 100 µl of PBS were added to each well and then 100 µl of the various fractions were added. Finally, 100 µl of the 3% blood suspension were added to each well. After 4 hours of incubation at room temperature the absorbance of the samples were measured at 650 nm (13).
TLC analysis and qualitative detection
For TLC, aluminum plates coated with silica gel were used. Ten µl of the various fractions and standard saponin were loaded onto the TLC plates. The plates were placed in n-butanol:water:acetic acid (84:14:7) until the solvent migrated 6 cm from the source, and dried. To identify the saponin fractions, ethanol:sulfuric acid (90:10), blood reagent, and lieberman-burchard reagent were used. After drying, the plate was sprayed with a fresh solution of ethanol:sulfuric acid (90:10) and heated at 110 ºC for 10 min.
For the lieberman-burchard assay, after development and drying, the TLC plate was sprayed with a freshly-prepared solution of lieberman-burchard reagent and heated as above.
For detection by blood reagent, a 3% blood solution was prepared as described above. The blood suspension was added to a glass tray and the TLC plate was placed in the blood suspension for 20 seconds. The plates were removed from the tray and kept vertical for 30 sec. Finally, to remove excess blood, the plate was dipped in PBS for 30 sec and kept vertical for 30 min (14). To evaluate the results hRst was calculated using the formula below:
hRst = Distance of the sample from the origin/Distance of the solvent from the origin × 100.
Fourier Transform Infrared Spectroscopy (FTIR)
The 80% ethanol saponin fraction was compared to standard saponins using FTIR spectroscopy. For this purpose the dried 80% ethanol saponin fraction and standard saponin were powdered and analyzed as potassium bromide (KBr) pellets using a Perkin Elmer spectrometer (absorbance mode from 4000 to 400 cm-1) (13).
Evaluation of saponin toxicity
Hemolytic assay
The hemolytic activity of saponin was performed using a 2% concentration of human red blood cells. Saponin was diluted with distilled water at concentrations ranging from 125 to 1000 µg/ml. PBS was used as a negative control. The hemolytic effect was expressed as the concentration producing 50% of the maximum hemolysis (HD50) using a spectrophotometric process that measured the samples’ optical densities (OD’s).
Cytotoxicity assay
A549 cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. The cells were incubated at 37 °C with 5% CO2. The MTT assay was conducted to investigate the cytotoxic effect of saponins on A549 cells. The cells were seeded at a density of 1 x 105 cells/ml in 96-well plates and incubated for 24 h. After incubation, the cells were treated with various concentrations of the saponin (0.5-4 μg/ml) for 24, 48, and 72 hours. Then, the MTT assay was conducted and the percent of cell viability was estimated as follows: % of cell viability = (OD of treated cells/OD of control cells) × 100
Statistical analysis:
Statistical analyses were performed using SPSS 16 software. One-way ANOVA and least significant difference (LSD) were used to compare group means. All data were expressed as means ± standard deviations (SD) and P values < 0.05 were considered significant.
Results
Phytochemical analysis
Among the fractions obtained, only the fraction isolated using 80% ethanol produced stable foam that persisted for 30 min.
Quantitative hemolytic activity assay of sea cucumber extracts
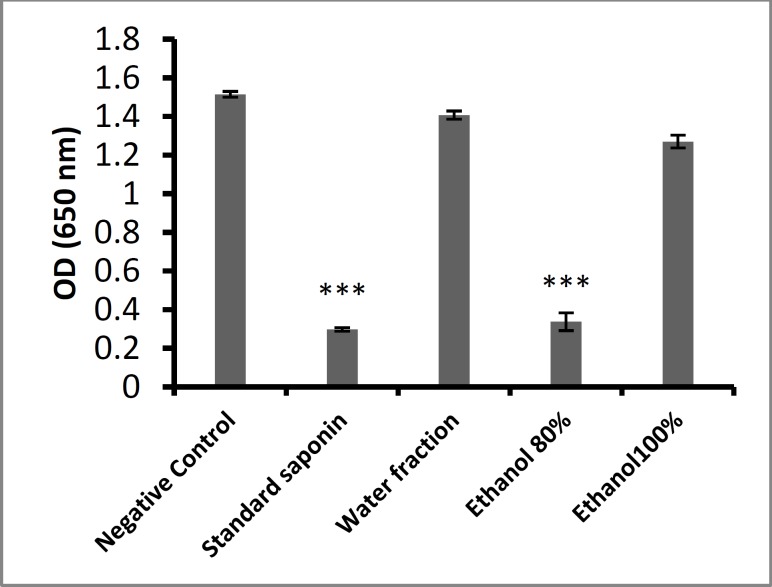
The hemolytic activity of the water, 80% ethanol, and 100% ethanol fractions from H. leucospilota and
standard saponin were evaluated using human red blood cells (RBCs) (Fig. 1). Hemolytic activity was observed only in the 80% ethanol fraction and standard saponin. The water and 100% ethanol fractions showed no hemolytic activity.
Fig. 1.
Hemolytic effect of H. leucospilota fractions. ***: P < 0.001Mean ± SD.
Thin Layer Chromatography (TLC) and qualitative detection
TLC was performed to verify the presence of saponins in extracted fractions. Ethanol:sulfuric acid (90:10), blood reagent, or lieberman-burchard reagent were sprayed onto the TLC plates. After development, intensive spots appeared on the TLC plates.
Liebermann-Burchard reagent
Saponins were detected in H. leucospilota extract by TLC, which exhibited a blue-green band with liebermann-burchard reagent spray, indicating the presence of a steroidal nucleus (Fig. 2).
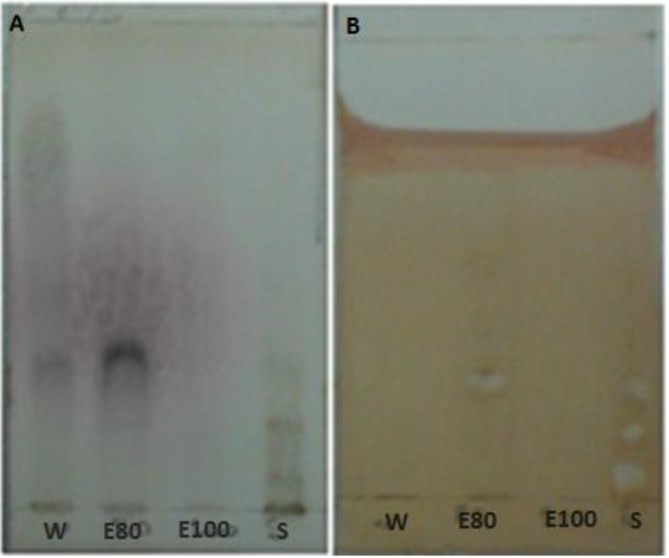
Fig. 2.

TLC of the 80% ethanol fraction extract from H. leucospilota and standard saponin with liebermann-burchard reagent. Right lane: standard saponin, Left lane: saponin extracted from H. leucospilota. Three bands are seen in the left lane (arrows). The lowest bond is related to saponin. The upper bands represent other steroidal compounds
Ethanol: sulfuric acid reagent
After ethanol-sulfuric acid was sprayed on the TLC plate, a spot appeared, indicating the presence of steroids, triterpenoids, and bile acids compounds (Fig. 3a).
Fig. 3.
TLC chromatogram of extracts from H. Leucospilota fractions: W: water fraction. E80: 80% ethanol fraction: E100: 100% ethanol fraction, S: standard saponin. Detection: (a) spraying with ethanol:sulfuric acid (90:10) and (b) blood solution
Erythrocytes reagent
Chromatograms of fractions from the extraction process and standard saponin with erythrocytes reagent is shown in Fig. 3b. The appearance of white spots in the chromatogram indicates hemolytic activity of saponin compounds. The hRst values were 32.35% for the 80% ethanol faction and 4.41, 16.17, and 30.88 for the three standard saponin spots.
Comparison of the chromatograms shows that the use of ethanol:sulfuric acid is not suitable to detect saponins due to the detection of non-saponin compounds.
Fourier Transform infrared spectroscopy (FTIR)
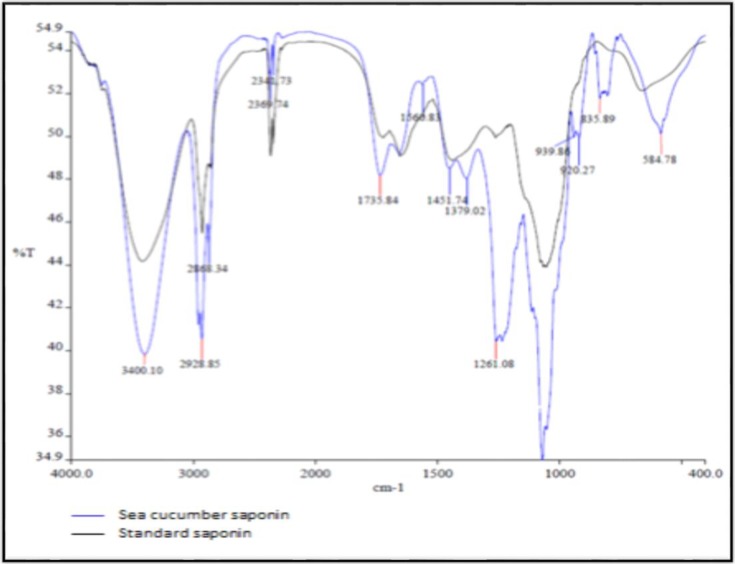
The FTIR spectrum fraction of 80% ethanol fraction of H.leucospilota showed some peaks (Fig. 4) and absorption between 4000 and 400 cm-1. The presence of the long sharp peak at 3400.10 cm-1 indicates the presence of hydroxyl groups (-OH), acidic groups are characterized by the presence of a broad peak between 3400 and 2400 cm-1 (2369.74 cm-1 for carboxylic acids), and the peak at 2928.85 cm-1 represents alkyl groups (C-H). Ether (C-O) and ester (–C=O) groups are characterized by the presence of sharp peaks between 1300 and 1000 cm-1. Oligosaccharide linkage absorption to sapogenins, that is C-O-C were apparent between 1054 to 1261.08 cm-1 The identification of hydroxyl, alkyl, and ether and ester groups in the FTIR spectrum from the 80% ethanol fraction indicate the presence of saponin.
Fig. 4.
FTIR spectral data of saponins in the 400-4000 cm-1 region. The absorption spectrum of standard saponin (Quillaja saponin) is shown in black and the absorption spectrum of the 80% ethanol fraction from H.leucospilota is shown in blue
Table 1.
The HD50 of the saponin extract was determined to be about 0.5 mg/ml on human RBC’s.
| Groups | Absorbance (Optical density) |
|---|---|
| Negative control (PBS) | 1.514±0.014 |
| Positive control (Standard saponin) | 0.297±0.009 |
| 1000 µg/ml | 0.025±0.021 |
| 750 µg/ml | 0.100±0.040 |
| 500 µg/ml | 0.490±0.008 |
| 250 µg/ml | 1.002±0.020 |
| 125 µg/ml | 1.310±0.032 |
Cytotoxic effect of saponin on A549 cells
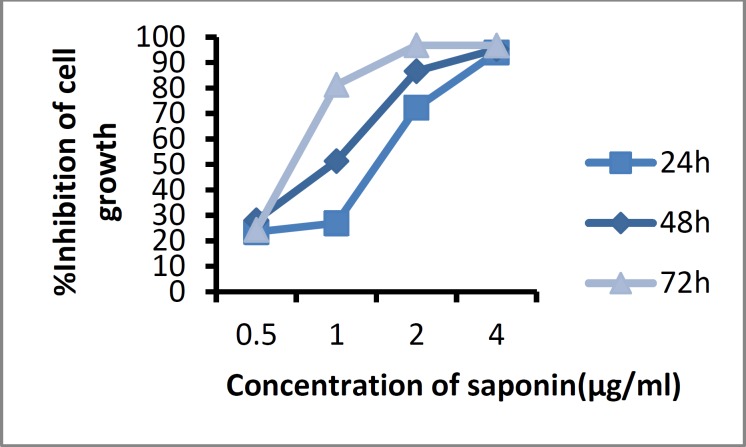
The cytotoxic effect of saponin on A549 cells was analyzed at concentrations of 0.5, 1, 2, and 4 μg/ml by the MTT assay after 24, 48, and 72 hours as shown in Fig. 5. The saponin extract inhibited cell proliferation in a dose and time -dependent manner. The half-maximal inhibitory concentration (IC50) was observed at a concentration of 1 μg/ mL after 48 hours of treatment and the inhibitory effect increased with increasing saponin concentrations.
Fig. 5.
Growth-inhibitory effect of saponin isolated from H. leucospilota on A549 cells at 24, 48, and 72 h.
Discussion
Echinoderms belong to a phylum of marine invertebrates that includes about 6000 living species divided into five classes: Crinoidea, Holoturoidea, Echinoidea, Asteroidea, and Ophiuroidea. Compounds from these organisms have been shown to have antibacterial, antifungal, antiviral, antitumor, anti-coagulant, cytotoxic, hemolytic, and antithrombotic effects (13). Currently, 187 species of sea cucumbers have been described. The presence of bioactive compounds with therapeutic properties in sea cucumbers has made them an attractive source of these compounds. Some important compounds include triterpene glycosides (saponins), chondroitin sulfates, glycosaminoglycans (GAGs), polysaccharides, phenolics, and essential fatty acids (3). Saponins are the main bioactive compounds that exhibit a wide range of biological activities and have many therapeutic effects (15). Saponins vary in the amount of sapogenin and in the lengths, linkages, and substituents of their sugars (14). These compounds have been studied in many sea cucumber species. For example, Silchenko et al. (2008) identified saponins in Mediterranean, North Atlantic, and North Pacific sea cucumber species (16). To the best of our knowledge, saponins from Iranian sea cucumber species have not yet been characterized.
In the present investigation, crude saponin isolated from H.leucospilota was detected by rapid and simple methods. Due to the sensitivity of bioactive compounds in marine organisms, the choice of solvents and extraction methods are critical for evaluation of these compounds’ biological activities. Silchenko et al. (2008) and Avilov et al. (2008) suggested the use of the alcoholic solvents ethanol or methanol for the extraction of bioactive compounds from marine organisms. Alcoholic solvents allow separation based on polarity (16, 17). In this study, the crude saponins were isolated from H. leucospilota with organic solvents and an HP-20 resin column was used for further purification. After extraction, the water and 80 and 100% ethanol fractions were examined for the presence of saponins. The foam test, a hemolysis assay and the liebermann-burchard test were performed and saponins were identified in the 80% ethanol fraction. This fraction was then subjected to FTIR spectroscopy. The biological activity of crude extracts was evaluated using the MTT assay.
Foam formation and hemolysis tests are common methods to confirm the presence of saponin compounds. Saponins are generally characterized by their ability to make foam (18). The ability to lyse RBC’s is one of the most important characteristics of saponins; therefore, hemolytic assay are also used to detect saponins. A simple method to confirm the presence of saponins was introduced Sharma et al. (2012), which combined TLC and hemolysis (14). Inalegwu and Sodipo (2013) reported the hemolytic activity of crude and purified saponin extracts from Tephrosia vogelii leaves (6). Silveira et al. (2011) compared the hemolytic and cytotoxic effects of Quillaja brasiliensis (Q. brasiliensis) saponins and Quil A saponin and showed that the Quil A is more toxic than Q. brasiliensis on VERO cells. Values of HD50 for Quil A and Q. brasiliensis were 52.2 and 125.2 μg/mL, respectively (19). Haddad et al. (2012) extracted two previously unknown triterpene saponins, magnosides A and magnosides B5, from Cybianthus magnus (Mez) Pipoly roots. The hemolytic activities of the two saponins were 3.8 and 33.5 μM, respectively (20).
Our results indicate that saponins isolated from H. leucospilota have moderate hemolytic effects on human blood cells with an HD50 of 0.5 mg/ml. The lieberman-burchard test is a rapid and simple method used to confirm the presence of saponin compounds (21). FTIR was used to identify saponins isolated from Moringa oleifera ( Moringaceae) pods (22) and steroidal saponin from Agave attenuata (23). The FTIR spectrum exhibited sharp peaks at 3400.10 (-OH), 2928.85 (C-H), and 1054 (C-O) cm-1, confirming the presence of saponin compounds.
To date, several studies have analyzed the cytotoxic effects of saponins. Khanna et al. (2009) isolated saponins of gymnemagenol from Gymnema sylvestre and dasyscyphin C from Eclipta prostrata leaves and demonstrated their cytotoxic activity on HeLa cells (24). Waheed et al. (2012) studied the effect of the saponin glycoside on HepG2, MDA-MB-468, and Caco-2 cells and indicated that the IC50 values of the saponin glycoside were 12.5 μM on MDA-MB-468 and Caco-2 cells and 100 μM on MCF-7 cells. Also in this study, it was shown that this compound could lyse sheep red blood cells at concentrations greater than100 mM (25). Harinantenaina et al. (2012) found that two saponins of Tarenna grevei have moderate antiproliferative effects on A2780 ovarian cancer cells, with IC50 of 7.6 and 4 μM (26). He et al. (2012) showed that saponins isolated from the roots of Panax notoginseng are cytotoxic to LoVo and Caco-2 human colon cancer cells (27). In our study, the saponin extracted from sea cucumber was cytotoxic to A549 cells in a direct dose and time-dependent manner. Our data confirm the presence of saponins in the sea cucumber species. Evaluation of biological activity of saponins isolated from H.leucospilota demonstrates that these compounds have potent cytotoxic effects on lung cancer cells and may be useful as anti-cancer agents.
Acknowledgment
This work was performed in the Animal Development Applied Biology Research Center. The authors thank the Pharmacology faculty, Ferdowsi University of Mashhad.
References
- 1.Butler MS. The role of natural product chemistry in drug discovery. J Nat Prod. 2004 Nov;67(12):2141–53. doi: 10.1021/np040106y. [DOI] [PubMed] [Google Scholar]
- 2.Sarfaraj HM, Sheeba F, Saba A, Mohd S. Marine natural products: A lead for Anti-cancer. Indian J Mar Sci. 2012 Feb;41:27–39. [Google Scholar]
- 3.Bordbar S, Anwar F, Saari N. High-value components and bioactives from sea cucumbers for functional foods—a review. Mar Drugs. 2011 Oct;9(10):1761–805. doi: 10.3390/md9101761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soltani M, Baharara J. Antioxidant and antiprolifereative capacity of dichloromethane extract of Holoturia leucospilota sea cucumber. IJCMB. 2014;2014:1–9. [Google Scholar]
- 5.Moghimipour E, Kooshapour H, Rezaee S, Khalili S, Handali S. In vitro cholestrol binding affinity of total saponin extracted from Glycyrrhiza glabra. Asian J Pharm Clin Res. 2014 Nov;7(1) [Google Scholar]
- 6.Inalegwu B, Sodipo O. Phytochemical screening and haemolytic activities of crude and purified saponins of aqueous and methanolic extracts of leaves of Tephrosia vogelii Hook F. Asian J Plant Sci Res. 2013;3(5):7–11. [Google Scholar]
- 7.Tian F, Zhang X, Tong Y, Yi Y, Zhang S, Li L, et al. Research Paper PE, a New Sulfated Saponin from Sea Cucumber, Exhibits Anti-Angiogenic and Anti-Tumor Activities In Vitro and In Vivo. Cancer Biol Ther. 2005 Aug;4(8):874–82. doi: 10.4161/cbt.4.8.1917. [DOI] [PubMed] [Google Scholar]
- 8.Adibpour N, Nasr F, Nematpour F, Shakouri A, Ameri A. Antibacterial and Antifungal Activity of Holothuria leucospilota Isolated From Persian Gulf and Oman Sea. Jundishapur J Microbiol. 2014 Jan;7(1) doi: 10.5812/jjm.8708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moghimipour E, Sadaghi-nejad B, Handali S, Ameri A, Ramezani Z, Azemi ME. In vitro screening of anti-candida activity of saponins extracted from Glycyrrhiza glabra and Quillaja saponaria. Asian J Pharm Clin Res. 2014 Jun;7(1) [Google Scholar]
- 10.Hu X-Q, Wang Y-M, Wang J-F, Xue Y, Li Z-J, Nagao K, et al. Dietary saponins of sea cucumber alleviate orotic acid-induced fatty liver in rats via PPARa and SREBP-1c signaling. Lipids Health Dis. 2010 Mar;9 doi: 10.1186/1476-511X-9-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y-X, Himaya S, Kim S-K. Triterpenoids of Marine Origin as Anti-Cancer Agents. Molecules. 2013 Jul;18(7):7886–909. doi: 10.3390/molecules18077886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bondoc KGV, Lee H, Cruz LJ, Lebrilla CB, Juinio-Meñez MA. Chemical fingerprinting and phylogenetic mapping of saponin congeners from three tropical holothurian sea cucumbers. Comp Biochem Physiol B. 2013 Sep;166(3):182–93. doi: 10.1016/j.cbpb.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Prabhu K, Bragadeeswaran S. Biological properties of brittle star Ophiocnemis marmorata collected from Parangipettai, Southeast coast of India. J Microbiol Antimicrob. 2013 Oct;5(10):110–8. [Google Scholar]
- 14.Sharma OP, Kumar N, Singh B, Bhat TK. An improved method for thin layer chromatographic analysis of saponins. Food Chem. 2012 May;132(1):671–4. doi: 10.1016/j.foodchem.2011.10.069. [DOI] [PubMed] [Google Scholar]
- 15.Sottorff I, Aballay A, Hernández V, Roa L, Muñoz LX, Silva M, et al. Characterization of bioactive molecules isolated from sea cucumber Athyonidium chilensis. Rev biol mar oceanogr. 2013 Apr;48(1):23–35. [Google Scholar]
- 16.Silchenko AS, Avilov SA, Kalinin VI, Kalinovsky AI, Dmitrenok PS, Fedorov SN, et al. Constituents of the Sea Cucumber Cucumaria okhotensis Structures of Okhotosides B1–B3 and Cytotoxic Activities of Some Glycosides from this Species⊥. J Nat Prod. 2008 Feb;71(3):351–6. doi: 10.1021/np0705413. [DOI] [PubMed] [Google Scholar]
- 17.Avilov SA, Silchenko AS, Antonov AS, Kalinin VI, Kalinovsky AI, Smirnov AV, et al. Synaptosides A and A1, triterpene glycosides from the sea cucumber Synapta maculata containing 3-O-methylglucuronic acid and their cytotoxic activity against tumor cells. J Nat Prod. 2008 Feb;71(4):525–31. doi: 10.1021/np070283+. [DOI] [PubMed] [Google Scholar]
- 18.Das T, Banerjee D, Chakraborty D, Pakhira M, Shrivastava B, Kuhad R. Saponin: role in animal system. Vet World. 2012;5(4):248–54. [Google Scholar]
- 19.Silveira F, Cibulski S, Varela A, Marqués J, Chabalgoity A, De Costa F, et al. Quillaja brasiliensis saponins are less toxic than Quil A and have similar properties when used as an adjuvant for a viral antigen preparation. Vaccine. 2011 Oct;29(49):9177–82. doi: 10.1016/j.vaccine.2011.09.137. [DOI] [PubMed] [Google Scholar]
- 20.Haddad M, Lelamer A, Banuls LMY, Carraz M, Vasquez P, Vaisberg A, et al. In vitro growth inhibitory effects of 13, 28-epoxyoleanane triterpene saponins in cancer cells. Planta medica. 2012 Feb;78(11) [Google Scholar]
- 21.Olugbenga AM, Musa AE, Oluwatoyin AH. Toxicological activity of crude saponin extract of Ficus platyphylla. Asian J Pharm Clin Res. 2012 Sep;5(1):30–3. [Google Scholar]
- 22.Sharma V, Paliwal R. Isolation and characterization of saponins from Moringa oleifera (moringaceae) pods. Int J Pharm Pharm Sci. 2013 Jan;5(1):179–83. [Google Scholar]
- 23.da Silva BP, de Sousa AC, Silva GM, Mendes TP, Parente JP. A new bioactive steroidal saponin from Agave attenuata. Z Naturforsch. 2002 Feb;57(5/6):423–8. doi: 10.1515/znc-2002-5-603. [DOI] [PubMed] [Google Scholar]
- 24.Khanna VG, Kannabiran K. Anticancer-cytotoxic activity of saponins isolated from the leaves of Gymnema sylvestre and Eclipta prostrata on HeLa cells. Int J Green Pharm. 2009 Jul;3(3) doi: 10.4103/0253-7613.48891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waheed A, Barker J, Barton SJ, Owen CP, Ahmed S, Carew MA. A novel steroidal saponin glycoside from Fagonia indica induces cell-selective apoptosis or necrosis in cancer cells. Eur J Pharm Sci. 2012 Jul;47(2):464–73. doi: 10.1016/j.ejps.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 26.Harinantenaina L, Brodie PJ, Callmander MW, Razafitsalama LJ, Rasamison VE, Rakotobe E, et al. Two antiproliferative saponins of Tarenna grevei from the Madagascar dry forest [] Nat Prod Commun. 2012 Jun;7(6) [PMC free article] [PubMed] [Google Scholar]
- 27.He N-W, Zhao Y, Guo L, Shang J, Yang X-B. Antioxidant, antiproliferative, and pro-apoptotic activities of a saponin extract derived from the roots of Panax notoginseng (Burk) FH Chen. J Med Food. 2012 Feb;15(4):350–9. doi: 10.1089/jmf.2011.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]