Abstract
Background:
The rapid increase of HIV-1 strains resistant to current antiretroviral drugs is a challenge for successful AIDS therapy. This necessitates the development of novel drugs, and to this end, availability of screening systems for in vitro drug discovery is a priority. Herein, we report the modification of a previously developed system for increased sensitivity, ease of use, and cost-efficiency, based on the application of the EGFP marker.
Methods:
A PCR-amplified gfp gene (gfp) was cloned into pmzNL4-3, the plasmid already designed to produce single-cycle replicable virions, in frame with the reverse-transcriptase gene to construct the pmzNL4-3/GFP plasmid. GFP-mzNL4-3 pseudo-typed virions, as the first progeny viruses, were recovered from the culture supernatant of HEK293T cells co-transfected with pmzNL4-3/GFP and the helper plasmids pSPAX2 and pMD2G, which respectively encode HIV-1 Gag-Pol and vesicular stomatitis virus glycoprotein. Single-cycle replication and virion production were assessed by syncytia formation, p24 antigen assays, and electron and fluorescence microscopy.
Results:
The incorporation of EGFP into the viral particles allowed their quantification by fluorometry, flow-cytometry, and fluorescence microscopy; however, this modification did not affect the single-round infectivity or production rate of the GFP fluorescence-emitting virions.
Conclusions:
Our results certify the development of a rapid, inexpensive, and safe GFP-reporting single-cycle replicable system for anti-HIV drug discovery. Further experiments are needed to measure the validity and robustness of the assay.
Key Words: HIV-1, Drug discovery, EGFP, Single-round infection, Fluorometry
Introduction
Human immunodeficiency virus (HIV), the undisputed champion of persistent viral replication, causes acquired immunodeficiency syndrome (AIDS) and is a major threat to global health (1). Antiretroviral (ARV) drug therapy has been much more successful than any other HIV treatments and made HIV infection manageable; however, use of these drugs is limited due to their severe toxicities (2), drug resistance development (3), and more worryingly, the reality that some newly HIV-infected patients carry viruses that are already resistant to the currently approved ARV treatments (4-5). When the high price of antiretroviral therapy, especially in the case of newly approved drugs, is added to the aforementioned limitations, the search for natural compounds with potential antiretroviral effects becomes increasingly important.
To this end, the availability of safe and valid tools for ARV drug discovery is essential. In vitro assessment has been the routine approach for the discovery of antiviral compounds, which is typically accomplished by measuring infection in the presence of increasing concentrations of candidate compounds either in conventional multiround infectivity or newer single-cycle replication assays (6).
In multiround assays, replication-competent viruses are cultured on cell lines or mitogen-activated peripheral blood mononuclear cells (PBMCs) isolated from healthy individuals for days to weeks in the presence of antiretroviral compounds, and finally the amount of produced viruses is determined by quantification of viral antigens, such as p24 or reverse transcriptase (7-8). These assays measure cumulative infection outcomes in the presence of candidate compounds rather than their instantaneous inhibitory effects and hence, may over or under -estimate the antiviral effect (9). Overall, the major disadvantage of multiround infection assays is that they fail to detect low but clinically significant antiviral activity, and further may over or under -estimate a drug’s effect (6).
Single-round infection assays usually rely on initial production of genetically manipulated pseudo-virions, which are capable of infecting a wide range of permissive cells, in readily transfectable cell lines, such as HEK293T cells (10-12). Because of the defective genes in the exploited vectors, pseudotyped viral particles generated in such systems can replicate only for a single cycle. This feature is the most significant advantage of the assay as it allows the measurement of immediate inhibition by the candidate compound rather than cumulative effects in long-term cultures (6). Furthermore, time-saving, higher reproducibility, and greater biological safety are other advantages of single-round compared with multiround infection assays. If TCD4+ cells are used as the permissive cells, then the assay also has the advantage of being exploited to measure the antiviral activities of compounds with potential HIV entry-inhibitory effects.
The single-round assays have also the potential to be used in high-throughput fashion in 96-well plates (11), which is the ideal format for scaled-up drug screening. Assay readout in single-round infection systems may be performed based on the measurement of viral antigens (13). Also, due to gene manipulations in vector-producing plasmids, viral particles may be quantified based on the signals obtained from reporter gene products, such as chloramphenicol acetyl transferase (CAT) (14), luciferase (11, 15-16), green-fluorescent protein (GFP) (12), beta-galactosidase (β-Gal) (15), secreted alkaline phosphatase (SEAP) (17), or other markers incorporated into the viral particles. Accordingly, each reporting system has its own benefits and drawbacks.
In our previous study, we reported the development of SCR HIV-1 virions (13) with the potential to be used in single-round infection assays in HIV-1 drug discovery studies (18-19). Herein, we report the modification of this system by incorporating enhanced green fluorescent protein (EGFP) into the SCR virions and increasing the readout sensitivity of the assay from the population level to both population and single cell levels.
Materials and Methods
Single-cycle replicable HIV-1 virions
Single-cycle replicable HIV-1 virions were previously designed and constructed by introducing a BalI restriction enzyme-directed 2-kb deletion spanning the pol region containing reverse transcriptase and part of integrase (RT-INT) of the HIV-1 genome from the NL4-3 strain. Co-transfection of HEK293T cells with plasmids pmzNL4-3 containing the mutated genome, psPAX2, and pMD2G resulted in the production of G-mzNL4-3 HIV-1 virions that were able to replicate for one cycle (13 , 20).
Green Fluorescent Protein (GFP) gene amplification
An enhanced gfp gene (gfp) encoding a red-shifted variant of wild-type GFP (21-23) was PCR-amplified from plasmid pEGFP-N1 (Clontech, USA, GenBank Accession #U55762) as the initial source in three sequential PCR’s using Pfu DNA polymerase and the F1/R1, F2/R2, and F2/R3 primer pairs shown in Table 1. In each amplification reaction, the previous PCR product was used as the template. This approach led to the creation of several restriction enzyme sites, including BalI on both sides of gfp. Furthermore, the reverse primers were designed to add the nucleotide sequence corresponding to the “KVLFLDG” motif, which is the HIV-1 protease cleavage site at the RT/INT junction (24), to the 3’-end of the amplified fragment (Fig. 1B).
Table 1.
Sequences of primers used for gfp gene amplification
| Primer name | Primer sequence (5’-3’) | |
|---|---|---|
| Forward | F1 | AATCGATGTACCGCGGGCCCGG |
| F2 | ATATGGCCAATCGATGTACCGCGG | |
| Reverse | R1 | TCTAGAGTCGCGGCCGCTCTTGTACAG |
| R2 | GGAACAGCACCTTCCTTCTAGAGTCGCG | |
| R3 | ATATGGCCAGCCGTCCAGGAACAGCACCTTC | |
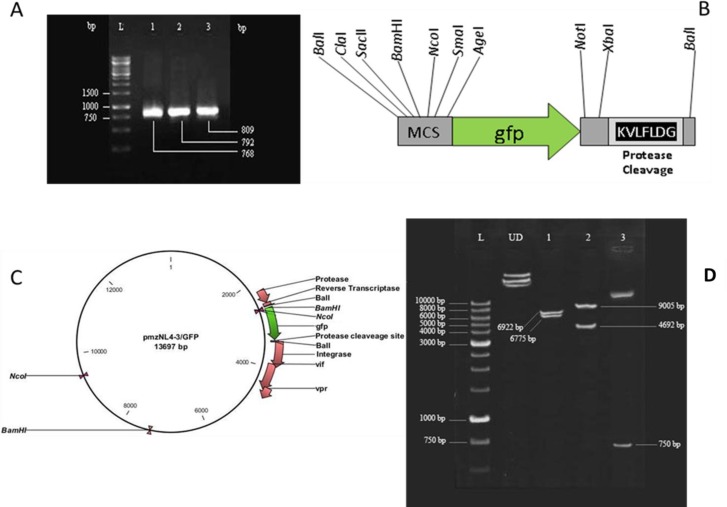
Fig. 1.
. pmzNL4-3/GFP plasmid construction and analysis. Agarose gel electrophoresis of three PCR products resulting from sequential amplification of gfp gene that led to the construction of the 809 bp final product (A). The scheme of the amplified gfp fragment in addition to the restriction enzyme recognition and protease cleavage sites created on its ends (B). pmzNL4-3/GFP plasmid map indicating BalI-mediated cloning of gfp in frame of reverse transcriptase-integrase fragments. Genes encoding protease and accessory proteins of HIV-1 (vif, vpr) in addition to the recognition sites of BamHI and NcoI enzymes are shown (C). Restriction enzyme analysis of pmzNL4-3/GFP plasmid with NcoI (lane 1), BamHI (lane 2) and SmaI/XbaI (lane 3) resulted in the creation of bands with expected sizes. UD and L refer to the undigested plasmid and DNA ladder, respectively (D).
The thermal program for all PCR amplifications consisted of 4 min of denaturation at 94 °C, 30 cycles of amplification (including 1 min at 94 °C, 30s at 55 °C, 1.5 min at 72 °C) and 5 min at 72 °C as the final extension. The last PCR product of 809 bp was first treated with Taq polymerase for 10 min at 72 °C, and then cloned into the pTZ57R/T cloning vector (Fermentas, Lithuania), which was confirmed by sequencing prior to further processing.
pmzNL4-3/GFP plasmid construction
Plasmid pTZ57R/T-GFP, extracted from Escherichia coli (E. coli) JM110 (dcm-), was double-digested with the BalI (MlsI) restriction enzyme and the isolated gfp fragment containing terminal restriction enzyme recognition sites and the 3’ protease cleavage signal (Fig. 1) was extracted from agarose gel using a High-Pure PCR Product Purification Kit (Roche, Germany) according to the vendor’s protocol. This 809 bp fragment was then cloned into the BalI-digested pmzNL4-3 plasmid (13, 20) in frame with the RT-INT genes to create plasmid pmzNL4-3/GFP, which was preserved in recombinase-deficient HB101 E. coli cells.
Production and evaluation of GFP-expressing HIV-1 SCR virions
To produce the first-generation viral particles, HEK293T cells were co-transfected with plasmids psPAX2, pMD2G (Addgene: www.addgene.org) and pmzNL4-3/GFP as previously described (13). Briefly, 1 x 105 cells/well were cultured in a 24-well plate and the next day 1 μg of the plasmid mixture at ratios of 33, 17, and 50%, respectively, was used for co-transfection with TurboFectTM reagent (Fermentas, Lithuania), according to the vendor’s procedure. Twenty four, 48, and 72 hrs post-transfection, cell pellets were analyzed for syncytia formation and GFP fluorescence emission by optical and fluorescence microscopy, respectively. Cells transfected with plasmid pEGFP-N1 (Clontech, USA) were used as GFP fluorescence emission controls. Culture supernatants of transfected cells were initially centrifuged at 700g for 10 min to deplete the floating cells. Cleared supernatants were further centrifuged at 60,000 g for 180 min and viral particles were collected as pellets, resuspended in 500 µl of Dulbecco’s modified eagle’s medium (DMEM) by gentle mixing overnight at 4 °C, and used for both p24 quantification by a commercial p24 enzyme-linked immunosorbent assay (ELISA) kit (Biomerieux, France), and direct observation of viral particles by transmission electron microscopy (TEM), using a previously described protocol (19).
To produce the second-generation SCR virions, 104 cells/well of HEK293T cells in 96-well plates containing 100 µl of DMEM with 10% fetal bovine serum (10% FBS-DMEM) were infected with 100 μl of the first-generation virus stock (p24 concentration range: 5-500 μg/ml) and maintained in 5% CO2 at 37 °C. Twenty-four hours post-infection the cells were washed three times with 10% FBS-DMEM at 1 hr time intervals, and resuspended in 200 μl of fresh medium (13). Similarly, 24, 48, and 72 hrs post-infection cell pellets and supernatants were analyzed for syncytia formation, GFP-emission, and p24 quantification as described above.
Results
pmzNL4-3/GFP plasmid construction and analysis
Sequential PCR amplifications using the F1/R1, F2/R2, and F2/R3 primer sets (Table 1) and pEGFP-N1 as the initial source resulted in the production of three fragments with the expected sizes of 768, 792, and 809 bp (Fig. 1A). The final PCR product contained the EGFP-encoding gene with additional bilateral sequences that carried several restriction enzyme recognition sites and the HIV-1 protease cleavage motif at the C-terminus (Fig. 1B). This fragment was T/A cloned into pTZ57R/T, confirmed by sequencing, and after digestion with BalI (MlsI), cloned into pmzNL4-3 in frame with the reverse transcriptase and integrase genes to create plasmid pmzNL4-3/GFP (Fig. 1C). Restriction enzyme analysis with BamHI, NcoI, SmaI, and XbaI confirmed the accuracy of pmzNL4-3/GFP (Fig. 1D).
Assessment of GFP expression and viral assembly of the first-generation SCR virions
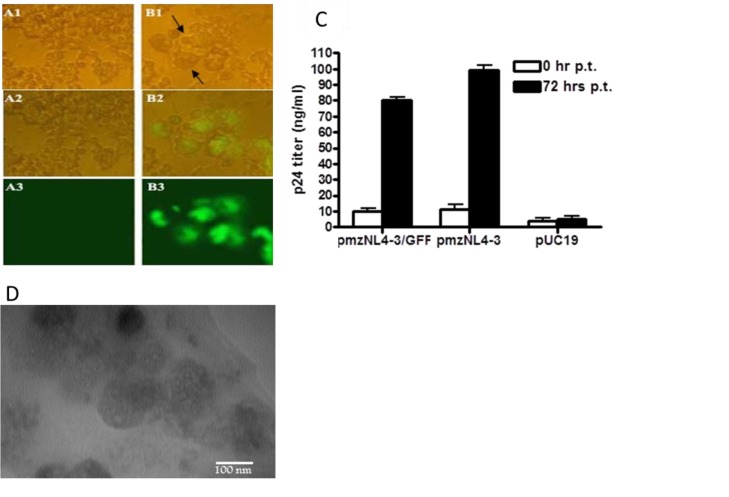
Production of SCR virions in HEK293T cells was previously reported via their co-transfection with plasmids pmzNL4-3, pSPAX2 encoding HIV-1 gag and pol, and pMD2G encoding the vesicular stomatitis virus surface glycoprotein (VSV-G) (13, 20). Here, we investigated the capacity of pmzNL4-3/GFP for the production of GFP fluorescence-emitting SCR virions. Accordingly, co-transfection of HEK293T cells with pmzNL4-3/GFP, pSPAX2 and pMD2G resulted in the formation of syncytial cells, with the highest fluorescence at 72 hrs post-transfection (Fig. 2; A1-B3). This fluorescence indicated expression of gfp, which was cloned into the BalI-recognition site of pmzNL4-3. Furthermore, formation of large round cells, referred to as syncytia (Figs. 2B1 and 2B2), was due to the production of HIV-1 virions, which was not observed in control cells transfected with plasmid pUC19 (Figs. 2A1 and 2A2). To confirm the assembly of viral particles, the amount of p24 protein in the transfected cell culture supernatants was quantified 72 hrs post-transfection. As expected, the p24 concentration was high, at 80±2 ng/ml, relative to the background amount of 10±3 ng/ml at the transfection time and in negative control cells transfected with pUC19 (4±2 ng/ml). This result was similar to the p24 concentration of 99±3 ng/ml obtained 72 hrs post-co-transfection of cells with pmzNL4-3, pSPAX2, and pMD2G, indicating the production of the first-generation HIV-1 virions by pmzNL4-3/GFP and no significant change in the viral assembly rate due to incorporation of gfp into pmzNL4-3 (Fig. 2C). Observation of rounded particles with heterogenic morphology with diameters of 120–150 nm by electron microscopy verified the formation of GFP-mzNL4-3 viral particles within the condensed supernatant of transfected cells (Fig. 2D).
Fig. 2.
Production of the first generation GFP-mzNL4-3 virions. In contrast to the cells transfected with pUC19 control plasmid (A1-A3), cells co-transfected with pmzNL4-3/GFP and helper plasmids (pSPAX2, pMD2G) produced large round syncytia (B1, shown with arrows) and GFP-emitting (B1-B3) cells at 72 hrs post-transfection, which were observed under the immunefluorescence microscope (400x). p24 ELISA assay of culture supernatant at 72 hrs post-transfection (p.t.) indicated the secretion of p24 for pmzNL-43/GFP-transfected cells comparable to the positive control cells transfected with pmzNL4-3 plasmid. Again cells receiving pUC19 produced a background level of p24. Bars show mean of p24 concentration ± SD in three transfected wells (C). Electron microscopy also confirmed the formation of 120-150 nm particles within the culture supernatant of pmzNL4-3/GFP cells (D).
Assessment of GFP expression, viral assembly, and non-infectivity of second-generation SCR virions
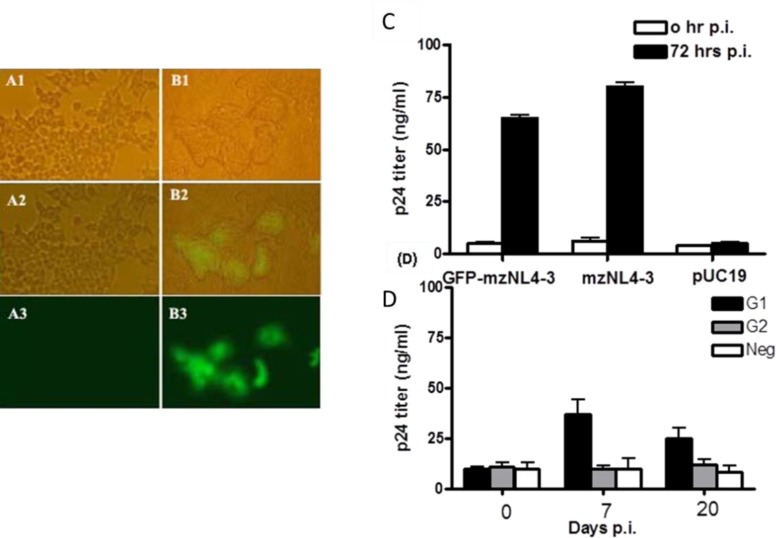
To investigate the replication potency of the fluorescence-emitting first-generation HIV-1 virions, a fresh culture of HEK293T cells was infected with the virions produced from the transfected cells in a range of 0.5-50 μg of p24 protein. Analysis of the infected cells by light and fluorescence microscopy, and measurement of p24 antigen in the supernatant verified syncytia formation, fluorescence (Fig. 3, A1-B3), and p24 antigen secretion into the culture media were shown (Fig. 3C). Overall, these findings confirmed that the first-generation HIV-1 virions carried the GFP protein inside the particles and further underwent at least one cycle of replication, which led to the generation of the second progeny viruses in the culture media. Of note, p24 production of 65±4 ng/ml by the second-generation viruses was similar to that of the mzNL4-3 virions (80±5 ng/ml) at 72 hrs post-infection (Fig. 3C). GFP fluorescence with at least 2 green cells/400x microscopic field was detected only when the cells were infected with a high dose of primary inoculum (50 μg of p24 protein).
Fig. 3.
Production of the second generation GFP-mzNL4-3 virions. 72 hrs post-infection (p.i.) HEK293T cells infected with the first generation GFP-mzNL4-3 virions (50 μg of p24) produced syncytium and showed GFP emission (B1-B3), in contrast to control cells treated with the supernatant of pUC19-transfected cells (A1-A3). GFP-mzNL4-3 infection (0.5 μg of p24) resulted in the secretion of p24 antigen as efficient as infection with mzNL4-3 virions (C). Cells infected with 0.5 μg of either the first generation (G1) or the second generation (G2) GFP-mzNL4-3 virions were assayed for p24 production up to 20 days p.i. Contrary to G1, the amount of measured p24 for G2 infected cells was not higher than that for non-infected control cells (Neg) in different time points. Bars represent mean of p24 concentration ± SD in three infected wells (D).
To ensure that cloning of the gfp fragment did not cause these virions to undergo more than one replication cycle, as was previously described (13), freshly-cultured HEK293T cells were infected with 500 ng of the second-generation viruses, and p24 antigen in the culture supernatant was measured at 7 and 20 days post-infection (Fig. 3D). Here, the first-generation virions were also applied as the positive control. Interestingly, the amount of detected p24 antigen was similar to that of the non-infected control cells and did not increase over the time. To confirm this result, the experiment was repeated using 50 μg of p24, a 100-fold greater inoculation dose, and similar results were obtained (data not shown). This data verified that the second-generation virions were replication-deficient and thus, newly-developed GFP-mzNL4-3 virions preserved their single-cycle replication capacity while remaining GFP-positive.
Discussion
Retroviral packaging vectors generally produce viruses that may be used for drug discovery studies. In this regard, systems generating replication-deficient or replication-limited viruses have obvious safety advantages. Previously, we reported the development of a retroviral packaging system that produced SCR virions (13, 20). In this system, production of the pseudotyped SCR viruses was achieved by co-transfectingHEK293T producer cells with pmzNL4-3, a plasmid with RT-IN deleted from the proviral genome of HIV-1, pSPAX2, a plasmid expressing the HIV-1 gag-pol region, and pMD2G, a plasmid encoding VSV-G. Due to the presence of the packaging signal in pmzNL4-3, only the transcript from this plasmid was packaged into the capsid (25). During the process of budding from the producer cells, capsids were enveloped by both HIV-1 ENV and the VSV-G proteins expressed on the cell surface prior to their release into the medium. This inclusion of VSV-G on the surface of the virions resulted in the production of pseudotyped viruses capable of infecting both lymphocytic cells and a wide range of cell types from a variety of organisms, and the production of viruses in high titers within the HEK293T cells (13, 26-27).
This system, which was designed based on p24 quantification, was used for in vitro screening of anti-retroviral compounds (18-19). However, measurement of HIV-1 p24 antigen by ELISA is costly, time-consuming, and informative only at the cell population level. Furthermore, the experimental design for the HIV-1 p24 assay requires the transfer of HIV-1-containing supernatants, which is a biohazard risk. One alternative to this approach is the use of reporter markers such as SEAP (17), CAT (14), luciferase (11, 15-16), β-Gal (15), or GFP (12), which allow conversion of the assays to readout methods. However, most of these reporting systems still require additional manipulations, such as cell lysis, supernatant transfer, or addition of enzyme substrate to obtain the final quantitative readout, which again renders these assays relatively expensive and time-consuming.
In the present study, we report the modification of a previously developed SCR system to improve its readout sensitivity from a population level using a p24 assay to both population and single-cell levels by direct detection and quantification of EGFP with a wide dynamic range devoid of additional manipulations. Accordingly, inclusion of the EGFP reporter to the SCR virions provides the advantage of studying individual infection events and reflects the number of infected cells. Therefore, application of this system for drug discovery through a fluorometric-based, high-throughput quantitation of EGFP, via the flow cytometric enumeration of EGFP-expressing cells, or by microscopic counting of EGFP-positive colonies will most likely facilitate the measurement of drug inhibition at clinically relevant concentrations. Of note, EGFP is directly quantified in cell culture and the reporter cell line requires no manipulation prior to the assay. In addition, the EGFP reporter allows for data acquisition at an optimal time point by prescreening selected positive control wells by fluorescence microscopy (28). Application of such a rapid and inexpensive marker makes it possible to avoid the costly and time-consuming quantification of viral gene products such as HIV-1 p24 or reverse transcriptase.
Results of the current study demonstrate that EGFP was successfully packaged into the assembled viruses (Fig. 3 A1-B3). Of note, the design of pmzNL4-3 was based on the deletion of a 2 kb fragment containing parts of the reverse transcriptase and integrase genes and the insertion of the EGFP coding sequence. The observation that exogenous EGFP was packaged into the virions showed that the remaining 23 N-terminal residues of reverse transcriptase, which were fused to EGFP (Fig. 1C), preserved its ability to incorporate into the assembled particles. Moreover, analysis of the second-generation GFP-mzNL4-3 virions verified their non-infectivity and safety when the deleted RT-IN fragment was substituted with the GFP coding sequence (Fig. 3D).
To detect the EGFP fluorescence from the cells infected with the first progeny viruses carrying EGFP, it was necessary to infect the cells with 50 μg of p24 protein per 104 cells; a relatively high concentration of viral particles. This was 100-fold greater multiplicity of infection (MOI) than the 0.5 μg of p24 protein per 104 cells that was used to detect p24 antigen in the culture supernatants of the infected cells. The reason for this may be due to the fact that for approximately 1500 copies of p24 protein, which shapes the viral capsid, only two copies of the RT-IN molecule, and hence, two copies of the RT-fused EGFP protein, will be assembled into a mature HIV-1 virion (29). This requirement for a high MOI may be addressed by alternative approaches such as placement of an exogenous promoter upstream of gfp to direct its strong and/or inducible expression (30). In our work, insertion of several restriction enzyme recognition sites at both ends of the gfp fragment (Fig. 1B) allows for similar modification of pmzNL4-3/GFP and/or substitution of gfp with other reporter markers.
The life cycle of the GFP-mzNL4-3 virions produced in HEK293T cells can be divided into two phases; the first phase includes receptor binding and entry, and the second phase contains the rest of the cycle leading to the production of the second-generation non-infective virions, which includes viral uncoating, reverse transcription, nuclear import, genome integration, viral protein expression, viral assembly and maturation, RNA encapsidation, and viral budding (30). The novel concept of a single-cycle, replicable GFP-mzNL4-3 theoretically allows us to distinguish between potential inhibitors of these two phases, so that when the virus and compound are simultaneously added to the target cells, a lack of EGFP fluorescence-emitting cells would indicate a fusion and/or entry -inhibitory effect of the candidate compound; however, if the cells produced EGFP but no p24, stages of the second phase would have been prohibited. If the putative drug was added to the previously infected cells (i.e. by around 2 hrs lag time), the assay would be expected to quantify only the inhibitory effects on the second phase.
Overall, the results reported here validate the development of a rapid, inexpensive, and safe GFP-reporting SCR system for anti-HIV drug discovery. The validity and robustness of the present system needs to be further characterized by its practical application for screening the antiviral activity of putative HIV-1 inhibitors. In addition, whether this system is fully amenable to high-throughput screening remains to be studied.
References
- 1.Chun TW, Fauci AS. HIV reservoirs: pathogenesis and obstacles to viral eradication and cure. AIDS. 2012;26(10):1261–8. doi: 10.1097/QAD.0b013e328353f3f1. [DOI] [PubMed] [Google Scholar]
- 2.Carr A. Toxicity of antiretroviral therapy and implications for drug development. Nat Rev Drug Discov. 2003;2(8):624–34. doi: 10.1038/nrd1151. [DOI] [PubMed] [Google Scholar]
- 3.Martinez-Picado J, DePasquale MP, Kartsonis N, Hanna GJ, Wong J, Finzi D, et al. Antiretroviral resistance during successful therapy of HIV type 1 infection. Proc Natl Acad Sci U S A. 2000;97(20):10948–53. doi: 10.1073/pnas.97.20.10948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Little SJ, Holte S, Routy JP, Daar ES, Markowitz M, Collier AC, et al. Antiretroviral-drug resistance among patients recently infected with HIV. N Engl J Med. 2002;347(6):385–94. doi: 10.1056/NEJMoa013552. [DOI] [PubMed] [Google Scholar]
- 5.Mehellou Y, De Clercq E. Twenty-six years of anti-HIV drug discovery: where do we stand and where do we go? J Med Chem. 2010;53(2):521–38. doi: 10.1021/jm900492g. [DOI] [PubMed] [Google Scholar]
- 6.McMahon MA, Shen L, Siliciano RF. New approaches for quantitating the inhibition of HIV-1 replication by antiviral drugs in vitro and in vivo. Curr Opin Infect Dis. 2009;22(6):574–82. doi: 10.1097/QCO.0b013e328332c54d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Japour AJ, Mayers DL, Johnson VA, Kuritzkes DR, Beckett LA, Arduino JM, et al. Standardized peripheral blood mononuclear cell culture assay for determination of drug susceptibilities of clinical human immunodeficiency virus type 1 isolates The RV-43 Study Group the AIDS Clinical Trials Group Virology Committee Resistance Working Group. Antimicrob Agents Chemother. 1993;37(5):1095–101. doi: 10.1128/aac.37.5.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schinazi RF, McMillan A, Cannon D, Mathis R, Lloyd RM, Peck A, et al. Selective inhibition of human immunodeficiency viruses by racemates and enantiomers of cis-5-fluoro-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine. Antimicrob Agents Chemother. 1992;36(11):2423–31. doi: 10.1128/aac.36.11.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferguson NM, Fraser C, Anderson RM. Viral dynamics and anti-viral pharmacodynamics: rethinking in vitro measures of drug potency. Trends Pharmacol Sci. 2001;22(2):97–100. doi: 10.1016/s0165-6147(00)01615-1. [DOI] [PubMed] [Google Scholar]
- 10.Kellam P, Larder BA. Recombinant virus assay: a rapid, phenotypic assay for assessment of drug susceptibility of human immunodeficiency virus type 1 isolates. Antimicrob Agents Chemother. 1994;38(1):23–30. doi: 10.1128/aac.38.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petropoulos CJ, Parkin NT, Limoli KL, Lie YS, Wrin T, Huang W, et al. A novel phenotypic drug susceptibility assay for human immunodeficiency virus type 1. Antimicrob Agents Chemother. 2000;44(4):920–8. doi: 10.1128/aac.44.4.920-928.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang H, Zhou Y, Alcock C, Kiefer T, Monie D, Siliciano J, et al. Novel single-cell-level phenotypic assay for residual drug susceptibility and reduced replication capacity of drug-resistant human immunodeficiency virus type 1. J Virol. 2004;78(4):1718–29. doi: 10.1128/JVI.78.4.1718-1729.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zabihollahi R, Sadat SM, Vahabpour R, Aghasadeghi MR, Memarnejadian A, Ghazanfari T, et al. Development of single-cycle replicable human immunodeficiency virus 1 mutants. Acta Virol. 2011;55(1):15–22. doi: 10.4149/av_2011_01_15. [DOI] [PubMed] [Google Scholar]
- 14.Terwilliger EF, Godin B, Sodroski JG, Haseltine WA. Construction and use of a replication-competent human immunodeficiency virus (HIV-1) that expresses the chloramphenicol acetyltransferase enzyme. Proc Natl Acad Sci U S A. 1989;86(10):3857–61. doi: 10.1073/pnas.86.10.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia-Perez J, Sanchez-Palomino S, Perez-Olmeda M, Fernandez B, Alcami J. A new strategy based on recombinant viruses as a tool for assessing drug susceptibility of human immunodeficiency virus type 1. J Med Virol. 2007;79(2):127–37. doi: 10.1002/jmv.20770. [DOI] [PubMed] [Google Scholar]
- 16.Jarmy G, Heinkelein M, Weissbrich B, Jassoy C, Rethwilm A. Phenotypic analysis of the sensitivity of HIV-1 to inhibitors of the reverse transcriptase, protease, and integrase using a self-inactivating virus vector system. J Med Virol. 2001;64(3):223–31. doi: 10.1002/jmv.1040. [DOI] [PubMed] [Google Scholar]
- 17.Tang RY, Su Y. Construction of a cell-based high-flux assay for the rev protein of HIV-1. J Virol Methods. 1997;65(2):153–8. doi: 10.1016/s0166-0934(97)02176-9. [DOI] [PubMed] [Google Scholar]
- 18.Zabihollahi R, Namazi R, Aghasadeghi MR, Esfahani AF, Sadat SM, Modarressi MH. The in vitro anti-viral potential of Setarud (IMOD), a commercial herbal medicine with protective activity against acquired immune deficiency syndrome in clinical trials. Indian J Pharmacol. 2012;44(4):448–53. doi: 10.4103/0253-7613.99301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zabihollahi R, Vahabpour R, Hartoonian C, Sedaghati B, Sadat SM, Soleymani M, et al. Evaluation of the in vitro antiretroviral potential of some Biginelli-type pyrimidines. Acta Virol. 2012;56(1):11–8. doi: 10.4149/av_2012_01_11. [DOI] [PubMed] [Google Scholar]
- 20.Rezaei A, Zabihollahi R, Salehi M, Moghim S, Tamizifar H, Yazdanpanahi N, et al. Designing a non-virulent HIV-1 strain: potential implications for vaccine and experimental research. Journal of Research in Medical Sciences. 2007;12(5):227–34. [Google Scholar]
- 21.Chalfie M, Tu Y, Euskirchen G, Ward WW, Prasher DC. Green fluorescent protein as a marker for gene expression. Science. 1994;263(5148):802–5. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- 22.Inouye S, Tsuji FI. Aequorea green fluorescent protein Expression of the gene and fluorescence characteristics of the recombinant protein. FEBS Lett. 1994;341(2-3):277–80. doi: 10.1016/0014-5793(94)80472-9. [DOI] [PubMed] [Google Scholar]
- 23.Prasher DC, Eckenrode VK, Ward WW, Prendergast FG, Cormier MJ. Primary structure of the Aequorea victoria green-fluorescent protein. Gene. 1992;111(2):229–33. doi: 10.1016/0378-1119(92)90691-h. [DOI] [PubMed] [Google Scholar]
- 24.de Oliveira T, Engelbrecht S, Janse van Rensburg E, Gordon M, Bishop K, zur Megede J, et al. Variability at human immunodeficiency virus type 1 subtype C protease cleavage sites: an indication of viral fitness? J Virol. 2003;77(17):9422–30. doi: 10.1128/JVI.77.17.9422-9430.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vorkunova GK, Lupandin SI, Bukrinskaia AG. [HIV-1 assembly is initiated by p17 matrix protein] Mol Biol (Mosk) 2011;45(5):879–83. [PubMed] [Google Scholar]
- 26.Burns JC, Friedmann T, Driever W, Burrascano M, Yee JK. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc Natl Acad Sci U S A. 1993;90(17):8033–7. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zabihollahi R, Sadat SM, Vahabpour R, Salehi M, Azadmanesh K, Siadat SD, et al. Introducing a frameshift mutation to the POL sequence of HIV-1 provirus and evaluation of the immunogenic characteristics of the mutated virions (RINNL4-3) Mol Biol (Mosk) 2012;46(3):519–24. [PubMed] [Google Scholar]
- 28.Ochsenbauer-Jambor C, Jones J, Heil M, Zammit KP, Kutsch O. T-cell line for HIV drug screening using EGFP as a quantitative marker of HIV-1 replication. Biotechniques. 2006;40(1):91–100. doi: 10.2144/000112072. [DOI] [PubMed] [Google Scholar]
- 29.Goff SP. Retroviridae: The Retroviruses and Their Replication. In: Knipe DM, Howley PM, editors. Fields Virology. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2007. 2005 pp. [Google Scholar]
- 30.delson ME, Pacchia AL, Kaul M, Rando RF, Ron Y, Peltz SW, et al. Toward the development of a virus-cell-based assay for the discovery of novel compounds against human immunodeficiency virus type 1. Antimicrob Agents Chemother. 2003;47(2):501–8. doi: 10.1128/AAC.47.2.501-508.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]