Abstract
The goal of this study was to determine the degree to which ex vivo diffusion tensor imaging (DTI) parameters correlate to one another in white matter regions on very high resolution MR scans. Specifically, we hypothesized that radial diffusivity (RD) and apparent diffusion coefficient (ADC) would correlate more closely than either would correlate with fractional anisotropy (FA).
We performed post mortem DTI imaging on three canine brains on a 7 T MR scanner (TR = 100 ms, NEX = 1, gradient amplitude = 600 mT/m, b = 1492–1,565 s/mm2) and generated maps of FA, RD, and ADC. We measured RD, FA and ADC within 14 regions of interest representative of various portions of white matter. We compared the three combinations of values, i.e., FA vs ADC, FA vs RD and ADC vs RD, using linear regression models.
Linear regression demonstrated that RD was significantly correlated with FA (p << 0.01; R2 = 0.3053) and also with ADC (p << 0.01; R2 = 0.6755), but to a much greater degree. However, ADC was not significantly correlated with FA (p = 0.526; R2 = 0.101).
Our findings suggest that both RD and ADC reflect similar cytoarchitectural features, most likely that of myelination, whereas FA values likely reflect both myelination and additional microstructural features that constrain the diffusion of water in white matter.
Keywords: diffusion tensor imaging, canine brain
Introduction
Diffusion tensor imaging (DTI) is an advanced MR imaging technique that allows for quantitative measurements of the magnitude and direction of microscopic water motion. These measurements can be used to infer important characteristics of microscopic structures. In the context of the human brain, DTI has become an important tool to study the mechanisms of brain development and the physiology and pathology of the central nervous system (CNS), particularly that of white matter (WM).
The major DTI metrics that are routinely measured include apparent diffusion coefficient (ADC, a measure of rate of microscopic water motion), fractional anisotropy (FA, the measure of tendency of water motion to diffuse in a non-random manner), radial diffusivity (RD, a measure of microscopic water motion perpendicular to the major eigenvalue), and axial diffusivity (a measure of microscopic water motion parallel to the major eigenvalue).
The specific cellular correlates of DTI metrics are still a matter of investigation. Initial DTI studies showed that FA is significantly higher in white matter compared to gray matter, suggesting that myelination might be a primary component of diffusional anisotropy.1 However, it was shown later that even CNS regions having a paucity of myelination can have relatively high FA values. For example, investigators studying a garfish model showed that the unmyelinated olfactory nerve has anisotropy values comparable to that of the well-myelinated optic nerve.2 The investigators indicated that the coherence of axons in these structures, i.e., the extent to which their course is parallel rather than crossing, may also be an important contributing factor. Other investigators showed in a shiverer mouse model of dysmyelination that lack of myelination was associated with a significant increase in RD without corresponding significant alterations in other DTI metrics, such as FA.3 They concluded that RD is more closely associated with the degree of myelination than FA. The same investigators later showed that serial changes in myelination in a mouse model correlated with changes in RD but not in FA.4,5
Both an increase in FA and a decrease in ADC have been shown to reflect ongoing WM maturation in the brain.6 However, the rates of increase in FA and decrease in ADC during brain maturation have been shown to differ substantially, suggesting that the two parameters might reflect different aspects of WM maturation.7 Furthermore, in studies of subjects throughout childhood, the rate of change of RD and ADC values has been shown to correlate more closely with the rate of myelination than rate of FA increase.8
DTI in living individuals is subject to a number of limitations, such as respiratory motion, inadequate voxel size that prevents sufficient calculation of DTI parameters due to partial volume effects and, on occasion, a poor signal-to-noise ratio. In this study, we performed MR scans of three normal ex vivo canine brains using an imaging technique that allowed very high spatial resolution and a high signal-to-noise ratio. The intent was to provide data that could better assess the degree to which various DTI parameters are correlated compared to human brain imaging. As noted earlier, previous studies in humans have shown a stronger correlation between RD and ADC with MR findings consistent with myelination than FA values.7 In a previous investigation that used one of the dogs in the present study, we correlated DTI parameters with degree of myelination as determined from optical density of a myelin stain.9 In that study, we found that ADC values and RD values more closely correlated with the optical density of a myelin stain than did FA values. Based on those studies, we hypothesized that ADC values in the canine brain would also more closely correlate with RD values than FA values. RD, FA and ADC values have been widely considered important factors in myelination, whereas axial diffusivity has been thought to chiefly reflect axonal density. For that reason, axial diffusivity was not considered in this investigation.
The canine brain is a viable, but relatively underutilized, model for studying the organization of white matter and the pathologic states that affect the human brain. For instance, like the human brain, the aging canine brain is characterized by the frequent development of amyloid plaques and amyloid angiopathy.10 Dogs are subject to many of the same brain diseases that affect the human brain, such as stroke, neoplasms, and infections.11 Importantly, these models can be used to test novel therapies for central nervous system diseases in a relatively accessible population that simulates human disease,12 thereby facilitating the introduction of such therapies for treatment of humans. However, such empirical studies that could exploit advanced MR imaging modalities are predicated on a better understanding of the relationship of water diffusion and the microstructure of white matter in the canine brain. The broad goal of this investigation is to fill this gap in knowledge, building on our previous observations.9
Methods
Specimen acquisition and preparation
Three purpose-bred male neutered adult research beagles were used for this study. The protocol was approved and performed under the guidelines of the University of Georgia's Institutional Animal Care and Use Committee (IACUC). The dogs were part of an unrelated terminal study and had been confirmed as normal based on physical and neurological examinations, hematology, serum chemistry and urine analyses in addition to CSF analysis and cranial MRI evaluations. Each dog was subject to humane euthanasia using intravenous pentobarbital. Upon expiration (as measured by cessation of breathing and reflex activity), each animal underwent transcardial perfusion with 0.1 M sodium phosphate buffer to exsanguinate the carcass, followed by perfusion with buffered 4% paraformaldehyde solution and 10% buffered sucrose. After this procedure, each dog's brain was removed intact from the cranial cavity in a standard post mortem extraction. The brains were placed in 10% sucrose buffered with 0.1 M sodium phosphate.
During the two weeks prior to imaging, the brains were soaked in 1% MR contrast material (gadoteridol; Bracco pharmaceuticals) in 0.1 M phosphate-buffered saline. This process allows the contrast material to diffuse into brain tissue, which shortens the T1 relaxation time of tissue, thereby allowing MR scans with a shorter TR and, thus, decreased scan time.
Imaging
The brains were scanned on a 7 T small animal MRI system (Magnex Scientific, Yarnton, Oxford, England) equipped with 670 mT/m Resonance Research gradient coils (Resonance Research, Inc., Billerica, MA, USA), and controlled with a General Electric Signa console (GE Medical Systems, Milwaukee, WI, USA). RF transmission and reception was achieved using a 6.5 cm diameter quadrature RF coil (M2M Imaging, Cleveland, OH, USA).
We acquired diffusion-weighted images using a custom-designed, spin-echo diffusion-weighted pulse sequence (TR = 100 ms, NEX = 1, gradient amplitude = 600 mT/m, b = 1492–1,565 s/mm2). The acquisition matrix was adjusted according to the dimension of each canine brain for a field of view producing a Nyquist-limited isotropic voxel size of 100 µm. Diffusion preparation was accomplished using a modified Tanner-Stejskal diffusion-encoding scheme with a pair of unipolar, half-sine diffusion gradient waveforms. Two b0 images and 6 high b-value images were acquired with diffusion sensitization along each of six non-collinear diffusion gradient vectors: [1, 1, 0], [1, 0, 1], [0, 1, 1], [−1, 1, 0], [1, 0, −1], and [0, −1, 1]. Total acquisition time was approximately 40 hours for each brain. We used a six-direction spin-echo approach because it provides high SNR and, compared to multi-shot EPI, is less prone to artefacts and distortions due to magnetic field inhomogeneity. In addition, the use of gadoteridol in preparation of the specimen for imaging reduces T2* to the extent that multi-shot approaches such as EPI are no longer feasible.
Data processing
Following imaging, we smoothed the data using the SUSAN denoising algorithm implemented in FSL with a 3 × 3 × 3 voxel kernel radius. Although the entire brain was imaged intact, we chose to focus our analysis on the right hemisphere because analyzing the entire brain was found to exceed the computational capabilities of our hardware. FA, ADC, eigenvalues, and eigenvector maps were reconstructed using Diffusion Toolkit, version 0.6.2. Axial diffusivity (AD) maps are equivalent to the primary eigenvalue maps. Radial diffusivity (RD) maps were calculated based on the average of the secondary and tertiary eigenvalue maps. We pooled RD values for all ROIs, FA values for all ROIs, and ADC values for all ROIs.
Coregistration of DTI maps and ROI placement
Transverse DTI metric maps from each brain consisted of 32-bit images. The two transverse slices from each DTI metric map that contained the 14 ROIs (see below) were acquired; subsequently a 2D affine registration for all DTI metric maps was performed against a common corresponding reference histological image. After affine registration, the registered metric map images were super-stacked into position and aligned to one another. ROIs were then drawn according to the designation specified in the Appendix and the metrics data acquired using the Analyze tool on ImageJ v1.47 n.
Histology
The brain was immersed in dry ice for ten minutes and hemi-sectioned in the sagittal plane. The right hemisphere was then bisected in the transverse plane at the midpoint of the corpus callosum. The anterior half of the right hemisphere was mounted on a sledge-style freezing microtome (American Optical, Southbridge, MA, USA) and maintained at the optimal temperature for sectioning with dry ice. Fifty micrometer transverse sections were taken continuously throughout the block. From these sections, we opted to mount a series consisting of one section per 500 µm (i.e., every tenth transverse section). These sections were mounted on glass slides and stained with 2.0% (w/v) gold chloride (Electron Microscopy Sciences, PA, USA) buffered in 0.02 M phosphate with 0.9% sodium chloride for one hour. The stain was then fixed and slides were coverslipped.
ROI placement
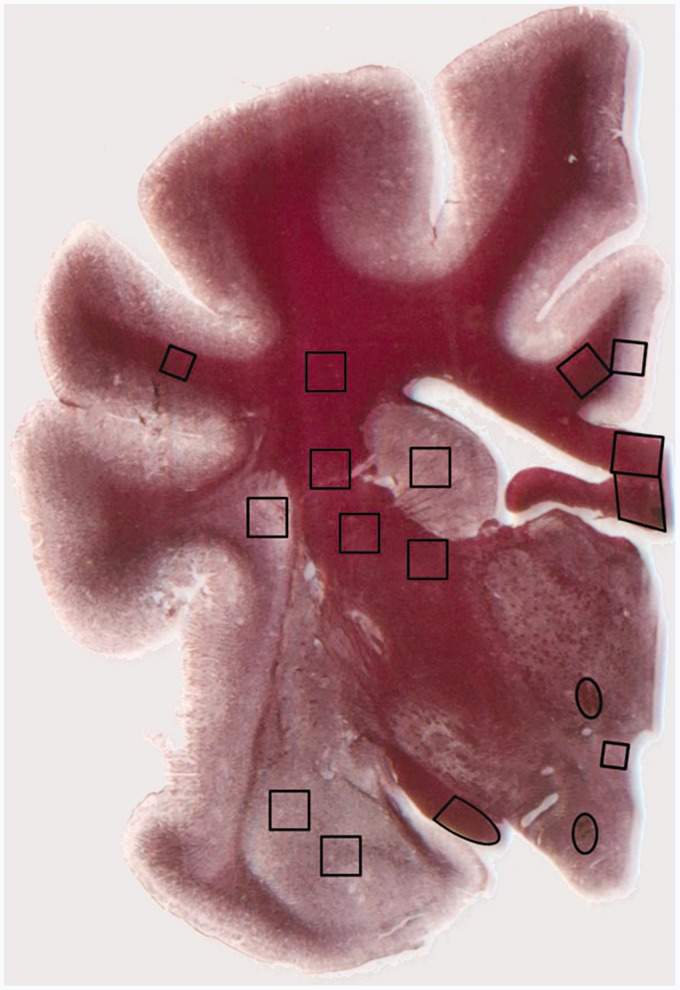
Fourteen white matter ROIs were designated in each brain. These ROIs were the postcommissural fornix, mammilothalamic tract, anterior and posterior limbs of the internal capsule, suprasylvian gyrus subcortical white matter, corona radiata, cingulate gyrus subcortical white matter, corpus callosum, fornix, optic tract, optic radiation, inferior cerebellar peduncle, and middle cerebellar peduncle (Figure 1). The ROIs were defined based on their relation to anatomic landmarks (see Appendix for full definitions) and chosen in order to represent a diverse collection of white matter structures ranging from subcortical white matter (an area from which we were like to sample voxels containing axons with low levels of spatial coherence) to deep compact white matter tracts (in which we were likely to sample voxels containing parallel axons with relatively high spatial coherence). The areas of the ROIs were as follows: optic tract- 4,267 pixels, suprasylvian gyrus subcortical white matter- 1,975 pixels, postcommissural fornix- 737 pixels, mammilothalamic tract- 678 pixels, and all other regions- 3,481 pixels.
Figure 1.
Transverse brain slice of the right hemisphere stained with gold chloride showing placement of regions of interest for DTI-histology correlation.
We obtained the average parameter value across all pixels in each ROI and treated each ROI as an independent sample.
Data analysis and statistics
We performed linear regression for all pooled RD, FA and ADC values. This test allowed measurement of correlations between RD, FA, and ADC. We then reported both p-values and Pearson correlation coefficients. Statistical analysis was performed in RStudio v0.97.237.
Inter-rater and intra-rater reliability assessments were taken to determine the reliability of ROI designations. Two raters independently measured FA, RD, and ADC across a total of 42 ROIs (three brains of 14 ROIs each) at two separate sessions one week apart. For each ROI, the average intra-rater and inter-rater measurement difference for three brains was found to be less than 10%.
Results
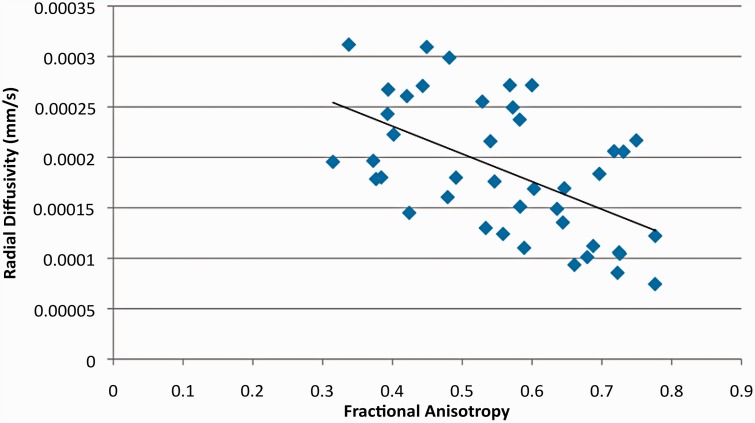
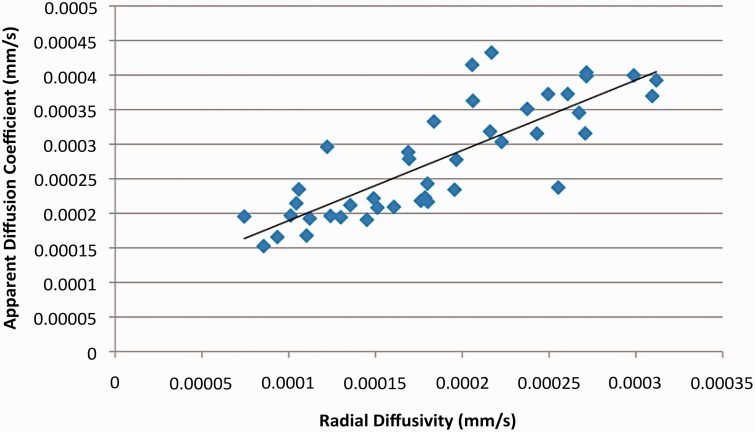
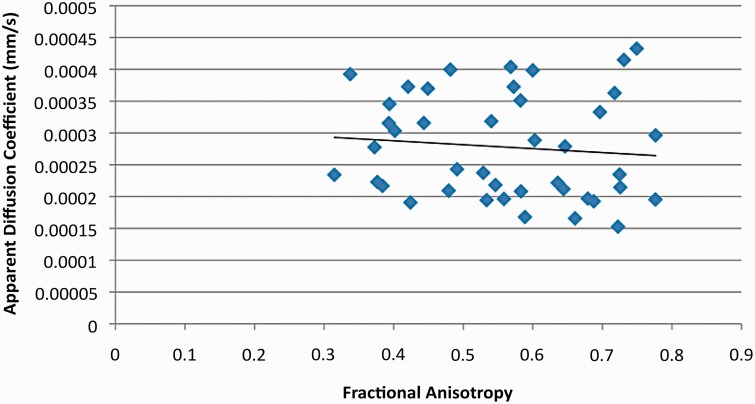
RD was significantly correlated with both FA (p << 0.01 R2 = 0.3053; Figure 2) and ADC (p< < 0.01, R2 = 0.6755; Figure 3). The specific p value for the RD vs FA correlation was p = 1.48 × 10−4 and that for the RD vs. ADC correlation was p = 2.52 × 10−11. However, ADC was not significantly correlated with FA (p = 0.526, R2 = 0.101; Figure 4). These findings support our hypothesis that RD values in the different regions of the canine brains would more closely reflect the variation in ADC values than FA values.
Figure 2.
Scatter plot comparing radial diffusivity (RD) and fractional anisotropy (FA) values. A significant correlation between RD and FA (p = 0.000148) was found; R-squared value = 0.3053.
Figure 3.
Scatter plot comparing apparent diffusion coefficient values (ADC) and radial diffusivity (RD) values. A highly significant correlation between ADC and RD (p < 0.01) was seen, which was much stronger than for the correlation of RD and FA shown in Figure 2; R-squared value = 0.6755.
Figure 4.
Scatter plot comparing ADC and fractional anisotropy (FA) values. The correlation between ADC and FA was not statistically significant.
Discussion
RD and ADC are highly correlated variables likely reflecting myelination
This study expands on the findings of a previous ex vivo canine imaging study involving a single canine brain, in which we found that (1) RD more closely correlated with ADC than with FA and (2) upon multivariate analysis, RD (but not FA) correlated with the histological features of myelination and fiber coherence.9 In the present study, we extended our examination by directly correlating RD, ADC, and FA values across three normal canine brains. RD values were again shown to be significantly and very strongly correlated with ADC values, confirming our hypothesis and supporting our previous findings. The strong correlation of ADC and RD suggests that they both reflect very similar histological (i.e., microstructural) features. Given our previous finding that RD is highly correlated with the degree of myelination, it is reasonable to propose that ADC values are also highly influenced by the degree of myelination. Recent investigations using human ex vivo studies have confirmed such an association.13
RD is correlated with FA
We found that RD significantly correlated with FA. However, the correlation was substantially weaker than for the relationship of RD with ADC. The finding that RD was more strongly correlated with ADC is not surprising given the fact that a strong mathematical relationship exists between the two parameters, i.e., ADC is the average of the three principal eigenvalues (i.e., the major eigenvalue and two minor eigenvalues) and RD is the average of the two minor eigenvalues. However, the close correlation cannot fully be explained by this mathematical relationship.
The correlation of RD and FA in this study of three canine brains reinforces the correlation of the two parameters that we found in a single brain.9 The physiological factors underlying this correlation are a matter of investigation. Some studies have shown a strong correlation between FA, on the one hand, and area of the myelin sheath and area and number of myelinated axons.14 However, that same study also showed a strong correlation between FA and (1) amount of extracellular water and (2) number of unmyelinated axons. Previous studies by other investigators have also shown a poor correlation of FA with degree of myelination.3
FA is not strongly correlated with ADC
In our study, FA and ADC were not significantly correlated. Both parameters have been described to represent maturity in white matter.6 In a previous study in humans, we found the rates of change in FA and ADC differ substantially during early infancy, with the rate of change in FA being slower.7 These differences suggest that FA and ADC measure different histological features. The lack of correlation between the two parameters in our study supports this contention. A recent study using high-angular resolution diffusion imaging (HARDI) and the orientation distribution function, which allow reconstruction of multiple underlying fibers per voxel, indicates that FA correlates poorly with individual fiber anisotropy.15 However, in that study, mean diffusivity, as reflected in ADC values, appeared to more accurately reflect individual fiber anisotropy. The finding of a poor correlation of FA with individual fiber anisotropy is commensurate with findings from our previous study in which multivariate analysis showed that RD, but not FA, significantly correlated with both degree of myelination (as reflected by optical density of a myelin stain) and fiber coherence.9
Limitations
Our work has a number of limitations. First, our data were gathered in mature dogs at a single time point; a longitudinal study of living dogs or imaging studies of ex vivo canine brains of dogs at various ages might find different results. Second, although readings were taken using numerous ROIs allowing for a robust sample size, the number of individual dogs from which specimens were obtained was only three. As such, the presence of inter-subject variability in brain structure at the micro- or even the ultra-structural levels could have resulted in different DTI metrics even if the same ROI is measured. Additional studies using a larger number of brains are warranted. Third, we treated each ROI in each brain as an independent sample. Some might argue that the different samples within a brain are somewhat dependent on each other, and that this lack of independence might account, in part, for the high degree of statistical significance seen in some of our analyses. Finally, we did not perform histological analysis in all brains, so we have not yet compared DTI metrics to histological metrics in a robust sample of canine brains.
In summary, using very high resolution DTI, we found a very strong correlation of RD values with ADC values; although a significant correlation was found between RD values and FA values, it was not as strong as that between RD values and ADC values. Our findings suggest that RD values and ADC values reflect similar microstructural features. Further studies including histological correlations between DTI metrics and histological features of myelination are warranted.
Appendix: ROI definitions
ROI designations
Fourteen common ROIs containing white matter were drawn on two MR transverse slices for each brain. The specific designations for the ROIs were taken from a study that compared the histology of canine brain (one of the three brains scanned in our study) with its corresponding DTI metrics.9 The same rules can be applied to the other two canine brains in this study to obtain its respective DTI metrics.
The following 11 ROIs were designated on the transverse slice at the level in which the mamillothalamic tract was at the same axial level as the inferior aspect of the optic tract.
Postcommissural region of the fornix: the ROI was drawn as an ellipse demarcated by the edges of the postcommissural fornix.
Mammilothalamic tract: the ROI was designated by an ellipse demarcated by the edges of the mammilothalamic tract.
Inferior posterior limb of the internal capsule: this ROI was drawn as a square with its superior aspect determined by the most inferior part of the caudate, centered laterally by the midpoint of the caudate nucleus, with an edge length of the vertical height of the corpus callosum at the cut edge.
Middle posterior limb of the internal capsule: this ROI was located equal parts inferior and lateral to the center of the caudate, centered at the midpoint of the white matter of the internal capsule and with an edge length of the vertical height of the corpus callosum at the cut edge.
Superior posterior limb of the internal capsule: this ROI was placed lateral to the center of the caudate, centered at the midpoint of the white matter of the internal capsule with an edge length of the vertical height of the corpus callosum at the cut edge.
Suprasylvian gyrus white matter: this ROI was placed midway within the white matter in this gyrus. It represents a square with edge length determined by the width of the subcortical white matter in this location.
Corona radiata: this ROI was drawn as a square at the midpoint between the lateral ventricle and the suprasylvian sulcus, with an edge length of the vertical height of the corpus callosum at the cut edge.
Cingulum bundle: this ROI was configured as a square at the centroid of the white matter tract in the core of the cingulate gyrus, with an edge length of the vertical height of the corpus callosum at the cut edge.
Corpus callosum: this ROI was drawn as a parallelogram with one edge defined by the sagittal cut edge of the corpus callosum and another running parallel to the cut edge at the point where the corpus callosum diverges from the fornix laterally.
Fornix: this ROI was configured as a square centered at the widest point of the fornix in the sagittal plane and made as large as possible without including regions outside the fornix.
Optic tract: this ROI was defined by the medial half of the optic tract, cut along an axis perpendicular to the major axis of the ellipsoid of the optic tract in this plane.
The following three ROIs were designated on the transverse slice at which the middle and inferior cerebellar peduncles had equal cross-sectional diameters.
Optic radiation: this ROI was designated by a square centered at the centroid of the medial parietal gyrus, encompassing as much white matter as possible without running into gray matter.
Inferior cerebellar peduncle: the medial and lateral boundaries of the peduncle were defined as the inflection points where the peduncle joins the pons and middle cerebellar peduncle. This ROI was a quarter-ellipse comprising the lateral half of the peduncle.
Middle cerebellar peduncle: the medial and lateral boundaries of the peduncle were defined by the points where it joins the inferior cerebellar peduncle and the cerebellum. This ROI was a quarter-ellipse comprising the medial half of the peduncle.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Zhai G, Lin W, Wilber KP, et al. Comparisons of regional white matter diffusion in healthy neonates and adults performed with a 3.0-T head-only MR imaging unit. Radiology 2003; 229: 673–681. doi: 10.1148/radiol.2293021462. [DOI] [PubMed] [Google Scholar]
- 2.Beaulieu C, Allen PS. Determinants of anisotropic water diffusion in nerves. Magn Reson Med 2005; 31: 394–400. doi: 10.1002/mrm.1910310408. [DOI] [PubMed] [Google Scholar]
- 3.Song SK, Sun SW, Ramsbottom MJ, et al. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage 2002; 17: 1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- 4.Song SK, Sun SW, Ju WK, et al. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage 2003; 20: 1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Song SK, Yoshino J, Le TQ, et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage 2005; 26: 132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 6.Mukherjee P, Miller JH, Shimony JS, et al. Normal brain maturation during childhood: developmental trends characterized with diffusion-tensor MR imaging. Radiology 2001; 221: 349–358. doi: 10.1148/radiol.2212001702. [DOI] [PubMed] [Google Scholar]
- 7.Provenzale JM, Isaacson J, Stinnett S, et al. Analysis of corpus callosum diffusion tensor imaging parameters in infants. Neuroradiol J 2012; 2: 429–437. [DOI] [PubMed] [Google Scholar]
- 8.Provenzale JM, Isaacson J, Chen S. Progression of corpus callosum diffusion-tensor imaging values during a period of signal changes consistent with myelination. Am J Roentgenol 2012; 198: 1403–1408. doi: 10.2214/AJR.11.7849. [DOI] [PubMed] [Google Scholar]
- 9.Wei PT, Leong D, Calabrese E, et al. Diffusion tensor imaging of neural tissue organization: correlations between radiologic and histologic parameters. Neuroradiol J 2013; 26(5): 501–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Czasch S, Paul S, Baumgartner W. A comparison of immunohistochemical and silver staining methods for the detection of diffuse plaques in the aged canine brain. Neurobiol Aging 2006; 27: 293–305. doi: 10.1016/j.neurobiolaging.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 11.Garosi LS, McConnell JF. Ischaemic stroke in dogs and humans: a comparative review. J Small Anim Pract 2005; 46: 521–529. doi: 10.1111/j.1748-5827.2005.tb00281.x. [DOI] [PubMed] [Google Scholar]
- 12.Dickson PI, Pariser AR, Groft SC, et al. Research challenges in central nervous system manifestations of inborn errors of metabolism. Mol Genet Metab 2011; 102: 326–338. doi: 10.1016/j.ymgme.2010.11.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klawiter EC, Schmidt RE, Trinkaus K, et al. Radial diffusivity predicts demyelination in ex vivo multiple sclerosis spinal cords. Neuroimage 2001; 55: 1454–1460. doi: 10.1016/j.neuroimage.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jito J, Nakasu S, Ito R, et al. Maturational changes in diffusion anisotropy in the rat corpus callosum: comparison with quantitative histological evaluation. J Magn Reson Imaging 2008; 28: 847–854. doi: 10.1002/jmri.21496. [DOI] [PubMed] [Google Scholar]
- 15.Zhan L, Leow AD, Zhu S, et al. A novel measure of fractional anisotropy based on the tensor distribution function. Med Image Comput Comput Assist Interv 2009; 12(Pt 1): 845–852. [DOI] [PubMed] [Google Scholar]