Abstract
Endovascular treatment is one of the treatment options considered for acute stroke in many primary stroke centers. Outcome from such treatment can be very successful and gratifying if the intervention is timely and patient selection is appropriate. There are however certain pitfalls that need to be kept in mind which, if the interventionalist is not careful, can adversely affect the outcome. We describe such a case where the patient presented with acute stroke due to basilar artery thrombosis but also had an aneurysm in the affected vessel. We also make certain recommendations to reduce the chances of complications arising during treatment of patients with such a condition.
Keywords: stroke, aneurysm, thrombolysis, hyperdensity
Introduction
Endovascular treatment is one of the treatment options considered for acute stroke in many primary stroke centers. Outcome from such treatment can be very successful and gratifying if the intervention is timely and patient selection is appropriate. There are however certain pitfalls that need to be kept in mind which, if the interventionalist is not careful, can adversely affect the outcome. We describe such a case where the patient presented with acute stroke due to basilar artery thrombosis but also had an aneurysm in the affected vessel. We also make certain recommendations to reduce the chances of complications arising during treatment of patients with such a condition.
Case Report
A 53-year-old woman was found unresponsive and quadriparetic with right-side affected more than the left. Her NIHSS score was 20 on admission. She underwent plain CT head scan which ruled out intracranial hemorrhage and showed hyperdensity of the distal basilar artery. She then underwent CT angiogram of her brain and neck including the aortic arch which showed thrombosis of distal basilar artery (BA) with no filling of either posterior cerebral artery (PCA). It also showed occlusion of the right vertebral artery (VA) with significant stenosis of the dominant left VA (L-VA) origin. She was then transferred to the endovascular suite for treatment of the BA thrombosis. Her initial cerebral angiogram showed complete occlusion of the hypoplastic right VA origin with some reconstitution distally at C4/5 level but with no filling of intracranial VA or BA. Angiogram of left common carotid artery (CCA) and left subclavian artery showed significant stenosis of her left internal carotid artery (ICA) (80% as per North American Symptomatic Carotid Endarterectomy Trial (NASCET) criteria) and L-VA at the origin (90% measured as per NASCET study).
To improve flow and facilitate access to basilar artery she underwent left VA angioplasty and stenting using a 4X12mm balloon mounted Multilink stent (Abbott Vascular, Abott park, IL, USA). There was 25% residual stenosis of L-VA origin post stenting/angioplasty. We accessed the distal left VA/BA using a synchro 2 soft microwire and Prowler select micro catheter (Codman & Shurtleff, Raynham, MA, USA). When we attempted to pass the microwire into one of the P1 branches we noticed that the microwire was looping in the midline instead of going into one of the PCAs. At this point we abandoned further attempts to negotiate the microwire distal to the thrombus as we were unsure of the anatomic path the microwire was taking. We decided to review the plain CT head prior to attempting any further intervention. On reviewing her plain CT head we noticed that the hyperdensity at the BA apex was bulbous suggesting a possible aneurysm in that location (Figure 1). We suspected that the aneurysm at the BA apex was causing the microcatheter to loop in the midline instead of going into either of the PCAs. We decided to thrombolyze the clot from the proximal end with intra-arterial tissue plasminogen activator (tPA) as mechanical thrombectomy could have been hazardous. After thrombolysis we noticed both PCAs filling and also noticed some linear contrast extension in the midline between both PCAs (Figure 2). This was interpreted as filling of the now partially thrombosed BA aneurysm and not as perforation of the vessel as the patient was neurologically and hemodynamically stable. Having established antegrade flow in BA and both PCAs and residual thrombus in the aneurysm we stopped the intervention at that point. She received a loading dose of Plavix 300 mg during the procedure and Plavix 75 mg/day and Aspirin 81 mg/day was continued after the procedure. Over the next few days the patient remained stable but developed right-sided transient ischemic attack (TIA) secondary to her left ICA stenosis. As she had developed TIA in spite of being treated with dual antiplatelet therapy she underwent a repeat cerebral angiogram and stenting of the left ICA stenosis. During that procedure a small (<5 mm) BA apex aneurysm was noted (Figure 3). Due to its small size it was not treated, kept under surveillance with yearly scans and has remained stable (Figure 3).
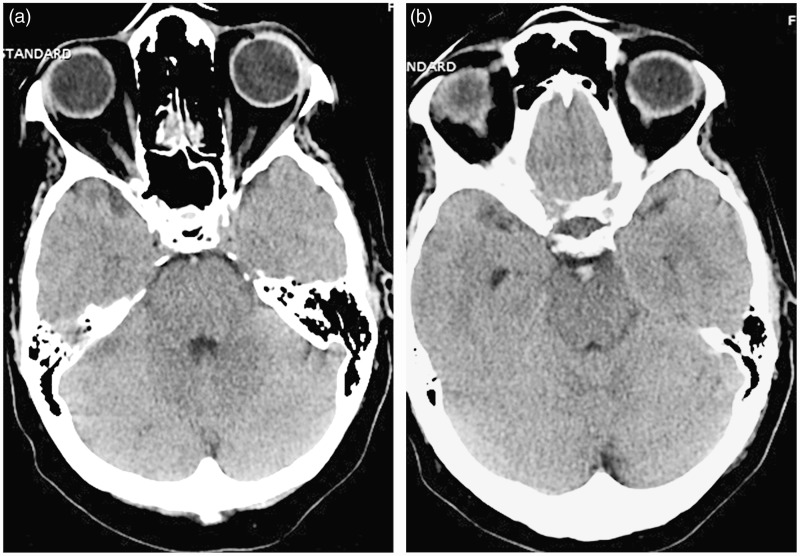
Figure 1.
Hyperdense, thrombosed normal-sized basilar artery (left) and thrombosed, bulbous basilar apex (right).
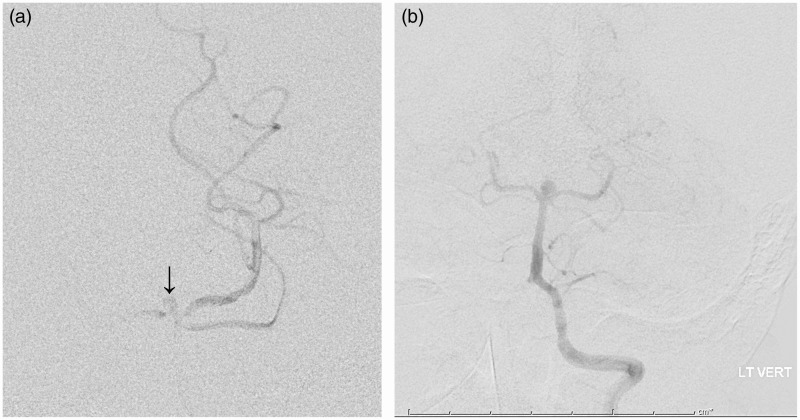
Figure 2.
Extravasation of dye in the midline (arrow) with filling of the left posterior cerebral artery (PCA) and partial filling of the right PCA.
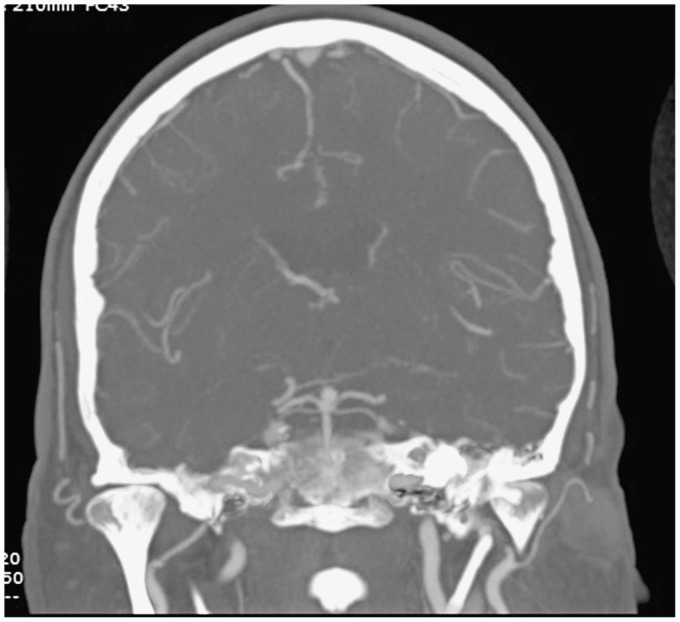
Figure 3.
The basilar apex aneurysm on DSA a few days later (left) and stable similar sized basilar apex aneurysm on CTA 10 months after thrombectomy.
Discussion
Mechanical thrombectomy for acute stroke, although unproven, is increasingly considered a treatment option for acute stroke patients in most primary stroke centers.2,3 This procedure involves mechanical removal of the clot using either aspiration alone or in combination with stent retrievers like Solitaire or with chemical thrombolytic agents like tPA. The stent retriever method involves passing microwire/microcatheter distal to the clot into a patent vessel and then deploying the stent across the thrombus before retrieving the thrombus with the stent. Passing the microwire is often done blind as there is no possibility of obtaining a road map due the thrombus obstructing the vessel involved. This can be hazardous. One of the complications with treating cerebral aneurysms via endovascular coiling is intra-procedural rupture of the aneurysm. This can happen when passing the microwire if adequate care is not exercised. This is especially a risk if the aneurysm is not visualized due to thrombus obstructing flow in the parent vessel hosting the aneurysm. Although it is possible to have such a combination, the exact incidence of such occurrence is not known. This is of particular concern in certain situations where an aneurysm is located at a vessel bifurcation such as the ICA terminus, BA apex or M1 bifurcation. If the anatomy of the vessels in these locations is not clear due to obstruction, passing a microwire beyond the thrombus into one of the distal branches becomes potentially dangerous. A high level of suspicion is needed to be able to identify aneurysms in these locations under such circumstances and to avoid perforating them by overzealous attempts at negotiating a microwire/microcatheter beyond the thrombus. There are very few cases of such incidental aneurysms identified during treatment of stroke.4–6 In these cases, as in our case, the actual sequence of thrombosis in the parent vessel and aneurysm is not clear.8 The presence of the aneurysms was also not realized prior to starting endovascular stroke treatment. In our case we first realized the possibility of an aneurysm at the BA apex when we noticed the microwire looping in the midline and not easily tracking into either of the PCAs. At that point we reviewed the plain CT head which showed a slightly bulbous hyperdensity at the basilar artery apex. This we felt could represent a thrombosed BA apex aneurysm. We hence decided to abandon further attempts to pass the microwire/microcatheter beyond this point and manage the clot from the proximal end. We used IA tPA for thrombolysis until both PCAs were filling. We also noticed partial filling of the aneurysm as there was residual thrombus in the aneurysm. We did not attempt to lyse the thrombus in the aneurysm sac. Using the steps described above we believe we avoided perforating the aneurysm. We recommend the following steps in the endovascular treatment of stroke in any patient. 1) Imaging technique: careful review of plain CT head (thin slices) as it is known that the rate of identifying the hyperdense middle cerebral artery sign increases significantly with thin slice non-enhanced CT scan of the brain.6,7 It should also be noted that the hyperdensity of the thrombosed vessel should not exceed the expected diameter of the parent vessel. Hyperdensity exceeding the expected diameter of the parent vessel should raise suspicion of a possible aneurysm in that location. So it is important not only to have a high level of suspicion for the presence of aneurysms in these situations but also to maximize the ability to identify them by appropriate thin slice non-enhanced CT scans of the brain. 2) Guidewire technique: passing the guidewire with its tip shaped like an inverted “U” to avoid penetration of the aneurysm even if the guide wire were to go into the aneurysm sac. In our case although the guidewire tip seemed to go in the midline beyond the normal perceived anatomy of the basilar artery or its branches, we feel we did not perforate the aneurysm as the tip of the guidewire was shaped to be blunt. 3) Recanalization technique: if an aneurysm is suspected we recommend treating the thrombus from the proximal end either using aspiration alone or in combination with thrombolytic agents rather than attempting a stent retrieval of the clot. No deliberate attempt should be made to lyse the thrombus inside the aneurysm.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interest
E.M. Deshaies MD is a physician consultant for MicroVention, ev3/Covidien Neurovascular, and Integra LifeSciences Corporation.
References
- 1.Kang D-H, Kim Y-S, Park J, et al. Rescue forced-suction thrombectomy using the reperfusion catheter of the penumbra system for thromboembolism during coil embolization of ruptured cerebral aneurysms. Neurosurgery 2012; 70: ons89–ons94. [DOI] [PubMed] [Google Scholar]
- 2.Chimowitz MI, et al. Endovascular treatment for acute ischemic stroke- still unproven. N Engl J Med 2013; 368(10): 952–955. doi: 10.1056/NEJMe1215730. [DOI] [PubMed] [Google Scholar]
- 3.Broderick JP, Palesch YY, Demchuk AM, et al. Endovascular therapy after intravenous t-PA versus t- PA alone for stroke. N Engl J Med. 2013; 368(10): 893–903. doi: 10.1056/NEJMoa1214300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saito N, Hayashi N, Okubo T, et al. Internal carotid artery aneurysm visualized during successful endovascular treatment of carotid embolization. Am J Neuroradiol 2000; 21(3): 546–548. [PMC free article] [PubMed] [Google Scholar]
- 5.Kühn AL, Hou SY, Spilberg G, et al. Visualization of a small hidden intracranial aneurysm during endovascular thrombectomy for acute MCA occlusion. J Vasc Interv Neurol 2014; 7(1): 47–49. [PMC free article] [PubMed] [Google Scholar]
- 6.Riedel CH, Jensen U, Rohr A, et al. Assessment of thrombus in acute middle cerebral artery occlusion using thin-slice non enhanced computed tomography reconstructions. Stroke 2010; 41(8): 1659–1664. doi: 10.1161/STROKEAHA.110.580662. [DOI] [PubMed] [Google Scholar]
- 7.Kim EY, Lee SK, Kim DJ, et al. Detection of thrombus in acute ischemic stroke: value of thin-section non contrast computed tomography. Stroke 2005; 36(12): 2745–2747. doi: 10.1161/01.STR.0000185720.03803.41. [DOI] [PubMed] [Google Scholar]
- 8.Kato M, Kaku Y, Okumura A, et al. Thrombosed unruptured cerebral aneurysm causing brain infarction followed by subarachnoid hemorrhage–case report–. Neuro Med Chir (Tokyo) 2005; 45: 472–475. doi: 10.2176/nmc.45.472. [DOI] [PubMed] [Google Scholar]