Abstract
We report a case of a 7-week-old boy with bilateral leukocoria and asymmetric microphthalmia who was found to have Norrie disease. Symmetrically hyperdense globes with no evidence of calcification were seen on CT scan. The MRI showed bilateral retinal hemorrhages resulting in conical vitreous chambers—narrow at the optic disc and widened toward the lens—characteristic of persistent fetal vasculature. Genetic evaluation revealed a previously undescribed mutation in the Norrie disease protein gene.
Keywords: Persistent fetal vasculature, Norrie disease, leukocoria
Introduction
Persistent fetal vasculature (PFV), previously known as persistent hyperplastic primary vitreous (PHPV), is the second most common cause of infantile leukocoria following retinoblastoma.1 PFV is often (>90%) unilateral, and due to failure of the embryonic hyaloid vascular system to regress.2 The disease is characterized by triangular retrolental tissue, which represents the persistent fetal hyaloid canal. Rare bilateral cases of PFV have been reported in association with Norrie disease.3
Norrie disease is an X-linked genetic syndrome characterized by congenital visual defect and progressive hearing loss.4 The disease is caused by mutation in the Norrie disease protein (NDP) gene, which encodes “Norrin,” a growth factor with a key role in vascular development of the eye, inner ear, and brain. We present a rare case of bilateral PHPV who was found to have a rare mutation in his NDP gene.
Case report
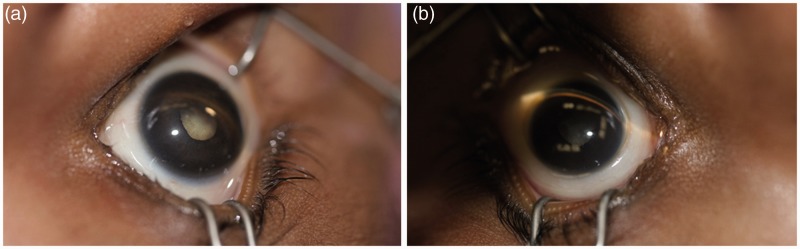
A 7-week-old term boy presented to our tertiary care referral center for bilateral leukocoria. The patient’s mother first noticed unusually rapid eye movements and that his left eye was smaller than the right when the patient was 4 weeks old. Subsequently, the primary pediatrician found bilateral leukocoria and discrepancy in globe size (Figure 1). The patient was referred to our center with concerns for retinoblastoma.
Figure 1.
The patient showed bilateral leukocoria, with the slightly smaller left eye globe (b) compared to the right (a). Photographs were taken from above the patient’s head, with the nose at the top of the image and the forehead at the bottom.
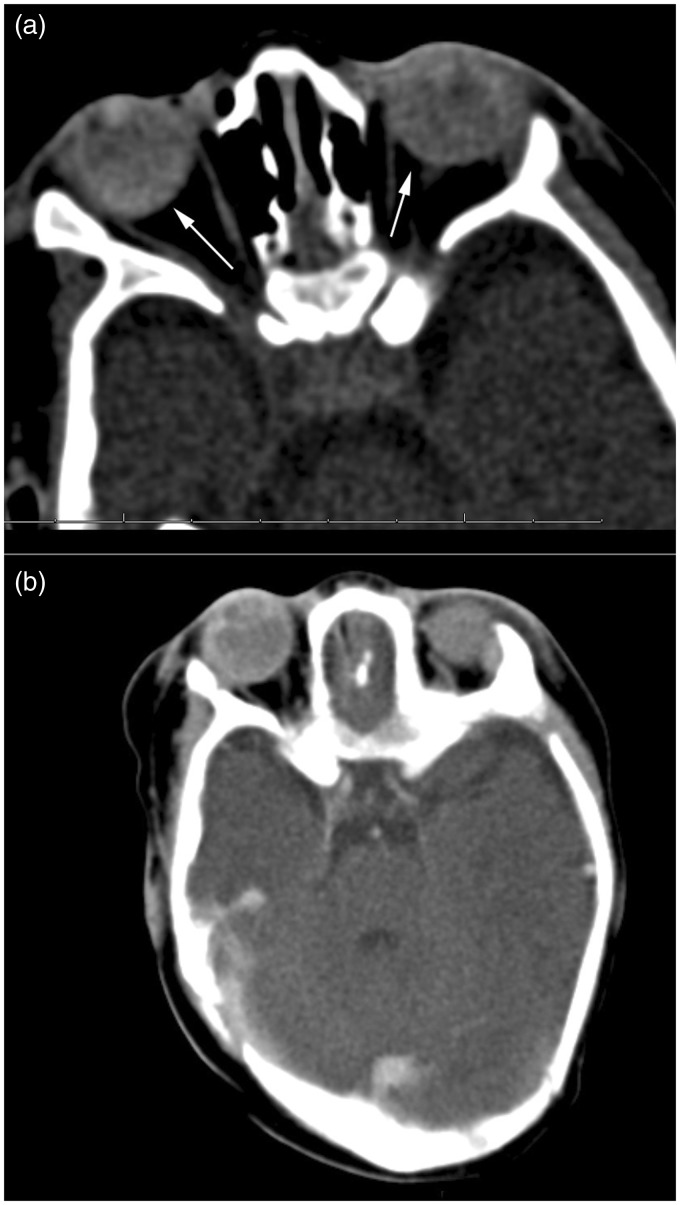
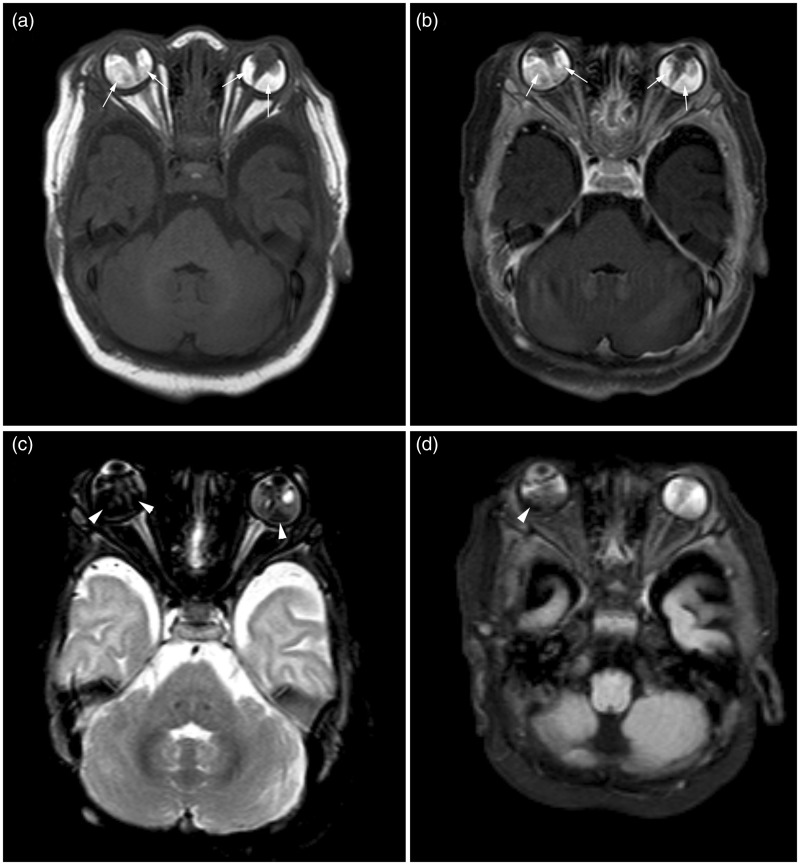
The orbital computed tomography (CT) scan demonstrates diffusely hyperdense globes bilaterally, without calcification (Figure 2). The left globe was slightly smaller than the right. The magnetic resonance imaging (MRI) showed cone-like tapering soft tissue extending posteriorly from the lens to the central retina, in both globes. There was surrounding T1 hyperintense and T2 hypointense fluid with associated susceptibility artifact suggestive of retinal detachment and hemorrhage (Figure 3). The left eye globe was also smaller than the right with mild hypertelorism on the MRI scan. There was no abnormal enhancement on post-contrast CT or MRI studies. No other cerebral abnormality was visualized.
Figure 2.
Mild hyperdensity of both globes (arrows), with no calcification visualized on non-contrast orbital CT (a). There was no abnormal mass-like enhancement on post-contrast images (b). The left globe is slightly smaller than the right. CT: computed tomography.
Figure 3.
The T1-weighted imaged without contrast (a) and post-contrast with fat saturation (b) showed heterogeneously hyperintense soft tissue in bilateral globes surrounding the Cloquet’s canal (arrows). The T2-weighted (c) and FLAIR (d) images, both with fat saturation, showed mild T2 hypointensity and mixed intensity on FLAIR with susceptibility changes suggestive of associated retinal hemorrhage (arrowheads). FLAIR: fluid-attenuated inversion recovery.
The sequence analysis of the patient revealed a thymine to cytosine nucleotide mutation in exon 3 (c.185) of the NDP gene. The thymine to cytosine nucleotide change in this location is expected to replace the codon for the amino acid leucine with proline at the codon 62 of the “Norrin” growth factor.
Discussion
Differential diagnosis of leukocoria in an infant includes tumors, phakomatoses, congenital malformations, vascular diseases, inflammatory diseases, and trauma.1 The most common cause of leukocoria is retinoblastoma, followed by PFV, coloboma, sclerosing endophthalmitis, Coats’ disease, and medulloepithelioma.2
The absence of calcification in our case predominantly excluded retinoblastoma from differential diagnosis. Foci of calcification are present in more than 90% of retinoblastoma cases, and are highly characteristic of the tumor, especially in children younger than 3 years of age.5 Bilateral retinoblastomas account for 20%–34% of cases; however, the proportion of bilateral tumors in patients younger than age 1 year, and those with the hereditary form of the disease, is higher.3 Based on the two-hit hypothesis, pathogenesis of retinoblastoma requires biallelic inactivation of Rb1 genes. Patients with heritable retinoblastomas have a new germline mutation in the Rb1 gene, or inherit the mutation from a parent. The second hit occurs as an acquired event, which explains why these patients commonly present with bilateral retinoblastoma and at a younger age.6,7 Retinoblastomas usually present as a hyperattenuating mass in the posterior globe, with contrast enhancement seen in 28% of cases.3 MRI can be used to determine choroidal invasion, optic nerve invasion posterior to the lamina cribrosa, and involvement of the anterior eye segment—all of which are associated with a worse prognosis.8
PFV is the most common benign mimic of retinoblastoma.1 The pathogenesis involves failure of normal regression of the embryonic hyaloid vascular system and its replacement by abnormal retinal vasculature—that normally occurs after four months of gestation.9 Abnormal retinal vascularization can also occur with many other pediatric vitreoretinopathies such as Norrie disease, familial exudative vitreoretinopathy, Coats’ disease, and retinopathy of prematurity.9
PFV patients often present with moderate microphthalmia, white vascularized retrolental tissue, severe intraocular hemorrhage, and different degrees of lenticular opacification.9 Although most cases of PFV are sporadic, it can also be inherited as an autosomal dominant or recessive trait.10,11 However, no candidate gene that can account for a substantial number of PFV patients has been reported yet.9 There are case reports of unilateral and bilateral PFV with mutations in the NDP and the Frizzled Class Receptor 4 (FZD4) genes.12–14 Norrin is a high-affinity ligand of FZD4 receptor in the Wnt signaling pathway, and it is hypothesized that a defect in Wnt signaling or apoptosis may play a role in development of PFV.15
The imaging findings in our case were most consistent with PFV. The eye globe in PFV usually reveals a stalk extending from the optic nerve to the posterior lens as a remnant of Cloquet’s canal that carries hyperdense vitreous and may contain the hyaloid artery.16 The vitreous body is usually hyperdense on CT scan, with abnormally high T1 and T2 signal on MRI scan.3,16 Microphthalmia, hemorrhage, and layering debris are usually visualized in the vitreous body. Calcification is rare. Post-contrast images may reveal enhancement of the vascular retrolental mass. Almost 90% of cases are unilateral; whereas bilateral cases have been associated with Norrie disease, and trisomy 13 syndromes.3,9,17
The uncommon presentation of bilateral PFV in our case raised the concern for Norrie disease, which was confirmed with a genetic test. Common presentations of Norrie disease include retinal malformation, deafness, and mental retardation.18,19 The majority of patients suffer from early-onset sensorineural hearing loss.20 Approximately 30% to 50% of these patients have some degree of cognitive retardation.4 The visual deficit in Norrie disease is due to deficient sprouting of the retinal vascular plexus.19,21 Norrie disease has been associated with bilateral PFV, hypoplastic optic nerves, and abnormal lenses.22 In a review of the literature, we found eight patients with proven bilateral PFV;13,18,21–25 among these patients, all except two had NDP gene mutations.13
The NDP gene encodes the Norrin protein, which is a secretory growth factor and plays a role in vascular development and maintenance through activation of the Wnt/β-catenin signaling cascade in the retina, stria vascularis of the inner ear, and brain.15 Norrin belongs to a family of cysteine-rich growth factors such as von Willebrand factor and transforming growth factor-β that all share a cysteine knot motif.19 Those mutations affecting the cysteine-knot motif of Norrin protein can lead to severe retinal dysgenesis and Norrie disease, like the presented case.26 Although the molecular mechanism of PFV is still unknown, it is conceivable that PFV and Norrie disease share overlapping molecular mechanisms.
Patients with PFV usually suffer from early cataract and closed-angle glaucoma. Enucleation may be inevitable in these patients following terminal glaucoma, intra-ocular hemorrhage, retinal detachment, and uveitis.1 Bilateral PHPV particularly has a poor prognosis in the absence of early vitrectomy.26 Unfortunately, the patient presented in this report had inconsistent treatment follow-ups with eventual blindness by the age of 3 years.
Other etiologies of leukocoria tend to present in children older than 3 years of age, including Coats disease, optic nerve glioma, toxocariasis, and medulloepithelioma.1–3,5 Coats disease is generally characterized by T1 and T2 hyperintense subretinal exudate (with high fat content).1–3 Patients with sclerosing endophthalmitis (toxocariasis) usually have a central vitreous granuloma, which is isointense to vitreous on T1-weighted images and hyperintense to vitreous on T2-weighted images.1–3 Medulloepitheliomas typically present as dense irregular masses in the ciliary body, although they may also arise in the retina.1–3
The presented case highlights the differential diagnosis of infantile leukocoria. The absence of calcification, characteristic persistent Cloquet’s canal remnant, and retinal hemorrhage were suggestive of PFV. Notably, bilateral PFV should raise the possibility of genetic disorders—most likely Norrie disease.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
None declared.
References
- 1.Balmer A, Munier F. Differential diagnosis of leukocoria and strabismus, first presenting signs of retinoblastoma. Clin Ophthalmol 2007; 1: 431–439. [PMC free article] [PubMed] [Google Scholar]
- 2.Razek AA, Elkhamary S. MRI of retinoblastoma. Br J Radiol 2011; 84: 775–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chung EM, Specht CS, Schroeder JW. From the archives of the AFIP: Pediatric orbit tumors and tumorlike lesions: Neuroepithelial lesions of the ocular globe and optic nerve. Radiographics 2007; 27: 1159–1186. [DOI] [PubMed] [Google Scholar]
- 4.Smith SE, Mullen TE, Graham D, et al. Norrie disease: Extraocular clinical manifestations in 56 patients. Am J Med Genet A 2012; 158A: 1909–1917. [DOI] [PubMed] [Google Scholar]
- 5.Apushkin MA, Apushkin MA, Shapiro MJ, et al. Retinoblastoma and simulating lesions: Role of imaging. Neuroimaging Clin N Am 2005; 15: 49–67. [DOI] [PubMed] [Google Scholar]
- 6.Knudson AG., Jr Mutation and cancer: Statistical study of retinoblastoma. Proc Natl Acad Sci U S A 1971; 68: 820–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Provenzale JM, Weber AL, Klintworth GK, et al. Radiologic-pathologic correlation. Bilateral retinoblastoma with coexistent pinealoblastoma (trilateral retinoblastoma). AJNR Am J Neuroradiol 1995; 16: 157–165. [PMC free article] [PubMed] [Google Scholar]
- 8.de Jong MC, de Graaf P, Noij DP, et al. Diagnostic performance of magnetic resonance imaging and computed tomography for advanced retinoblastoma: A systematic review and meta-analysis. Ophthalmology 2014; 121: 1109–1118. [DOI] [PubMed] [Google Scholar]
- 9.Shastry BS. Persistent hyperplastic primary vitreous: Congenital malformation of the eye. Clin Experiment Ophthalmol 2009; 37: 884–890. [DOI] [PubMed] [Google Scholar]
- 10.Khaliq S, Hameed A, Ismail M, et al. Locus for autosomal recessive nonsyndromic persistent hyperplastic primary vitreous. Invest Ophthalmol Vis Sci 2001; 42: 2225–2228. [PubMed] [Google Scholar]
- 11.Galal AH, Kotoury AI, Azzab AA. Bilateral persistent hyperplastic primary vitreous: An Egyptian family supporting a rare autosomal dominant inheritance. Genet Couns 2006; 17: 441–447. [PubMed] [Google Scholar]
- 12.Dhingra S, Shears DJ, Blake V, et al. Advanced bilateral persistent fetal vasculature associated with a novel mutation in the Norrie gene. Br J Ophthalmol 2006; 90: 1324–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pendergast SD, Trese MT, Liu X, et al. Study of the Norrie disease gene in 2 patients with bilateral persistent hyperplastic primary vitreous. Arch Ophthalmol 1998; 116: 381–382. [PubMed] [Google Scholar]
- 14.Robitaille JM, Wallace K, Zheng B, et al. Phenotypic overlap of familial exudative vitreoretinopathy (FEVR) with persistent fetal vasculature (PFV) caused by FZD4 mutations in two distinct pedigrees. Ophthalmic Genet 2009; 30: 23–30. [DOI] [PubMed] [Google Scholar]
- 15.Xu Q, Wang Y, Dabdoub A, et al. Vascular development in the retina and inner ear: Control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell 2004; 116: 883–895. [DOI] [PubMed] [Google Scholar]
- 16.Castillo M, Wallace DK, Mukherji SK. Persistent hyperplastic primary vitreous involving the anterior eye. AJNR Am J Neuroradiol 1997; 18: 1526–1528. [PMC free article] [PubMed] [Google Scholar]
- 17.Kaste SC, Jenkins JJ, 3rd, Meyer D, et al. Persistent hyperplastic primary vitreous of the eye: Imaging findings with pathologic correlation. AJR Am J Roentgenol 1994; 162: 437–440. [DOI] [PubMed] [Google Scholar]
- 18.Lev D, Weigl Y, Hasan M, et al. A novel missense mutation in the NDP gene in a child with Norrie disease and severe neurological involvement including infantile spasms. Am J Med Genet A 2007; 143A: 921–924. [DOI] [PubMed] [Google Scholar]
- 19.Parzefall T, Lucas T, Ritter M, et al. A novel missense NDP mutation [p.(Cys93Arg)] with a manifesting carrier in an Austrian family with Norrie disease. Audiol Neurootol 2014; 19: 203–209. [DOI] [PubMed] [Google Scholar]
- 20.Halpin C, Owen G, Gutiérrez-Espeleta GA, et al. Audiologic features of Norrie disease. Ann Otol Rhinol Laryngol 2005; 114: 533–538. [DOI] [PubMed] [Google Scholar]
- 21.Hatsukawa Y, Nakao T, Yamagishi T, et al. Novel nonsense mutation (Tyr44stop) of the Norrie disease gene in a Japanese family. Br J Ophthalmol 2002; 86: 1452–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mafee MF, Goldberg MF, Valvassori GE, et al. Computed tomography in the evaluation of patients with persistent hyperplastic primary vitreous (PHPV). Radiology 1982; 145: 713–717. [DOI] [PubMed] [Google Scholar]
- 23.Halpin C, Sims K. Twenty years of audiology in a patient with Norrie disease. Int J Pediatr Otorhinolaryngol 2008; 72: 1705–1710. [DOI] [PubMed] [Google Scholar]
- 24.LaRussa F, Wesson MD. Norrie’s disease vs. PHPV: One family’s dilemma. J Am Optom Assoc 1992; 63: 404–408. [PubMed] [Google Scholar]
- 25.Meire FM, Lafaut BA, Speleman F, et al. Isolated Norrie disease in a female caused by a balanced translocation t(X,6). Ophthalmic Genet 1998; 19: 203–207. [DOI] [PubMed] [Google Scholar]
- 26.Walsh MK, Drenser KA, Capone A, Jr, et al. Early vitrectomy effective for bilateral combined anterior and posterior persistent fetal vasculature syndrome. Retina 2010; 30(4 Suppl): S2–S8. [DOI] [PubMed] [Google Scholar]