Abstract
We present the magnetic resonance imaging findings of an eight-year-old boy with Epstein–Barr virus (EBV) encephalitis, with special attention to lesion neuroanatomic distribution, diffusion-weighted images, and proton magnetic resonance spectroscopy (MRS). T2 and FLAIR-weighted images showed bilateral and symmetric basal nuclei lesions, with diffusion facilitation. MRS of the lesions demonstrated elevated lactate/lipid and excitatory neurotransmitters. The purpose of this report is to alert to this imagiologic pattern of EBV infection, and in particular to the fact that facilitated diffusion does occur on EBV encephalitis.
Keywords: Encephalitis, Epstein–Barr virus, ADC map, magnetic resonance spectroscopy
Introduction
The Epstein–Barr virus (EBV) is a ubiquitous pathogen found in almost all people by the end of their second decade.1 The infection is usually benign, but in some limited situations (less than seven percent) neurologic complications can occur.2 The clinical outcome varies from complete recovery to death. The virus may have special tropism for the basal nuclei like some other infectious, metabolic or hypoxic, and ischemic diseases.
We report a child with EBV encephalitis showing bilateral, symmetrical, and diffuse lesions of basal ganglia, with facilitated diffusion, elevated lactate/lipid, and excitatory neurotransmitters on magnetic resonance spectroscopy (MRS). The combination of these findings may be quite distinctive of the disease.
Case report
An eight-year old boy was admitted to our hospital with a one week history of headaches and fever for three days. On the day of admission he developed seizures. He had no other history of disease and was immunocompetent. On arrival he was drowsy, had amygdaline hyperemia, and a stiff neck. He did not show focal neurological deficits.
Lab results showed leukocytosis, elevated AST (127 U/L), and ALT (105 U/I).3 Lumbar puncture revealed 76 white cells per mm (87% monocytes), glucose 3 mmol/L, protein 118 mg/dL, and lactate 2.6 mmol/L. The cerebrospinal fluid was negative for EBV, enterovirus, herpes simplex, mycoplasma, influenza A or B, and Chlamydia pneumoniae antigens. Serologic analysis, including EBV antigen at polymerase chain reaction (PCR), was also unrevealing. Computed tomography of the brain on the admission day was normal. Electroencephalography (EEG) showed slow bihemispheric activity with paroxistic activity on the right frontocentral region.
The diagnosis of encephalitis was presumed and the patient was treated with acyclovir 20 mg/kg/dose 8-8 h ev, ceftriaxone 100 mg/kg ev id, and ciprofloxacin 20 mg/kg 12-12 h ev. For the following two days he had generalized tonicoclonic seizures, which responded to antiepileptic drugs.
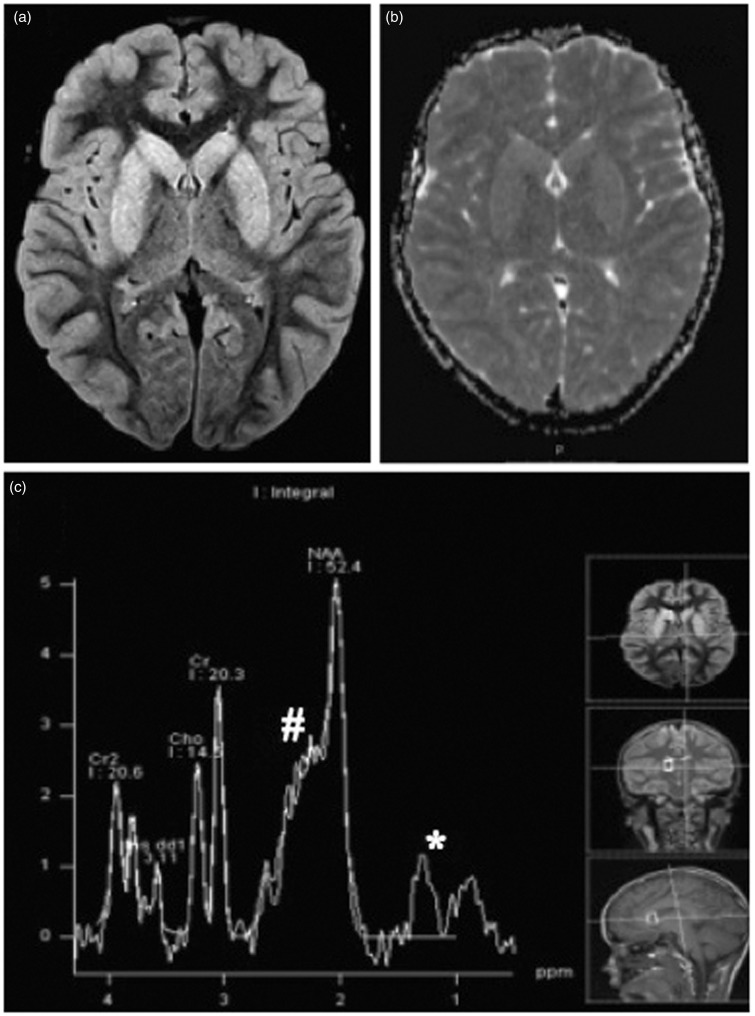
Brain MR performed on the second day in hospital showed diffuse and symmetrical increased signal intensity of the basal ganglia on T2 and FLAIR-weighted images, involving the all caudate and lenticular nuclei (Figure 1(a)). Basal ganglia showed facilitated diffusion, with increase signal on apparent diffusion coefficient (ADC) maps (Figure 1(b)). There was also slight diffuse edema of the cerebral cortex, most prominent on right frontal lobe, without signs of restricted or facilitated diffusion in the cortex. There was no white matter involvement and no contrast enhancement was noted. Multiple-voxel MRS acquired using short echo time (30 ms) showed elevation of excitatory amino acids levels and lactate/lipid peak on basal ganglia (Figure 1(c)).
Figure 1.
(a) Axial FLAIR image shows hyperintense signal of the basal nuclei, bilateral and symmetric. (b) ADC map demonstrates diffusion facilitation in the basal nuclei. (c) Proton MRS from right caudate nuclei at TE of 30 ms reveals the presence of lactate/lipid (*) and elevation in excitatory neurotransmitters (#). The case originally appeared on the AJNR website.
Positive EBV antigen in cerebrospinal fluid at PCR was found on the twelfth day after the beginning of the disease. Complete clinical recovery occurred over the course of the following months.
Discussion
EBV encephalitis has a wide range of both clinical and MR findings. Imaging abnormalities can range from normal to diffuse signal intensity changes either in gray or white matter.2 The regions most frequently involved in EBV encephalitis, as demonstrated by imaging, are cerebral hemispheres, basal ganglia, cerebellum, brain stem, thalamus, and limbic system.1,3
EBV may have a special tropism for the deep nuclei and therefore a characteristic pattern is that of increased T2-weighted signal in the thalami and basal ganglia.2 In our patient the tropism of the virus for basal ganglia is shown, but what is more notorious is its diffuse and totally symmetric involvement, as often seen on metabolic diseases. Many types of encephalitis have been reported to have restricted water motion. However, findings of diffusion-weighted images (DWI) in encephalitis are less predictable than in infarction.4 The use of DWI and ADC maps on EBV central nervous system infections has been reported in only a few articles. Reduced ADC values, representing cytotoxic edema, have been found in the splenium corpus callosum and cerebral cortex in EBV encephalitis.2,3,5 Facilitated diffusion, as found in our case, has been seldom reported.5,6 It indicates that the lesions might be associated with vasogenic edema instead of cytotoxic edema and may be predictive of a good outcome, as happened in the case presented. Primary inflammation, secondary autoimmune reaction or both are possible mechanisms of encephalitis by EBV and the presence of high ADC values might be related to an autoimmune pathogenic mechanism.6 There have been few reports of MRS in patients with viral encephalitis. The most common finding is a non-specific reduction in N-acetyl aspartate (NAA) and in some instances increased lactate.4 MRS is a useful magnetic resonance imaging adjunct, but non-specific, that on EBV infection has been reported to show decreased NAA and increased levels of myo-inositol, excitatory amino acids, and macromolecules, providing evidence for infection and inflammation.5,7 Our findings on MRS support cerebral injury by elevation of excitatory neurotransmitters. This report demonstrates EBV predilection for basal ganglia and highlights the occurrence of facilitated diffusion on EBV encephalic lesions, differentiating it from encephalitis most commonly associated with restricted diffusion. Whether this is a characteristic feature of this form of encephalitis or an individual variation remains to be proved.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
None declared.
References
- 1.Baskin HJ, Hedlund G. Neuroimaging of herpes virus infections in children. Pediatr Radiol 2007; 37: 949–963. [DOI] [PubMed] [Google Scholar]
- 2.Hagemann G, Mentzel HJ, Weisser H, et al. Multiple reversible MR signal changes caused by Epstein–Barr virus encephalitis. Am J Neuroradiol 2006; 27: 1447–1449. [PMC free article] [PubMed] [Google Scholar]
- 3.Abul-Kasim K, Palm L, Maly P, et al. The neuroanatomic localization of Epstein–Barr virus encephalitis may be a predictive factor for its clinical outcome: A case report and review of 100 cases in 28 reports. J Child Neurol 2009; 24: 720–726. [DOI] [PubMed] [Google Scholar]
- 4.Zimmerman RD. Physiological imaging in infection, inflammation and demyelination: Overview. In: Gillard JH, Waldman AD, Barker PB. (eds). Clinical MR neuroimaging: Physiological and functional techniques, New York: Cambridge University Press, 2005, pp. 415. [Google Scholar]
- 5.Özbek O, Koç O, Paksoy Y, et al. Epstein–Barr virus encephalitis: findings of MRI, MRS, diffusion and perfusion. Turkish J Pediatrics 2011; 53: 680–683. [PubMed] [Google Scholar]
- 6.Oh MJ, Lim SJ, Kim YJ, et al. A case of brainstem encephalitis associated with Epstein–Barr virus infection. J Korean Child Neurol Soc 2011; 18: 277–282. [Google Scholar]
- 7.Cecil KM, Jones BV, Williams S, et al. MRI and MRS of Epstein–Barr virus infection: Case report. Neuroradiology 2000; 42: 619–622. [DOI] [PubMed] [Google Scholar]