Abstract
Background
Hemimegalencephaly is a rare hamartomatous entity characterised by enlargement of all or part of the cerebral hemisphere ipsilaterally with cortical dysgenesis, large lateral ventricle and white matter hypertrophy with or without advanced myelination. Although conventional magnetic resonance imaging (MRI) is useful for detecting these diagnostic features, hemimegalencephaly is not always easily distinguished from other entities, especially when hemimegalencephaly shows blurring between the grey and white matter. Diffusion tensor imaging (DTI) is a functional MRI technique commonly used to assess the integrity of white matter. The usefulness of DTI in assessing hemimegalencephaly has not been fully elucidated. In this study, we clarified the characteristics of hemimegalencephaly with regard to DTI and its parameters including fractional anisotropy and apparent diffusion coefficient.
Methods
Three patients with hemimegalencephaly underwent MRI including DTI. We first visually compared fractional anisotropy mapping and conventional MRI. Next, we quantitatively measured the fractional anisotropy and apparent diffusion coefficient values in the subcortical white matter of the hemisphere with hemimegalencephaly and corresponding normal-appearing contralateral regions and analysed the values using the Mann–Whitney U test.
Results
On fractional anisotropy mapping, we could clearly distinguish the junction of grey and white matter and observed thicker white matter in the hemisphere with hemimegalencephaly, which was unclear on conventional MRI. The white matter in the hemisphere with hemimegalencephaly showed significantly higher fractional anisotropy (P < 0.0001) and lower apparent diffusion coefficient (P = 0.0022) values than the normal contralateral side.
Conclusion
DTI parameters showed salient hemimegalencephaly features and could be useful in its assessment.
Keywords: Hemimegalencephaly, magnetic resonance imaging, diffusion tensor imaging, fractional anisotropy, apparent diffusion coefficient
Introduction
Hemimegalencephaly (HME) is a rare hamartomatous entity characterised by enlargement of all or part of the cerebral hemisphere ipsilaterally with cortical dysgenesis, large lateral ventricle and white matter hypertrophy.1–3 Although conventional magnetic resonance imaging (MRI) is useful for detecting these diagnostic features, HME is not always easily distinguished from other entities, especially when HME shows blurring between the grey and white matter, which is also observed in other diseases including focal cortical dysplasia, tuberous sclerosis and glioma.3,4
Diffusion tensor imaging (DTI) is a functional MRI technique commonly used to assess the integrity of white matter by measuring water diffusion, and it has been applied to assess specific white matter bands using fibre tracking and to detect axonal impairment resulting from various diseases.5–7 Among several functional DTI parameters, fractional anisotropy (FA), apparent diffusion coefficient (ADC) and the fibre tracking method are widely used clinically to assess white matter conditions.8–12 Briefly, two studies investigated the fibre tracking method to assess white matter bundles in patients with HME,13,14 but the usefulness of FA and ADC for assessing HME was not reported. In the present study, we clarified the HME characteristics with regard to FA and ADC.
Materials and methods
The present study was approved by the institutional review board and informed consent was waived. We evaluated retrospectively the imaging findings of three patients with HME (1 month, 2 months and 18 years of age) who underwent MRI with a 3.0 Tesla unit (Intera Achieva 3.0T Quasar Dual; Philips Medical Systems, Best, The Netherlands) including conventional MRI and DTI. The conventional MRI sequences included T1-weighted imaging (TR/TE, 450/12 ms; 6 mm thickness; 7 mm intersection gap; number of acquisitions, 20; pixel matrix, 251) and T2-weighted imaging (TR/TE, 4000/100 ms; 6 mm thickness; 7 mm intersection gap; number of acquisitions, 20; pixel matrix, 268). For DTI analysis, we used single-shot spin-echo echo-planar sequences with TR/TE, 4461–5667/61 ms; 3 mm slice thickness and no intersection gap; field of vision, 224 × 224 mm; number of excitations, 4; pixel matrix, 128 × 128; voxel size, 1.75 × 1.75 × 3.00 mm; and 50 continuous transverse slices. We measured diffusion along 15 non-collinear directions using a diffusion-weighted factor b in each direction for 800 s/mm2.
First, two neuroradiologists with 5 and 17 years of experience visually compared the signal pattern of the affected cerebral hemisphere with the normal side on both conventional magnetic resonance images and the FA map. Next, we measured quantitatively the FA and ADC values in the subcortical white matter. Using image-processing software (Dr. View/Linux; Infocom Corporation, Tokyo, Japan), we placed regions of interest (ROIs) symmetrically at subcortical regions beneath the cortex in the affected hemisphere and the corresponding normal-appearing contralateral regions. For each subject, 24–36 ROIs (12–18 pairs) of 50 pixels each were placed onto the three representative slices of FA map (Figure 1), and mean FA and ADC values were calculated for each hemisphere by applying the identical ROI masks to both FA and ADC mappings.
Figure 1.
Representative locations of regions of interest.
ROIs are placed symmetrically at subcortical regions beneath the cortex in the affected hemisphere and the corresponding normal-appearing contralateral regions on (a) FA and (b) ADC mapping. 128 × 76 mm (300 × 300 DPI).
ROI: region of interest; FA: fractional anisotropy; ADC: apparent diffusion coefficient.
For statistical analyses of FA and ADC values, Mann–Whitney U tests were performed using the JMP Pro Version 9.0 software (SAS Institute, Inc., Cary, NC, USA). A P value less than 0.05 was considered to indicate statistical significance.
Results
We distinguished visually the junction of grey and white matter more clearly on FA mapping than on conventional MRI, and observed thicker white matter in the hemisphere with HME in both infants and the young adult patient (Figures 2 and 3). On conventional MRI, the junction of the grey and white matter was blurred on both T2 and T1-weighted images, which could be attributed to signal changes in the white matter. Compared with the normal contralateral side on the T2-weighted image, the white matter signal in the hemisphere with HME was decreased in the infant patients 1 and 2 (1 and 2 months of age, respectively), whereas in patient 3 (18 years of age), the signal of the affected white matter was relatively hyperintense.
Figure 2.
Patient 1. A 1-month-old boy with right-sided HME.
(a) On T2 and (b) T1-weighted images, the junction of cortical grey and white matter is inconspicuous as a result of the decreased signal of white matter on the T2-weighted image and increased signal on the T1-weighted image, suggesting advanced myelination. (c) FA and (d) colour FA maps clearly show the junction of grey and white matter as well as increased white matter volume in the right hemisphere. The colour FA map shows fibre directions; anteroposterior (green), right and left (red) and craniocaudal (blue).
HME: hemimegalencephaly; FA: fractional anisotropy.
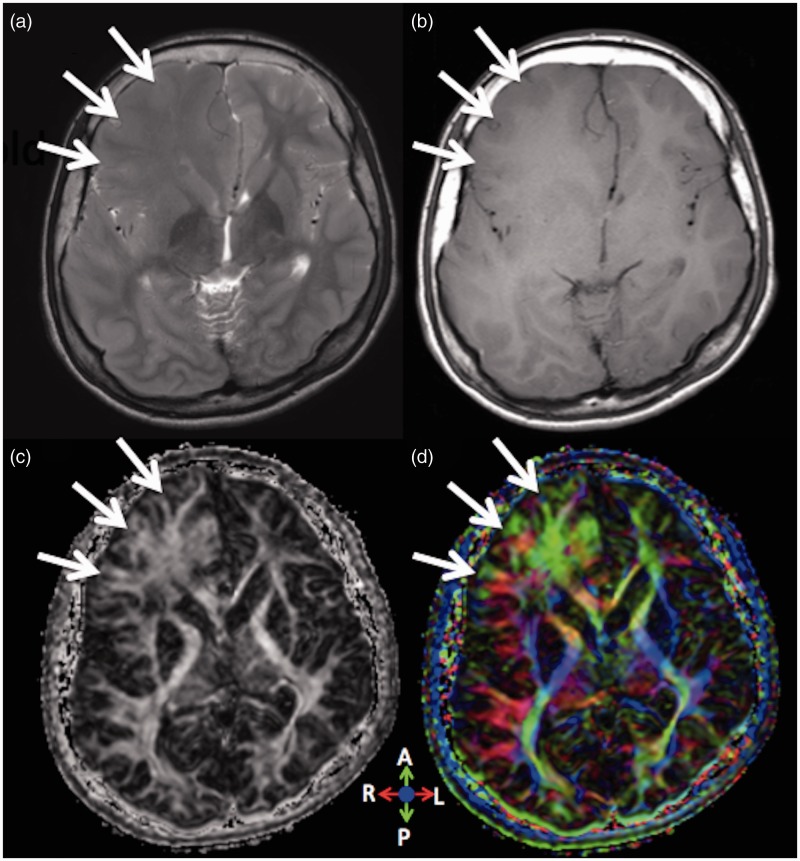
Figure 3.
Patient 3. An 18-year-old man with right-sided HME.
The diagnosis was uncertain when the patient visited our hospital with a tentative diagnosis of brain tumour. (a) On T2 and (b) T1-weighted images, the junction of cortical grey and white matter is inconspicuous in the right frontal lobe. (a) On the T2-weighted image, the right frontal white matter shows slight hyperintensity compared with the contralateral normal side. (c) FA and (d) colour FA maps clearly show the junction of grey and white matter as well as increased white matter volume in the right frontal lobe, indicating a diagnosis of HME, not a brain tumour. The colour FA map shows fibre directions; anteroposterior (green), right and left (red) and craniocaudal (blue).
HME: hemimegalencephaly; FA: fractional anisotropy.
In the hemisphere with HME, the mean FA value was 0.405 and the standard deviation (SD) was 0.129. In the normal contralateral side, the mean FA value was 0.34 and SD was 0.117. In the hemisphere with HME, the mean ADC value was 902.3 × 10−6 mm2/s and the SD was 210.4 × 10−6 mm2/s. In the normal contralateral side, the mean ADC value was 943.0 × 10−6 mm2/s and the SD was 223.1 × 10−6 mm2/s (Table 1). The white matter in the hemisphere with HME had significantly higher FA (P < 0.0001) and lower ADC (P = 0.0022) values than the normal contralateral side (Figure 4).
Table 1.
The mean FA and ADC values of each HME patient.
| Affected side | Contralateral side | |
|---|---|---|
| Mean FA (SD) | ||
| Patient 1 | 0.337 (0.036) | 0.275 (0.067) |
| Patient 2 | 0.296 (0.039) | 0.256 (0.032) |
| Patient 3 | 0.564 (0.073) | 0.477 (0.067) |
| Total | 0.405 (0.129) | 0.340 (0.117) |
| Mean ADC (SD) (×10−6 mm2/second) | ||
| Patient 1 | 1040.4 (56.0) | 1148.5 (82.2) |
| Patient 2 | 1065.6 (35.5) | 1008.7 (57.3) |
| Patient 3 | 624.5 (42.4) | 662.6 (35.8) |
| Total | 902.3 (210.4) | 943.0 (223.1) |
FA: Fractional anisotropy; ADC: apparent diffusion coefficient; HME: hemimegalencephaly; SD: standard deviation.
Figure 4.
Box plot of FA and ADC values of all HME patients. The white matter in the hemimegalencephalic hemisphere (affected side) showed (a) significantly higher FA values (P < 0.0001) and (b) lower ADC values (P = 0.0022) than the normal contralateral side.
FA: fractional anisotropy; ADC: apparent diffusion coefficient.
Discussion
Yagishita et al. reported that the characteristic signal change termed advanced myelination on conventional MRI was a decreased signal on T2-weighted imaging and an increased signal on T1-weighted imaging in the white matter of the affected hemisphere, which is an imaging feature of HME in patients aged 18 months or less.15 The same signal pattern consistent with advanced myelination was observed in the two infant patients 1 and 2 months of age, which indicated the diagnosis of HME using only conventional MRI. However, in the young adult patient 3 (18 years of age), blurring of the junction between grey and white matter without the advanced myelination signal pattern on conventional MRI led to a tentative diagnosis of a brain tumour with differential diagnoses of other disease entities including focal cortical dysplasia, tuberous sclerosis and HME (Figure 3). In such cases, conventional MRI alone might be inadequate to distinguish HME from other entities.
We attempted FA and ADC of DTI in three patients with HME. On visual inspection, the junction of grey and white matter on the affected side was clearly distinguishable on the FA mapping consistently but was less distinct on conventional MRI sequences (Figures 2 and 3). In addition to a clear delineation of the grey and white matter junction on the affected side, FA mapping showed increased volume of the white matter (as if subcortical white matter was broadly extending towards the affected gyri), which was consistent with documented reports that white matter hypertrophy or hypermyelination of the subcortical U-fibre is a pathological characteristic of HME.14,16
Despite the dissociation of signal patterns on conventional MRI between the two age groups, infant and young adult cases (Figures 2 and 3), ROI quantitative analyses consistently showed significantly higher FA and lower ADC values in the subcortical white matter of the affected hemisphere compared with the contralateral side in both groups. To the best of our knowledge, this is the first report of FA and ADC values in patients with HME. ADC and FA are two major parameter types of DTI used for the assessment of white matter integrity; ADC is a measurement of the rate of microscopic water motion without reference to any one direction and FA is a measurement of anisotropy.17 ADC decreases as more barriers to random water motion (e.g. cell membranes, myelinated axonal projections and extracellular molecules) increase in number,18 and FA values are reportedly positively correlated with axonal density and negatively correlated with myelin thickness.19 In a variety of diseases including glioma, cortical dysplasia, tuberous sclerosis, demyelinating disease and diffuse axonal injury, pathological white matter is usually associated with decreased FA values and increased ADC values.7–11,20 The altered FA and ADC values may reflect a pathological loss of integrity of white matter.21,22 Conversely, in the present study, increased FA values and decreased ADC values were observed consistently in the hemispheres with HME regardless of the age group. Several studies using the DTI fibre tracking method have detected aberrant (excessive) white matter bundles as well as excessive subcortical U-fibre in the affected hemispheres of HME patients.13,14 Therefore, we hypothesised that higher FA and lower ADC values at the subcortical regions beneath the HME cortices may reflect increased axonal density and decreased free water in the extracellular space due to the pathological excessive fibre bundles and increased volume of white matter (white matter hypertrophy). Thus, regardless of the patients’ age range, characteristic alteration of DTI parameters, higher FA and lower ADC values may indicate more precisely microstructural changes in the white matter associated with HME than conventional MRI, and therefore might be helpful in distinguishing HME from other diseases.
Our study had several limitations. First, the number of study patients was small. In addition, we did not conduct histopathological investigations and thereby validate the actual pathological background associated with the signal changes in conventional MRI and DTI parameters. Although progressive hemispheric shrinking due to age has been reported in HME patients,23 we did not evaluate longitudinal changes of FA and ADC values in the same patients with HME to investigate different phases of disease such as hypertrophic and shrinking phases. Time-sequential changes through different phases should thus be investigated.
Conclusions
DTI was useful for assessing HME, elucidating white matter hypertrophy, a main characteristic of HME, and demonstrating significantly higher FA and lower ADC values on the affected side than on the normal contralateral side.
Acknowledgements
The authors are especially grateful to Noriko Kurihara, Department of Diagnostic Radiology, and Hiroyoshi Suzuki, Department of Pathology, National Hospital Organization, Sendai Medical Center for their professional advice.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Broumandi DD, Hayward UM, Benzian JM, et al. Best cases from the AFIP: hemimegalecephaly. Radiographics 2004; 24: 843–848. [DOI] [PubMed] [Google Scholar]
- 2.Flores-Sarnat L. Hemimegalencephaly: part 1. Genetic, clinical, and imaging aspects. J Child Neurol 2002; 17: 373–384. [DOI] [PubMed] [Google Scholar]
- 3.Kalifa GL, Chiron C, Sellier N, et al. Hemimegalencephaly: MR imaging in five children. Radiology 1987; 165: 29–33. [DOI] [PubMed] [Google Scholar]
- 4.Yagishita A, Arai N. Cortical tubers without other stigmata of tuberous sclerosis: imaging and pathological findings. Neuroradiology 1999; 41: 428–432. [DOI] [PubMed] [Google Scholar]
- 5.Stadlbauer A, Nimsky C, Buslei R, et al. Diffusion tensor imaging and optimized fiber tracking in glioma patients: histopathologic evaluation of tumor-invaded white matter structures. NeuroImage 2007; 34: 949–956. [DOI] [PubMed] [Google Scholar]
- 6.Renoux J, Facon D, Fillard P, et al. MR diffusion tensor imaging and fiber tracking in inflammatory diseases of the spinal cord. Am J Neuroradiol 2006; 27: 1947–1951. [PMC free article] [PubMed] [Google Scholar]
- 7.Lee JS, Han MK, Kim SH, et al. Fiber tracking by diffusion tensor imaging in corticospinal tract stroke: topographical correlation with clinical symptoms. NeuroImage 2005; 26: 771–776. [DOI] [PubMed] [Google Scholar]
- 8.Widjaja E, Zarei Mahmoodabadi S, Otsubo H, et al. Subcortical alterations in tissue microstructure adjacent to focal cortical dysplasia: detection at diffusion-tensor MR imaging by using magnetoencephalographic dipole cluster localization. Radiology 2009; 251: 206–215. [DOI] [PubMed] [Google Scholar]
- 9.Karadag D, Mentzel HJ, Güllmar D, et al. Diffusion tensor imaging in children and adolescents with tuberous sclerosis. Pediatr Radiol 2005; 35: 980–983. [DOI] [PubMed] [Google Scholar]
- 10.Piao C, Yu A, Li K, et al. Cerebral diffusion tensor imaging in tuberous sclerosis. Eur J Radiol 2009; 71: 249–252. [DOI] [PubMed] [Google Scholar]
- 11.Provenzale JM, McGraw P, Mhatre P, et al. Peritumoral brain regions in gliomas and meningiomas: investigation with isotropic diffusion-weighted MR imaging and diffusion-tensor MR imaging. Radiology 2004; 232: 451–460. [DOI] [PubMed] [Google Scholar]
- 12.Stadlbauer A, Ganslandt O, Buslei R, et al. Gliomas: histopathologic evaluation of changes in directionality and magnitude of water diffusion at diffusion-tensor MR imaging. Radiology 2006; 240: 803–810. [DOI] [PubMed] [Google Scholar]
- 13.Sato N, Ota M, Yagishita A, et al. Aberrant midsagittal fiber tracts in patient with hemimegalencephaly. Am J Neuroradiol 2008; 29: 823–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamiya K, Sato N, Saito Y, et al. Accelerated myelination along fiber tracts in patients with hemimegalencephaly. J Neuroradiol 2014; 41: 202–210. [DOI] [PubMed] [Google Scholar]
- 15.Yagishita A, Arai N, Tamagawa K, et al. Hemimegalencephaly: signal changes suggesting abnormal myelination on MRI. Neuroradiology 1998; 40: 734–738. [DOI] [PubMed] [Google Scholar]
- 16.Kato M, Mizuguchi M, Sakuta R, et al. Hypertrophy of the cerebral white matter in hemimegalencephaly. Pediatr Neurol 1996; 14: 335–338. [DOI] [PubMed] [Google Scholar]
- 17.Beaulieu C, Allen PS. Determinations of anisotopic water diffusion in nerves. Magn Reson Med 1999; 31: 394–400. [DOI] [PubMed] [Google Scholar]
- 18.Provenzale JM, Isaacson J, Chen S, et al. Correlation of apparent diffusion coefficient and fractional anisotropy values in the developmental infant brain. Am J Roentgenol 2010; 195: W456–462. doi: 10.2214/AJR.10.4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Concha L, Livy DJ, Beaulieu C, et al. In vivo diffusion tensor imaging and histopathology of the fimbria-fornix in temporal lobe epilepsy. J Neurosci 2010; 30: 996–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Widjaja E, Blaser S, Miller E, et al. Evaluation of subcortical white matter and deep white matter tracts in malforations of cortical development. Epilepsia 2007; 48: 1460–1469. [DOI] [PubMed] [Google Scholar]
- 21.Whittall KP, MacKay AL, Graeb DA, et al. In vivo measurement of T2 distributions and water contents in normal human brain. Magn Reson Med 1997; 37: 34–43. [DOI] [PubMed] [Google Scholar]
- 22.Urbach H (ed). MRI in Epilepsy. Heidelberg: Springer Verlag; 2013. p. 145.
- 23.Becherini F, Pisano T, Castagna M, et al. Progressive hemispheric shrinking in hemimegalencephaly: a possible role for seizure-related neuronal loss. Dev Med Child Neurol 2008; 50: 553–557. [DOI] [PubMed] [Google Scholar]