Abstract
Posterior reversible encephalopathy syndrome (PRES) is a clinico-radiological condition, generally observed in conjunction with severe and acute hypertension, that involves mainly the posterior head areas (occipital and temporal lobes) and anterior “watershed” areas. In this syndrome it is rare to observe a predominant involvement of the brainstem. We describe the clinical and radiological findings in a patient with brainstem involvement, discussing its pathophysiological features and possible differential diagnosis.
Keywords: Hypertensive encephalopathy, brainstem posterior reversible encephalopathy, vasogenic oedema
Introduction
Posterior reversible encephalopathy syndrome (PRES) is a syndrome characterized by a series of symptoms that can be observed in the course of severe hypertension, such as headache, seizures, cortical blindness, papilledema and depression of consciousness.1 The imaging findings consist of vasogenic oedema that is predominantly seen in the white matter of the cerebral hemispheres, especially posterior regions2 (occipital lobe). If the establishment of hypertension treatment is well timed, the clinical and radiological features can regress; thus, this condition is known as “posterior reversible leukoencephalopathy” syndrome.3
A variant of this syndrome with predominant involvement of the brainstem has rarely been reported. We present a case and discuss pathophysiological, clinical, and radiographic features of this variant.
Case report
A 32-year-old Hispanic woman was admitted to our institution with a history of an abortion episode 1 month before, and of headache and fever during the last 10 days, who developed a decreased level of consciousness progressing to coma, without visual abnormalities or hypertension (blood pressure 120/70 mm Hg). The blood count revealed a haemoglobin level of 11.2 g/dl and a platelet count of 497,000/mm3. Laboratory data showed also increase of erythrocyte sedimentation rate, antithrombin III, D-dimer and C reactive protein.
Computed tomography (CT) of the head, performed 48 h after the onset of symptoms, showed diffuse and nuanced alteration of the signal (hypodensity) in the right basal ganglia region. The study protocol included: Turbo Spin Echo T2-weighted sequence (TSE T2) on coronal and axial plane, Turbo Spin Echo T1 weighted sequence (TSE T1) on axial plane, diffusion-weighted imaging (DWI) and apparent diffusion coefficient (ADC) map images on sagittal and coronal plane (Figure 1).
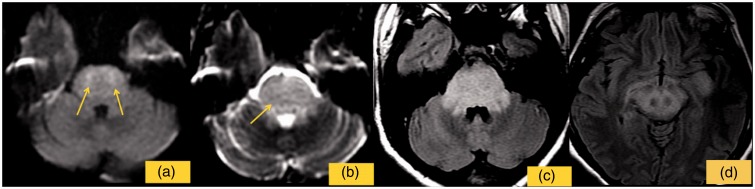
Figure 1.
The image shows the first control performed 48 h after clinical onset. DWI shows small restricted foci in the pons (a) high values of ADC map (b) and FLAIR shows high signal intensity in the brainstem and in the supra-tentorial region (c, d).
Magnetic resonance imaging (MRI) demonstrated extensive increased signal on fluid attenuation inversion recovery (FLAIR) and FSE T2 sequences in the pons, midbrain, and midbrain peduncles, with cranial and symmetric extension to the sub-thalamic, temporal, and basal ganglia regions bilaterally. The ADC map showed increased values in these regions, with restricted diffusion in the pons. Magnetic resonance angiography of the intra- and extra-cranial circulation was normal.
The last MRI follow-up, 5 months later, showed pontine high signal areas on T2-weighted and FLAIR sequences, representing the residual ischemic damage, with complete regression of signal alterations of the supra-tentorial regions (Figure 3).
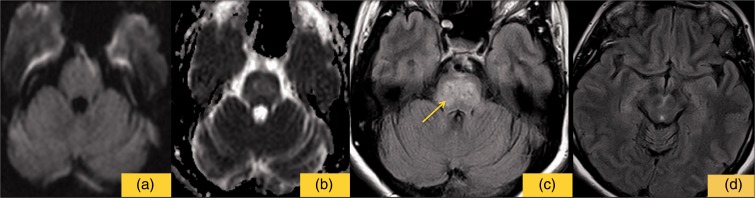
Figure 3.
The image shows the last MRI follow-up. (a) shows no restricted signal on DWI sequence. ADC values are slight increased (b). On FLAIR sequences pontine high signal areas (c), representing the residual ischemic damage, with complete regression signal alterations of the supra-tentorial regions (d).
Clinically the patient had a regression of the symptoms, with residual non-coordination between superior and inferior limbs.
The patient was treated with Clexane and magnesium sulphate, a drug used for the treatment of eclamptic seizures; this drug, unlike traditional antiepileptic drugs, does not present substantial harmful effects.4 The patient has also undertaken rehabilitation therapy for limb co-ordination.
Discussion
PRES is a clinical-radiological syndrome which presents with radiological findings of reversible vasogenic sub-cortical oedema, that affects especially the posterior head regions (occipital lobe), with symmetrical and bilateral involvement, associated with clinical symptoms secondary to hypertensive encephalopathy;3 this syndrome has a complete clinical and radiographic regression3 if timely treatment is established. Neuroimaging, but above all MRI, is fundamental for early diagnosis and differential diagnosis.
The alteration of the signal, on neuroimaging, is mainly due to vasogenic oedema in sub-cortical white matter,3 involving above all the posterior regions, in particular the parieto-occipital lobes and cerebellar hemispheres bilaterally;5 this abnormality is represented with high signal intensity areas on T2-weighted and FLAIR images.6
It is also important to differentiate vasogenic oedema (reversible form), the predominant abnormality, from cytotoxic oedema (irreversible form);6 for this purpose we can use DWI and ADC map images. Because vasogenic rather than cytotoxic oedema is the principal basis for the lesions, regions demonstrate high signal intensity on T2-weighted images, isointense or slightly increased signal intensity on diffusion-weighted images7 and increased ADC values (appear brighter)7 in ADC mapping. Most lesions do not enhance on T1-weighted images.
Clinical presentation of PRES is characterized by headache,8,9 papilledema, visual disturbances, seizures, decreased level of consciousness (including coma) and other neurologic disturbances.
The syndrome is correlated with many clinical conditions such as:
– preeclampsia/eclampsia/HELLP syndrome;
– immunosuppressive/cytotoxic drugs (e.g. antineoplastic drugs, cyclosporin, interferon-alfa, antiretroviral therapy);
– thrombotic thrombocytopenic purpura, haemolitic uraemic syndrome;
– acute and chronic renal diseases, such as glomerulonephritis;
– high-dose steroid therapy;
– liver failure;
– porphyria;
– endocrine dysfunctions;
– bone marrow transplantation;
– hypercalcaemia, hyperparathyroidism;
– erythropoietin therapy, massive blood transfusion;
– autonomic instability (in patient with spinal cord injuries, Guillain-Barré syndrome);
– other causes (e.g. intravenous globulin, exposure to sympathomimetic drugs, contrast media exposure, digitoxin intoxication).10
The clinical presentation reveals also cerebellar, bulbar, and/or long tract signs in about one-third of cases; retinal abnormalities have also been observed in a similar proportion of cases.11,12 Most patients improve clinically and radiologically within a brief period of days to a few weeks (Figure 2).
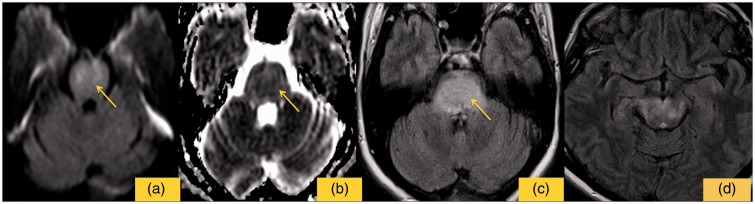
Figure 2.
The image shows no restricted signal on DWI sequence (a), values of ADC map are slightly increased (b), high signal of the pons on FLAIR sequence (c), while lower signal than the first control for supra-tentorial regions (d).
In this case, this condition occurred in an obstetric patient with abnormal pregnancy.13 The high prevalence of obstetric cases may in part be explained by the fluid accumulation often observed especially during the puerperium, which may support and accentuate the tendency to develop brain oedema.14 There are also some predisposing factors occurring during eclampsia, such as altered vascular reactivity, deficiency of vasodilating prostaglandins and endothelial dysfunction,14 that may predispose to PRES.
In our case, the patient showed symptoms belatedly a period after abortion, in the absence of previous pre-eclamptic signs, which is not frequently reported. The subclinical presentation with slight symptoms (headache, fever and diplopia) was undervalued, and the patient progressed to coma without immediately undergoing MRI. Some 48 h after clinical onset, MRI showed vasogenic and cytotoxic oedema confirmed by high values of ADC map associated with small restricted foci in the pons, probably due to the delay between clinical presentation and diagnostic evaluation.
Various neurologic pathologies must be considered in the differential diagnosis with the brainstem variant, such as brainstem infarction, pontine glioma, central pontine myelinolysis and infective encephalitis.4
The last MRI follow-up showed pontine high signal areas on T2-weighted and FLAIR sequences, representing residual ischemic damage, with complete regression of signal alterations of the supra-tentorial regions. This partial reversibility of the lesions can be explained by the different compensation between intracerebral supra-tentorial and brainstem circulation. Clinically the patient had regression of the symptoms with residual incoordination between superior and inferior limbs.
There are various theories behind the origin of these lesions that may explain the clinical and radiological abnormalities associated with PRES. According to the first hypothesis, ischemia and cytotoxic oedema, involving mainly the border-zone arterial regions, is produced by a spasm of the cerebral vasculature in response to acute hypertension (i.e. “overregulation”).15 A more recent hypothesis suggests that the syndrome results from passive overdistension of cerebral arterioles due to a breakthrough of autoregulation;16 this would lead to focal vasogenic (hydrostatic) oedema, in the peripheral vascular distribution of the involved vessel, for interstitial extravasation of fluid and proteins.
Reversible brainstem encephalopathy with characteristic MRI features can be caused not only by hypertension, but also by other causes; in this case the patient had normal pressure (120/70 mmHg), as well as an increased of d-dimer and an abortum history, that we consider the two main aspects. Thus, a brainstem variant of PRES, rather than hypertensive brainstem encephalopathy, was thought to be a more appropriate description in this patient.17 The brainstem is frequently involved in combination with supra-tentorial structures: in this case we observed the involvement of sub-thalamic, temporal and basal ganglia region bilaterally.
The clinical and radiological aspects can regress if the establishment of hypertension treatment is well timed; furthermore, this syndrome requires no additional therapy if the pressure is stabilized.18
Conclusion
Brainstem encephalopathy is a rare variant of PRES, causing slight clinical presentation until coma. The association between sometimes nuanced clinical (as in this case) and MRI findings is fundamental to allow an early diagnosis and avoid the worst consequences.
MRI is the gold standard technique to obtain differential diagnosis and follow-up of the condition.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Vaughan CJ, Delanty N. Hypertensive emergencies. Lancet 2000; 356: 411–417. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz RB, Jones KM, Kalina P, et al. Hypertensive encephalopathy: Findings on CT, MR imaging, and SPECT imaging in 14 cases. AJR Am J Roentgenol 1992; 159: 379–383. [DOI] [PubMed] [Google Scholar]
- 3.Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med 1996; 334: 494–500. [DOI] [PubMed] [Google Scholar]
- 4.Calhoun DA, Oparil S. Treatment of hypertensive crisis. N EngI J Med 1990; 323: 1177–1183. [DOI] [PubMed] [Google Scholar]
- 5.Lamy C, Oppenheim J, M´eder F, Mas JL. Neuroimaging in posterior reversible encephalopathy syndrome. J Neuroimag 2004; 14: 89–96. [PubMed] [Google Scholar]
- 6.Casey SO, Sampaio RC, Michel E, et al. Posterior reversible encephalopathy syndrome. AJNR Am J Neuroradiol 2000; 21: 1199–1206. [PMC free article] [PubMed] [Google Scholar]
- 7.Provenzale JM, Petrella JR, Cruz LC, Jr, et al. Quantitative assessment of diffusion abnormalities in posterior reversible encephalopathy syndrome. AJNR Am J Neuroradiol 2001; 22: 1455–1461. [PMC free article] [PubMed] [Google Scholar]
- 8.Ambrosetto P, Nicolini F, Zoli M, et al. Ophthalmoplegic migraine: From questions to answers. Cephalalgia 2014; 34: 914–919. [DOI] [PubMed] [Google Scholar]
- 9.Moscato G, Cirillo L, Dall'olio M, et al. Management of unruptured brain aneurysms: Retrospective analysis of a single centre experience. Neuroradiol J 2013; 26: 315–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Servillo G, Bifulco F, De Robertis E, et al. Posterior reversible encephalopathy syndrome in intensive care medicine. Intensive Care Med 2007; 33: 230–236. [DOI] [PubMed] [Google Scholar]
- 11.Lee VH, Wijdicks EF, Manno EM, et al. Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Arch Neurol 2008; 65: 205–210. [DOI] [PubMed] [Google Scholar]
- 12.Kitaguchi H, Tomimoto H, Miki Y, et al. A brainstem variant of reversible posterior leukoencephalopathy syndrome. Neuroradiology 2005; 47: 652–656. [DOI] [PubMed] [Google Scholar]
- 13.Servillo G, Striano P, Striano S, et al. Posterior reversible encephalopathy syndrome (PRES) in critically ill obstetric patients. Intensive Care Med 2003; 29: 2323–2326. [DOI] [PubMed] [Google Scholar]
- 14.Stott VL, Hurrell MA, Anderson TJ. Reversible posterior leukoencephalopathy syndrome: A misnomer reviewed. Intern Med J 2005; 35: 83–90. [DOI] [PubMed] [Google Scholar]
- 15.Trommer BL, Homer D, Mikhael MA. Cerebral vasospasm and eclampsia. Stroke 1988; 19: 326–329. [DOI] [PubMed] [Google Scholar]
- 16.Hauser RA, Lacey DM, Knight MR. Hypertensive encephalopathy: Magnetic resonance imaging demonstration of reversible cortical and white matter lesions. Arch Neurol 1988; 45: 1078–1083. [DOI] [PubMed] [Google Scholar]
- 17.The Magpie Trial Collaborative Group. Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo-controlled trial. Lancet 2002; 359: 1877–1890. [DOI] [PubMed] [Google Scholar]
- 18.Gifford AW. Management of hypertensive crises. JAMA 1991; 266: 829–835. [PubMed] [Google Scholar]