Abstract
Few systematic surveys have dealt with the potential procedural risks associated with the use of retrievable intracranial stents [Solitaire Flow Restoration (Solitaire FR)], which have become effective tools for recanalizing acutely occluded cerebral arteries. The aim of this study was to present the real-world experiences of Solitaire-FR-related adverse events by reviewing the MAUDE (Manufacturer and User Facility Device Experience) as published on the United States Food and Drug Administration website. In total, 85 adverse events related to the use of the Solitaire FR stent were reported between March 2012 and October 2014. In 80 patients these adverse events were attributable to inadvertent detachment of the device. Thirteen of these 80 patients (16%) died after the procedure. Morbidity data were available in 62 patients, among whom 11 (18%) had suffered a procedure-related injury. Detachment occurred at the first, second, and third pass in nine (21%), 21 (49%), and 13 (30%) of the 43 patients for whom this information was available, respectively. Resistance was perceived by the physician during retrieval of the device in 12 patients, and lesion characteristics were noted in 13. A rescue maneuver was reported in 20 (25%) of the 80 patients in whom the adverse event was attributable to detachment of the device, resulting in flow reestablishment in 13 (65%). The risk of inadvertent detachment during stent retrieval cannot be overemphasized in real-world scenarios, and careful consideration of the “dos and don’ts” is essential for the achievement of a safe procedure.
Keywords: acute stroke, endovascular procedure, stent, intraoperative complication, device failure, safety management
Introduction
The Solitaire Flow Restoration (Solitaire FR) stent (Covidien/ev3, Irvine, CA, USA) has been recognized as an effective tool for the recanalization of acute cerebral arterial occlusion since achieving the CE mark in 2009 and United States Food and Drug Administration (US FDA) approval in 2012.1–7 The device is based on the Solitaire AB Neurovascular Remodeling device, which was originally developed for stent-assisted embolization of wide-neck intracranial aneurysms.1–4 Deploying the Solitaire FR stent produces prompt flow restoration by displacing the thrombus along the vessel walls; the clot can then be retrieved by pulling the unfolded stent backward. Its closed-cell design and structural attachment to a nitinol pushwire allows successful resheathing and retrieval. However, withdrawing the stent when it is in contact with the endothelium may exert a shearing force that can result in intimal injury, and in the worst case inadvertent detachment may occur at the junction between the stent and the nitinol pushwire.1,4,7 However, no systematic surveys have evaluated the incidence, mechanism, prevention, and management principles for such an event. To identify these factors among real-world experiences of Solitaire-FR-related adverse events, this study reviewed the MAUDE (Manufacturer and User Facility Device Experience) as published on the US FDA website; this is a reporting system for physicians to recount adverse events incurred using medical devices.
Materials and Methods
The overall occurrence of inadvertent detachment of the Solitaire FR stent was surveyed by reviewing the MAUDE website, and all cases of inadvertent detachment were collected during the period from March 2012 to October 2014.8 This was the period during which the Solitaire FR device was approved by the US FDA, and as such MAUDE data showed that almost all mechanical thrombectomies were performed using this device. The patient outcomes reviewed included procedure-related mortality and morbidity. The lesion locations were evaluated where the procedure performed, and the number of stent passes until detachment was recorded. Possible causes of the inadvertent detachment were reported by physician, and the rescue maneuvers used to restore the flow, if performed, were registered for this retrospective review.
Results
In total, 85 adverse events related to the use of the Solitaire FR stent were reported between March 2012 and October 2014. In 80 patients these adverse events were attributable to inadvertent detachment of the Solitaire FR stent (Table 1), while the remaining five were not related to detachment (e.g., subarachnoid hemorrhage or intimal dissection). Thirteen of these 80 patients (16%) with inadvertent detachment died after the procedure: nine patients as a consequence of problems related to the procedure, and the other four due to medical illness not related to the procedure. Morbidity data were available for 78% (62/80) of the surviving patients, among whom 11 had suffered a procedure-related injury. Lesion location was clarified in only 47 of the 80 patients (59%). The lesions were distributed as follows: nine (19%) in the internal carotid artery (ICA), 17 (36%) in the middle cerebral artery (MCA), eight (17%) in the ICA and MCA, and three (6%) in the posterior circulation.
Table 1.
Inadvertent detachment of the Solitaire Flow Restoration (Solitaire FR) stent during acute stroke intervention reported on the MAUDE (Manufacturer and User Facility Device Experience) on the United States Food and Drug Administration website [8].
| Parameter | Percentage (number) |
|---|---|
| Patient outcome | |
| Death | 16% (13/80) |
| Procedure-related, n = 9; non-procedure-related, n = 4 | |
| Survival | 78% (62/80) |
| With injury, n = 11; without injury, n = 51 | |
| n.a. | 6% (5/80) |
| Lesion location | |
| Internal carotid | 19% (9/47) |
| Middle cerebral | 36% (17/47) |
| Internal carotid and middle cerebral | 17% (8/47) |
| Posterior cerebral (n = 1) + vertebral (n = 2) | 6% (3/47) |
| n.a. | 41% (33/80) |
| Number of passes before detachment | |
| First pass | 21% (9/43) |
| Second pass | 49% (21/43) |
| Third pass | 30% (13/43) |
| n.a. | 46% (37/80) |
| Possible cause of inadvertent detachment | |
| Perception of resistance during retrieval | 35%a (12/34) |
| Lesion characteristics | 38% (13/34) |
| Atherosclerotic plaque (heavy calcification, n = 3; large burden, n = 2) Thrombus (hard, n = 2; long segment, n = 1) Tortuous vascular course, n = 5 | |
| Snagged on a previously implanted stent | 24% (8/34) |
| Microcatheter exchange after device engagement | 3% (1/34) |
| n.a. | 58% (46/80) |
n.a., not accessible.
Oversized stent relative to the target vessel diameter was placed in one case.
The number of passes was recorded for 43 of the 80 patients (54%); stent detachment occurred at the first pass in nine patients (21%), the second pass in 21 patients (49%), and the third pass in 13 patients (30%).
The possible causes of the inadvertent detachment were recorded for 34 of 80 patients (43%). In 12 of these 34 cases (35%) the interventionalist reported feeling resistance from the device during the procedure. In one case it was mentioned that the Solitaire FR stent was too large relative to the diameter of the target vessel, although the exact size was not provided. Lesion characteristics were described in 13 of these 34 cases (38%), including a calcified or large-burden plaque (n = 5), a hard or lengthy thrombus (n = 3), and tortuous anatomy (n = 5). The Solitaire FR stent snagged on a previously implanted carotid stent in eight cases (24%). Uniquely, there was a single case in which separation was provoked by exchanging a second microcatheter after engagement of the stent retriever with the original microcatheter.
A rescue maneuver was attempted in 25% of the detachment cases (20/80), and flow was reestablished in 65% of these (13/20) (Table 2). Stent removal was successful in five out of nine attempted cases. Flow was successfully restored in two of those five balloon angioplasties, and the rescue was achieved by pushing the stent into the distal MCA using the guidewire in a unique single case. The patients received only anticoagulation treatment in four cases.
Table 2.
Management and outcomes of detachment of the Solitaire FR stent in selected literature.
| Reference | No. | Risk factors and number of passes | Management | TIMI (mRS) |
|---|---|---|---|---|
| Nayak et al.1 | 1/7 | Vessel tortuosity, first pass | Tirofiban Dual antiplatelet | 3 (mRS 4) |
| Miteff et al.4 | 1/26 | Vessel tortuosity, large clot burden, second pass | IA UK | 2 (mRS 3) |
| Dorn et al.7 | 1/104 | n.a. | n.a. | n.a. (no clinical influence) |
| SWIFT trial9 | 4/31a | n.a. | n.a. | n.a. |
| STAR trial10 | 15/202b | n.a. | n.a. | n.a. |
| Gascou et al.12 | 2/138 | n.a. | n.a. | n.a. |
| MAUDE8 | 80/n.a. | Risk factor indentified (34/80), First pass (9/43) Second pass (21/43) Third pass (13/43) | Attempted rescue maneuver (20/80) Flow reestablishment (13/20) Successful stent removal (5/9) Balloon angioplasty (2/5) Pushing stent into distal MCA (1/1) Suction thrombectomy(1/1) Anticoagulation only (4/4) | n.a. |
No., number of patients showing detachment/total study population; IA UK, intra-arterial urokinase infusion; MCA, middle cerebral artery; TIMI, Thrombolysis In Myocardial Infarction Recanalization Scale; mRS, modified Rankin Scale; SWIFT, Solitaire With the Intention For Thrombectomy; STAR, Solitaire Flow Restoration
Thrombectomy for Acute Revascularization.
Device- or procedure-related serious adverse event.
Device-related serious adverse event.
The devices involved in the adverse event were returned to the manufacturer for evaluation in only 17 of the 80 cases (21%); in eight cases the pushwire and a part of the stent were returned, and in nine cases only the pushwire was returned.
Discussion
Mechanical thrombectomy using the Solitaire FR stent has become a feasible option for recanalization in acute stroke, with an excellent success rate.1–7,9–12 Unlike other intracranial self-expanding stents, the Solitaire FR stent can be retrieved by withdrawing its connected pushwire. However, this capability represents a double-edged sword because unexpected separation between the stent and the pushwire can occur, with an incidence of 1.8% (5/275) reported for a few published case series.1,4,7,12 A recent single-center prospective study found an incidence of Solitaire-FR-related complications (stent fracture and spontaneous detachment) of 1% (2/138).12 Among several multicenter, randomized and prospective studies using the Solitaire FR device, the SWIFT (Solitaire With the Intention For Thrombectomy) study found incidences of device- and procedure-related serious adverse events of 13% (4/31) and 9% (5/58), respectively.9 The STAR (Solitaire Flow Restoration Thrombectomy for Acute Revascularization) trial found an incidence of device- or procedure-related serious adverse events of 7% (15/202).10 However, no information on the device- or procedure-related adverse events was available for the NASA (North American Solitaire Acute Stroke) registry.11 In contrast to the paucity of serious adverse events in several case series and multicenter registries,9–11 80 cases of inadvertent detachment of a Solitaire FR stent were collected in the present study from the MAUDE website during two years of clinical use.8
Procedure-related death was reported in 16% of cases, and 18% of the survivors had suffered from a procedure-related sequela. The manufacturers of the Solitaire FR stent recommend that this product should not be used for more than two passes because a larger number of passes is regarded as a risk factor for complications.13 However, it appears that there is already a substantial risk during the first pass, and extraordinary attention and care should be paid at every attempt. According to the MAUDE website, approximately three-quarters of recorded inadvertent separations occurred before the third pass, although the number of passes was not recorded in as many as 37 of the 80 cases (46%). These results are consistent with previous reports of separation occurring at the first or second attempt.1,4
Early recognition of the potential risk factors might be the clue to prevent this complication, although the possible causes were reported in only 42% (34/80) of cases. In total, 35% (12/34) of physicians perceived resistance from the device during the procedure. Lesion characteristics were described in 38% (13/34) of cases, and included a calcified or large-burden plaque, a hard or lengthy thrombus, and tortuous anatomy, which should be carefully evaluated on preintervention CT or MRI. The risk of engagement of the Solitaire FR stent must be considered after carotid stent placement. An example case experienced by the present authors (not reported to MAUDE) suggested that retrieving an oversized 6 mm × 30 mm stent at segment M2 of the MCA may increase the risk of detachment (Figure 1). While the use of stents with diameters is larger than the supposed optimal size for the artery has the advantage of exerting a greater radial force, it has also runs the risk of detachment by increasing the tensile force and fatigue at its weakest point when pulling the stent back. Therefore, our strategy has changed such that we now partially unfold the stent during retrieval.14,15 After positioning the microcatheter at the thrombus segment, the stent is partially unfolded so as not to expose the proximal marker band outside the microcatheter.15 The partial resheathing with the microcatheter appears to prevent inadvertent separation by protecting the stent-wire junction from excessive bending in tortuous arterial segments during retrieval. Although there is as yet insufficient clinical evidence to confirm the efficacy of this strategy, it is supported by an in vitro experiment showing that detachment occurs more easily when pushing the stent forward and forcing it to bend around the proximal marker band.14 That study tested five different conditions of detachment, and it was revealed that inadvertent detachment may occur either at the proximal or distal adhesive of the proximal marker band, which contains a ball-and-socket joint.14 However, in most cases the involved device was not returned for evaluation since the stent was placed within the patient’s cerebral artery and there were no available clinical data on the exact location of the detachment point on the device itself.8 Furthermore, it is almost impossible to observe very small ball-and-socket structures on fluoroscopy without the aid of a microscope. Therefore, a prospective registry study for microscopic evaluation is recommended to identify the exact inadvertent detachment zone and weak portion of the stent in real-world scenarios.
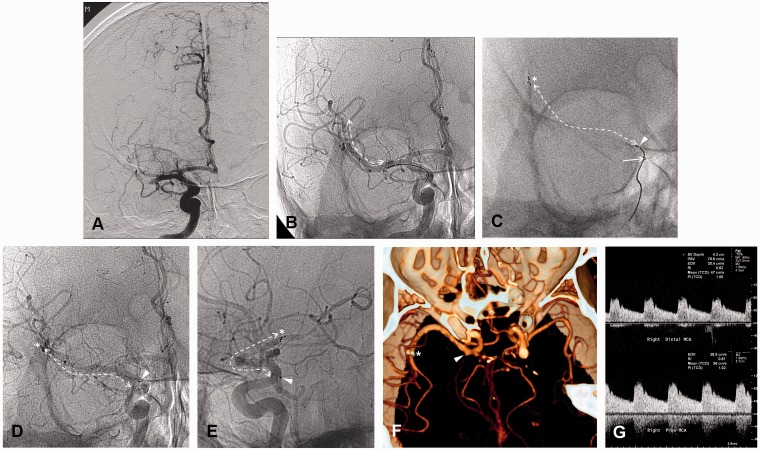
Figure 1.
Inadvertent detachment of a Solitaire FR stent during mechanical thrombectomy in a 74-year-old man who presented with sudden-onset dysarthria and left-sided hemiparesis. A) Initial angiogram showing acute occlusion of the right M1 segment of the middle cerebral artery. B) The entire length of the stent (arrow) was unfolded, immediately restoring anterograde flow through the stent, and displacing the clot. C) During device retrieval, the distal tip was evident (arrowhead) outside of the microcatheter (solid arrow) and some resistance was perceived by the interventionalist (the asterisk indicates the distal tip of the stent, and the dashed arrow indicates the entire length of the stent). D,E) The pushwire was withdrawn without resistance, but the proximal (arrowhead) and distal (asterisk) positions of the stent were unchanged. F) CT angiography image indicating flow through the patent stent (proximal portion, arrowhead; distal portion, asterisk) at 1 day after treatment. G) Transcranial Doppler sonography performed 7 months later indicated that the flow through the stent was normal, with a peak systolic velocity and resistance index of 98.3 cm/s and 0.61, respectively.
Rescue maneuvers were attempted in 25% of cases (20/80), and flow was restored successfully in 65% (13/20) of them. No standardized guideline was proposed regarding the indications and salvage regimen, and this issue remains to be resolved. Interventional rescue—including stent removal if possible and balloon angioplasty—might be considered, and the benefit of medication, including tissue plasminogen activator, antiplatelet drug, and/or anticoagulation, should be balanced against the risk of hemorrhagic transformation.
This retrospective review of the MAUDE website has some limitations, in that it provides restricted information regarding patient outcome, event type, event description, and manufacturer narratives, which were simply reported by the interventionalists. Since most of the information provided was not reported in a unified format, even simple counting can be misleading. However, the incidence of adverse events was presumed to be reliable even without precise data including patient gender, age, and other background characteristics.
Conclusion
The risk of inadvertent detachment during stent retrieval cannot be overemphasized in real-world clinical scenarios, and careful consideration of the “dos and don’ts” is essential for the achievement of a safe procedure.
Acknowledgments and Funding
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (grant number 2014R1A1A2057298).
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Nayak S, Ladurner G, Killer M. Treatment of acute middle cerebral artery occlusion with a Solitaire AB stent: preliminary experience. Br J Radiol 2010; 83(996): 1017–1022. doi: 10.1259/bjr/42972759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castaño C, Dorado L, Guerrero C, et al. Mechanical thrombectomy with the Solitaire AB device in large artery occlusions of the anterior circulation: a pilot study. Stroke 2010; 41(8): 1836–1840. doi: 10.1161/STROKEAHA.110.584904. [DOI] [PubMed] [Google Scholar]
- 3.Roth C, Papanagiotou P, Behnke S, et al. Stent-assisted mechanical recanalization for treatment of acute intracerebral artery occlusions. Stroke 2010; 41(11): 2559–2567. doi: 10.1161/STROKEAHA.110.592071. [DOI] [PubMed] [Google Scholar]
- 4.Miteff F, Faulder KC, Goh AC, et al. Mechanical thrombectomy with a self-expanding retrievable intracranial stent (Solitaire AB): experience in 26 patients with acute cerebral artery occlusion. Am J Neuroradiol 2011; 32(6): 1078–1081. doi: 10.3174/ajnr.A2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brekenfeld C, Schroth G, Mordasini P, et al. Impact of retrievable stents on acute ischemic stroke treatment. Am J Neuroradiol 2011; 32(7): 1269–1273. doi: 10.3174/ajnr.A2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Machi P, Costalat V, Lobotesis K, et al. Solitaire FR thrombectomy system: immediate results in 56 consecutive acute ischemic stroke patients. J Neurointerv Surg 2012; 4(1): 62–66. doi: 10.1136/jnis.2010.004051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dorn F, Stehle S, Lockau H, et al. Endovascular treatment of acute intracerebral artery occlusions with the solitaire stent: single-centre experience with 108 recanalization procedures. Cerebrovasc Dis 2012; 34(1): 70–77. doi: 10.1159/000338903. [DOI] [PubMed] [Google Scholar]
- 8.Manufacturer and User Facility Device Experience. US Food and Drug Administration website. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/textsearch.cfm. 2014.
- 9.Saver JL, Jahan R, Levy EI, et al. Solitaire flow restoration device versus the Merci Retriever in patients with acute ischaemic stroke (SWIFT): a randomised, parallel-group, non-inferiority trial. Lancet 2012; 380(9849): 1241–1249. doi: 10.1016/S0140-6736(12)61384-1. [DOI] [PubMed] [Google Scholar]
- 10.Pereira VM, Gralla J, Davalos A, et al. Prospective, multicenter, single-arm study of mechanical thrombectomy using Solitaire Flow Restoration in acute ischemic stroke. Stroke 2013; 44(10): 2802–2807. doi: 10.1161/STROKEAHA.113.001232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zaidat OO, Castonguay AC, Gupta R, et al. North American Solitaire Stent Retriever Acute Stroke registry: post-marketing revascularization and clinical outcome results. J Neurointerv Surg 2014; 6(8): 584–588. doi: 10.1136/neurintsurg-2013-010895. [DOI] [PubMed] [Google Scholar]
- 12.Gascou G, Lobotesis K, Machi P, et al. Stent retrievers in acute ischemic stroke: complications and failures during the perioperative period. Am J Neuroradiol 2014; 35(4): 734–740. doi: 10.3174/ajnr.A3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Instructions for Use of the Solitaire FR Revascularization Device supplied with the device in product package eV3. Neurovascular.
- 14.Kwon HJ, Chueh JY, Puri AS, et al. Early detachment of the Solitaire stent during thrombectomy retrieval: an in vitro investigation. J Neurointervent Surg. 2014 Jan 16. [Epub ahead of print]. doi: 10.1136/neurintsurg-2013-010942. [DOI] [PubMed]
- 15.Youn SW, Kim HK. Refinement of a thrombectomy technique to treat acute ischemic stroke: technical note on microcatheter advance during retrieving self-expandable stent. J Korean Soc Radiol 2012; 67(1): 1–6. [Google Scholar]