Abstract
Background and purpose
Intra-arterial therapy for acute ischaemic stroke has evolved rapidly in the last few years. Stent retrievers have now replaced ‘first-generation’ devices, which have been the principle devices tested in stroke trials.
Our aims were to determine the rates of successful recanalization and functional independence in acute stroke patients treated with stent retrievers. We also sought to assess the safety outcomes of stent retrievers by assessing the rates of mortality and intra-cranial haemorrhage.
Materials and methods
We conducted a systematic review and meta-analysis of studies which utilized stent retrievers as sole treatment or as part of a multi-modal approach in acute ischaemic stroke.
Results
We identified 20 eligible studies: 17 on Solitaire (ev3/Covidien, Irvine, California, USA) (n = 762) and three on Trevo (Stryker, Kalamazoo, Michigan, USA) (n = 210). The mean age of participants was 66.8 (range 62.1–73.0) years and the M:F ratio was 1.1:1. The average stroke severity score (National Institutes of Health Stroke Scale (NIHSS)) at presentation was 17.2. The weighted mean symptom onset to arterial puncture and procedural duration were 265.4 minutes and 54.8 minutes, respectively.
Successful recanalization was achieved in 84.5% of patients with a weighted mean of 2.0 stent retriever passes. Independent functional outcome was achieved in 51.2% and the mortality rate was 16.8%.
Conclusion
Stent retrievers have the potential to achieve a high rate of recanalization and functional independence whilst being relatively safe. They should be assessed in well-designed randomized controlled trials to determine their efficacy and assess whether they compare favourably with ‘standard treatment’ in stroke.
Keywords: Stroke, mechanical thrombectomy, intra-arterial stroke therapy
Introduction
Stroke is the third global leading cause of mortality and serious long-term disability.1 Early diagnosis and treatment are crucial to limit irreversible neuronal loss.2
Almost two decades ago, systemic intravenous recombinant tissue plasminogen activator (rt-PA) was licensed by the Food and Drug Administration (FDA) and is now considered ‘standard treatment’ in acute ischaemic stroke.3,4 Though the therapeutic window of rt-PA was extended to 4.5 h following the ECASS 3 trial,5 a large number of patients still fail to present within the therapeutic window.6
Intra-arterial strategies, namely pharmacological thrombolysis and mechanical embolectomy, aim to restore flow by recanalizing the occluded vessel.7 Successful recanalization has been shown to correlate with improved functional outcome in acute ischaemic stroke.8,9 A clear benefit of administering prourokinase directly into the primary arterial occlusive lesion (AOL) was demonstrated in the PROACT II trial.10
First-generation thrombectomy devices achieved better recanalization rates compared with rt-PA, and an independent association between vessel recanalization and improved functional outcomes was demonstrated in the MERCI11 and Multi MERCI trials.12 The association was even more pronounced with the use of second-generation retriever devices (SWIFT13 and TREVO 214).
However, three randomized controlled trials (RCTs) (MR RESCUE,15 SYNTHESIS Expansion16 and IMS III17) published in March 2013 failed to prove that endovascular therapy was superior to systemic rt-PA in acute ischaemic stroke. These trials had potential design flaws and utilized mainly first-generation devices. However, they have introduced clinical equipoise surrounding the perceived effectiveness of intra-arterial thrombectomy devices.
We therefore systematically reviewed published reports on the use of stent retrievers in the treatment of acute ischaemic stoke to determine the rates of recanalization and favourable functional outcomes in these patients. The importance of this review lies in the generation of evidence regarding stent retrievers which may subsequently guide the clinical management of acute stroke.
Materials and methods
Design
This review was carried out according to guidelines developed by the Cochrane Database of Systematic Reviews.18 We designed our research question using the PICO framework19 as follows: population – acute ischaemic stroke patients; intervention – stent retrievers; comparison(s) – alternative stroke therapies (including intravenous rt-PA and ‘first-generation’ devices); and outcome(s) – assessment of recanalization, functional and safety outcomes.
Data sources and study selection
An extensive search was performed using EMBASE, Cochrane CENTRAL and Medline without language restrictions. Years covered ranged from 1999 up to 10th October 2013 (search performance date). Free text and subject headings (MeSH and EMTREE) were utilized in order to enhance the search sensitivity.
Exploded headings relating to Stroke, Brain/Cerebral, Ischemia/Infarction/Hypoxia, Thrombectomy and Embolectomy with the Boolean operators ‘OR’ and ‘AND’ were used (Appendices 1–3). Reference lists of included studies and existing systematic reviews were also checked. In order to validate the search strategy, hand searching was carried out in the two journals which contributed to the largest number of papers in this review, namely the American Journal of Neuroradiology (AJNR) and Stroke: A Journal of Cerebral Circulation from 2007–2013.
Selection criteria
Primary research studies in any language with living human participants were sought for inclusion. The studies had to utilize stent retriever devices in isolation or as part of a multi-modal reperfusion therapy (MMRT) approach in stroke. We excluded in vitro and animal studies. We developed stringent inclusion and exclusion criteria (Table 1) to reduce bias, and the identified studies were further assessed using a validated quality assessment tool, namely the RTI item bank.20 The latter was used for both observational studies and RCTs in order to enhance consistency and uniformity in the appraisal.
Table 1.
Inclusion and exclusion criteria.
| Inclusion criteria |
|---|
| 1. Studies which include patients presenting with acute ischemic stroke (AIS) symptoms |
| 2. Age of participants > 18 years |
| 3. Number of participants included in the study (n) > 20 |
| 4. Patients who were treated with either (a) intravenous thrombolysis within 4.5 hours using rt-PA (Alteplase)*, (b) mechanical thrombectomy using a stentriever or (c) both in the acute setting. |
| 5. Studies which specify their outcome measures using either recanalization rates or functional outcomes (utilizing the modified Rankin Score @30 or 90 days) |
| 6. Study designs: RCTs, non-randomized controlled studies and observational studies |
| 7. Dates: 2007 to 2013 |
as recommended by the AHA/ASA guidelines 2011 for treatment of acute ischaemic stroke – also referred to as ‘standard treatment’.
Data extraction
A primary evaluation of studies was carried out by screening titles and abstracts. Full-text articles were then obtained for eligible studies as well as in cases where it was not clear whether a study should be included or not on the sole basis of the primary evaluation.
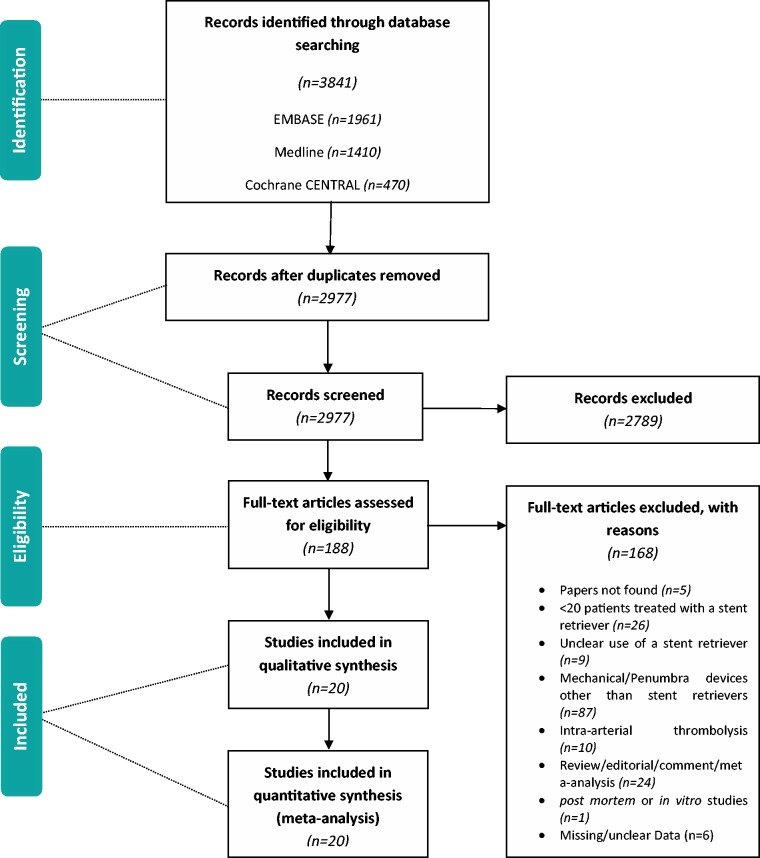
Inclusion and exclusion decisions were documented and used to complete a PRISMA flowchart (Figure 1). Articles that were not in the English language were translated. EndNote X6 was used as reference management software and to organize the articles retrieved. When a study was characterized by various reports, these were included, collated and treated as one unit. We excluded duplicate publications.
Figure 1.
PRISMA flow diagram.
Data were extracted using a standardized assessment form (Appendix 4) in order to allow for a consistent systematic approach. The latter evaluated the study design, characteristics and potential biases prior to data extraction. Data collected included stent retriever device utilized, relevant timings and data related to the thrombectomy procedure, recanalization rates and functional outcomes. In studies where there were multiple outcome measurement points, these were recorded separately. Duplicate publication bias was limited by careful review of the extracted data of included studies.
Study characteristics
Study characteristics assessed included their study design (RCT, non-RCT and observational designs), number of participating centres, data collection (prospective or retrospective) and adequacy of follow-up. The year and journal of publication were also recorded.
Baseline characteristics of patients
The number of participants, mean age and range, male to female ratio, stroke severity (as assessed by NIHSS) at presentation, treatment with intravenous rt-PA, and percentage of anterior circulation strokes were recorded.
Procedural characteristics
Extracted data included site of arterial occlusion, baseline occlusion scores, type of device used, mean number of device passes, time from stroke onset to arterial puncture, procedure time (defined as time from arterial puncture to successful recanalization), anaesthesia utilized and additional use of intra-arterial thrombolysis.
Outcomes
Our primary outcomes were successful recanalization (defined as TICI score of 2b–3 or TIMI score of 2–3) and functional independence at 90 days (defined as a score of ≤2 on the modified Rankin Score (mRS)). The secondary outcomes sought were rates of intra-cranial haemorrhage (ICH), symptomatic intra-cranial haemorrhage (sICH), and mortality (mRS = 6) at 90 days following the procedure. Whenever possible the stratified TIMI and TICI grade and the mRS score at 90 days were also recorded.
Statistical analysis
We extracted numeric data (when available) from the chosen studies related to our primary (recanalization rates and functional independence at 90 days) and secondary outcomes (rates of ICH, sICH and mortality at 90 days). The data were used to calculate weighted means (and standard deviations) of outcome measures. We used the random effects model to perform our meta-analysis.
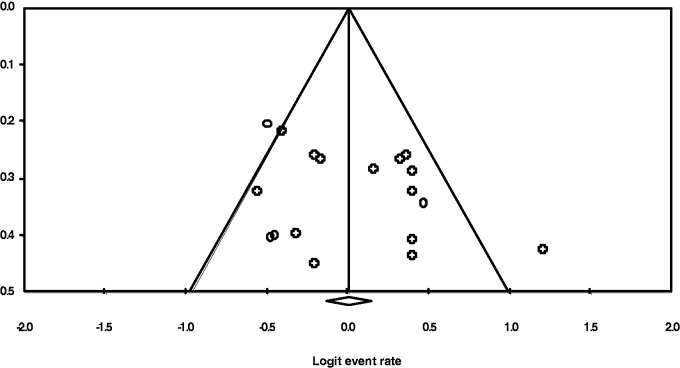
We tested for heterogeneity using the likelihood ratio χ2 test and by computing the I2 statistic for each outcome. We also constructed a funnel plot to assess for publication bias.
Results
Our initial search yielded 3841 primary studies identified through database searching. We did not identify any further studies by hand searching. Some 2977 records were screened after removing 864 duplicates that were identified either electronically or manually. In all, 188 full-text articles were assessed for eligibility, of which only 20 were included in the meta-analysis. Five papers could not be retrieved.
The reasons for exclusion after full-text article review were: < 20 participants in the study (n = 26), unclear use of a stent retriever (n = 9), mechanical/Penumbra devices other than stent retrievers (n = 87), intra-arterial thrombolysis as opposed to the use of stent retrievers (n = 10), review/editorial/comment/meta-analysis (n = 24), post mortem or in vitro studies (n = 1) and missing/unclear data (n = 6).
Characteristics of included studies
In total we identified 20 studies (Table 2) that utilized stent retrievers as a sole treatment or as part of a multi-modal approach in acute ischaemic stroke. The studies were published between 2010 and 2013. The smallest study (Castano et al., 2010) included 20 participants whereas the largest study (Dorn et al., 2012) had 104 patients. Of the studies, 19 (95%) were written in the English language and one (Kocher et al., 2013) study was in Czech. Three studies (Nogueira et al., 2012;14 Saver et al., 2012;13 Mokin et al. 2013) were multi-centre collaborations. The studies included a total of 972 patients with a median of 48.6 patients per study. Both heterogeneity statistics (Table 3) and the funnel plot (Figure 2) failed to demonstrate significant heterogeneity between the included studies. All studies (n = 20) assessed long-term functional outcome using the mRS.
Table 2.
Study characteristics.
| No. | Authors | Year of Publication | Journal | Device Used | Study Design | Single / Multi-centre | Data Collection |
|---|---|---|---|---|---|---|---|
| 1 | Miteff F, et al. | 2011 | AJNR | Solitaire | Observational | Single institution | Retrospective |
| 2 | Broussalis E, et al. | 2013 | AJNR | Trevo | Non-randomised controlled | Single institution | Prospective |
| 3 | Soize S, et al. | 2013 | AJNR | Solitaire | Observational | Single institution | Prospective |
| 4 | Dorn F, et al. | 2012 | Cerebrovascular diseases | Solitaire | Observational | Single institution | Unclear |
| 5 | Kocher M, et al | 2013 | Ces Radiol | Solitaire | Observational | Single institution | Unclear |
| 6 | Mohlenbruch M, et al. | 2012 | Clinical Neuroradiology | Solitaire | Observational | Single institution | Unclear |
| 7 | Mpotsaris A, et al. | 2013 | Clinical Neuroradiology | Solitaire | Observational | Single institution | Unclear |
| 8 | Costalat V, et al. | 2012 | European Journal of Radiology | Solitaire | Observational | Single institution | Prospective |
| 9 | Roth C, et al. | 2013 | JACC Cardiovasc Interv | Solitaire | Observational | Single institution | Prospective |
| 10 | Cohen JE, et al. | 2013 | Journal of Clinical Neuroscience | Solitaire | Observational | Single institution | Unclear |
| 11 | Machi P, et al. | 2012 | J Neurointerv Surg | Solitaire | Observational | Single institution | Unclear |
| 12 | Mpotsaris A, et al. | 2012 | JNNP | Solitaire | Observational | Single institution | Prospective |
| 13 | Sanak D, et al. | 2013 | J Vasc Interv Radiol | Solitaire | Observational | Single institution | Prospective |
| 14 | Nogueira RG, et al. | 2012 | Lancet | Trevo | RCT | Multi-centre | Prospective |
| 15 | Saver JL, et al. | 2012 | Lancet | Solitaire | RCT | Multi-centre | Prospective |
| 16 | Yoon YH, et al. | 2013 | Neuroradiology | Solitaire | Observational | Single institution | Prospective |
| 17 | Mokin M, et al. | 2013 | Neurosurgery | Solitaire | Observational | Multi-centre | Retrospective |
| 18 | Castano C, et al. | 2010 | Stroke | Solitaire | Observational | Single institution | Prospective |
| 19 | Leker RR, et al. | 2012 | Stroke | Solitaire | Non-randomised controlled | Single institution | Prospective |
| 20 | Roman LS, et al. | 2012 | Stroke | Trevo | Observational | Single institution | Prospective |
Table 3.
Heterogeneity tests.
| Outcome | Chi2 | df | I2 | |
|---|---|---|---|---|
| 1 | Recanalization | 17.969 | 19.000 | 0.000 |
| 2 | Functional independence | 18.071 | 18.000 | 0.392 |
| 3 | Mortality | 15.683 | 18.000 | 0.000 |
| 4 | ICH | 14.088 | 13.000 | 7.722 |
| 5 | sICH | 14.792 | 16.000 | 0.000 |
Figure 2.
Funnel plot (Outcome: Functional independence at 90 days).
Subject and patient characteristics
The mean age of participants was reported in all studies, but only 15 (75%) studies provided the age range of participants. One study (Dorn et al., 2012) failed to provide the mean NIHSS at presentation and the stroke severity score varied from 13 to 21.4. The male to female ratio could not be calculated in a single (Mpotsaris et al., 2012) study. The Solitaire (ev3/Covidien, Irvine, California, USA) and Trevo (Stryker, Kalamazoo, Michigan, USA) devices were used in 17 (Miteff et al., 2011; Soize et al., 2013; Dorn et al., 2012; Kocher et al., 2013; Mohlenbruch et al.,2012; Mpotsaris et al., 2012; Costalat et al., 2012; Roth et al., 2013; Cohen et al., 2013; Machi et al., 2012; Mpotsaris et al., 2012; Sanak et al., 2013; Saver et al., 2012;13 Yoon et al., 2013; Mokin et al., 2013; Castano et al., 2010; Leker et al., 2012) and three (Broussalis et al., 2013; Nogueira et al., 2012;14 Roman et al., 2012) studies, respectively. Eleven (55%) studies specified whether they included anterior and/or posterior circulation strokes, and the weighted percentage of anterior circulation strokes included in the analysis was 81%. The baseline patient characteristics are summarized in Table 4.
Table 4.
Summary of patient baseline characteristics, treatments and outcomes.
| N | ||||||
|---|---|---|---|---|---|---|
| Number of studies | 20 | |||||
| Number of participants | 972 |
| DEMOGRAPHICS | N | % | ||||
|---|---|---|---|---|---|---|
| Average NIHSS at presentation | 17.22 | |||||
| M:F ratio | 1.13:1 | |||||
| Age (mean) | 66.81 | |||||
| % Anterior circulation strokes | 81.83 | |||||
| TREATMENT(s) | N | % | ||||
|---|---|---|---|---|---|---|
| % iv rt-PA administered | 55.0% | |||||
| Procedure time (minutes) | 54.79 | |||||
| Symptom onset to puncture (minutes) | 265.41 | |||||
| Devices used | Solitaire | 17 | 85.0% | |||
| Trevo | 3 | 15.0% | ||||
| Mean device passes | 1.99 |
| OUTCOMES | Contributing Studies | Events\Total | % | 95% CI | p | |
|---|---|---|---|---|---|---|
| % successful recanalization | 20 | 821\972 | 85.4% | 0.811, 0.888 | <0.001 | |
| mRS<2 | 19 | 437\868 | 51.2% | 0.461, 0.562 | 0.653 | |
| mRS=6 | 19 | 157\868 | 16.8% | 0.132, 0.211 | <0.001 | |
| ICH | 14 | 156\666 | 22.0% | 0.169, 0.281 | <0.001 | |
| sICH | 17 | 57\816 | 7.6% | 0.056, 0.102 | <0.001 |
Procedural characteristics
Intravenous rt-PA was administered in 55% of the included patients (see Table 4) and was administered either before or during the embolectomy procedure. Ten (50%) studies specified the type of anaesthesia used for the procedure, with eight studies (Miteff et al., 2011; Broussalis et al., 2013; Mohlenbruch et al.,2012; Mpotsaris et al., 2012; Costalat et al., 2012; Cohen et al., 2013; Machi et al., 2012; Castano et al., 2010) opting for general anaesthetic. Intra-arterial thrombolysis was used as an adjunct treatment in six studies (Miteff et al., 2011; Broussalis et al., 2013; Nogueira et al., 2012;14 Saver et al., 2012;13 Yoon et al., 2013; Roman et al., 2012).
The mean symptom onset to arterial puncture time and mean procedure time were 265.4 (range 154–462.5) and 54.8 (range 33–95.9) minutes, respectively. Table 5 summarizes treatment characteristics.
Table 5.
Demographics and treatment characteristics.
| No. | Authors | N | NIHSS at presentation | Age (mean) | Age (range) | M:F | % Anterior circulation | % i.v. rt-PA | Dose of i.v. rt-PA | Anaesthesia | Symptom onset to arterial puncture (min) | Mean procedure time (min) | Device passes (mean) | Additional i.a. Thrombolysis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Miteff F, et al. | 26 | 21.4 | 65.0 | 35-88 | 1.3 | 61.5 | 0.0% | General | 462.5 | 95.6 | Yes | ||
| 2 | Broussalis E, et al. | 62 | 17.0 | 68.0 | 26-89 | 1.4 | 87.0 | 42.0% | General | 210.0 | 72.0 | 2.5 | Yes | |
| 3 | Soize S, et al. | 36 | 17.1 | 64.0 | 15-85 | 1.0 | 88.9 | 63.9% | 0.9mg/kg | Local | 269.0 | 33.0 | 1.5 | No |
| 4 | Dorn F, et al. | 104 | 67.3 | 31-96 | 1.0 | 55.7% | 0.9mg/kg | 47.0 | 2.5 | No | ||||
| 5 | Kocher M, et al. | 50 | 18.0 | 66.8 | 18-84 | 1.4 | 49.5 | 1.5 | No | |||||
| 6 | Mohlenbruch M, et al. | 25 | 14.0 | 67.0 | 42-85 | 1.5 | 52.0% | 0.6mg/kg | General | 54.0 | 2.0 | No | ||
| 7 | Mpotsaris A, et al. | 41 | 15.8 | 62.1 | 27-80 | 0.5 | 58.5% | General | 95.9 | No | ||||
| 8 | Costalat V, et al. | 50 | 14.7 | 67.6 | 1.0 | 50.0% | 0.9mg/kg | General | 54.0 | 2.0 | No | |||
| 9 | Roth C, et al. | 40 | 16.4 | 70.1 | 1.1 | 95.0 | 70.0% | 0.9mg/kg | 154.0 | 54.0 | 1.8 | No | ||
| 10 | Cohen JE, et al. | 31 | 19.5 | 63.3 | 26-92 | 1.2 | 19.0% | General | 228.0 | 46.9 | No | |||
| 11 | Machi P, et al. | 56 | 15.8 | 67.5 | 26-91 | 1.3 | 26.8 | 0.9mg/kg | General | 316.0 | 37.0 | 2.0 | No | |
| 12 | Mpotsaris A, et al. | 26 | 16.0 | 62.9 | 35-79 | 11.5 | 73.0% | 327.0 | 2.1 | No | ||||
| 13 | Sanak D, et al. | 50 | 18.0 | 66.8 | 18-84 | 1.4 | 90.0 | 100.0% | 0.9mg/kg | 189.0 | 49.5 | 1.5 | No | |
| 14 | Nogueira RG, et al. | 88 | 18.3 | 67.4 | 18-85 | 1.2 | 94.0 | 276.0 | Yes | |||||
| 15 | Saver JL, et al. | 58 | 17.3 | 67.1 | 22-85 | 1.1 | 98.0 | 293.0 | 36.0 | Yes | ||||
| 16 | Yoon YH, et al. | 26 | 13.0 | 73.0 | 23-87 | 1.2 | 65.0% | 0.9mg/kg | Local | 240.0 | 37.5 | Yes | ||
| 17 | Mokin M, et al. | 101 | 17.6 | 64.7 | 1.0 | 90.0 | 39.0% | 301.0 | No | |||||
| 18 | Castano C, et al. | 20 | 19.0 | 65.6 | 37-79 | 1.0 | 50.0% | General | 352.0 | 50.0 | 1.4 | No | ||
| 19 | Leker RR, et al. | 22 | 20.0 | 64.7 | 1.0 | 234.0 | 44.0 | No | ||||||
| 20 | Roman LS, et al. | 60 | 18.0 | 71.2 | 1.1 | 90.0 | 55.0% | 210.0 | 80.0 | Yes |
Recanalization rates
Recanalization was assessed using the TICI, TIMI and Mori et al. scores in 13 (65%), six (30%) and one (5%) studies, respectively.
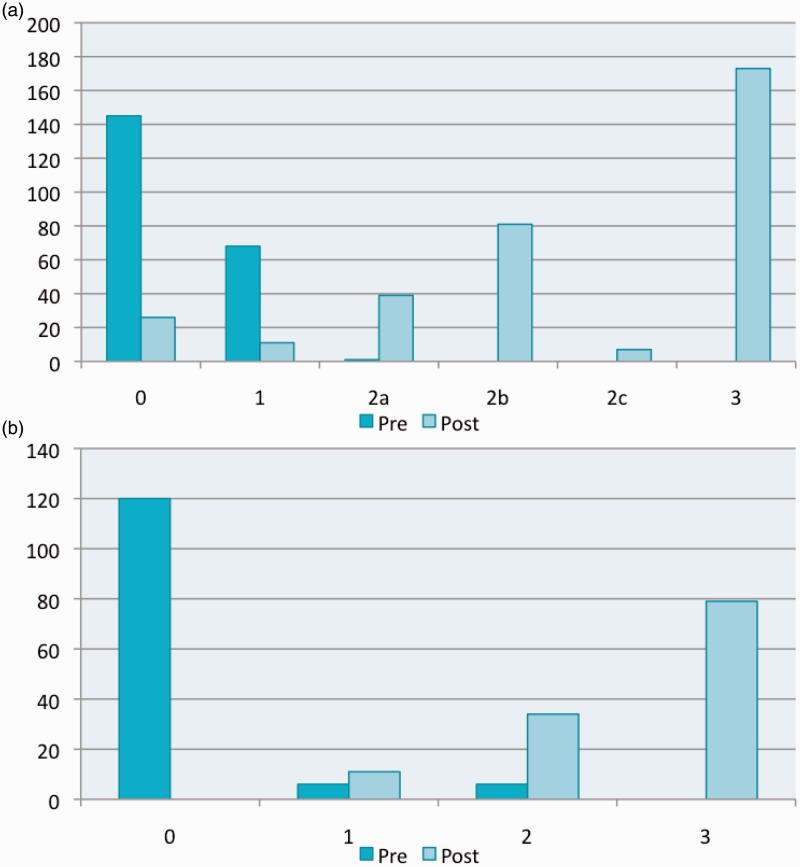
Twelve studies (Broussalis et al., 2013; Soize et al., 2013; Mpotsaris et al., 2013; Costalat et al., 2012; Roth et al., 2013; Cohen et al., 2013; Machi et al., 2012; Mpotsaris et al., 2012; Nogueira et al., 2012;14 Yoon et al., 2013; Mokin et al., 2013; Castano et al. 2010) provided a breakdown of pre- or post- procedure occlusion scores, and the results are summarized in Figure 3.
Figure 3.
Pre- and post-procedure TICI and TIMI scores. (a) TICI (Contributing studies – PRE: 2, 3, 9, 11 and 18; POST: 2, 3, 8, 11, 14, 16 and 18) (b) TIMI (Contributing studies – PRE:10 and 17; POST: 7, 10 and 12).
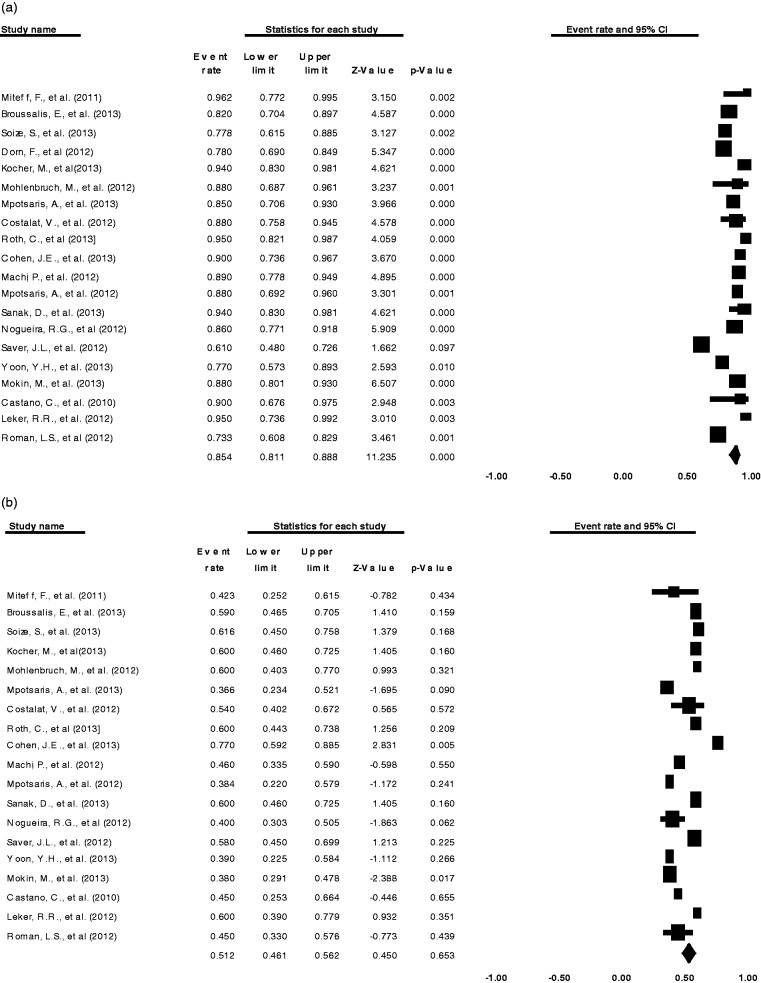
Successful recanalization was achieved in 84.5% (20 comparisons; 95%CI 0.811, 0.888; p < 0.001) of patients (Figure 4) with a mean of 2.0 (range 1.4–2.5) stent retriever passes. Table 6 summarizes the recanalization rates and functional outcomes.
Figure 4.
Meta-analysis (a) Forest plot of successful recanalization. (b) Forest plot of independent functional outcome.
Table 6.
Outcomes.
| No. | Authors | N | Recanalization score used | % Successful recanalization | Duration of follow-up (months) | % mRS<2 | % mRS=6 | % ICH | % sICH |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Miteff F, et al. | 26 | TIMI | 96.2 | 3 | 42.3 | 19.2 | 11.5 | 7.7 |
| 2 | Broussalis E, et al. | 62 | TICI | 82.0 | 3 | 59.0 | 8.0 | 31.0 | 10.0 |
| 3 | Soize S, et al. | 36 | TICI | 77.8 | 3 | 61.6 | 22.2 | 27.7 | 8.3 |
| 4 | Dorn F, et al. | 104 | TICI | 78.0 | 1 | ||||
| 5 | Kocher M, et al. | 50 | TICI | 94.0 | 3 | 60.0 | 14.0 | 28.0 | 6.0 |
| 6 | Mohlenbruch M, et al. | 25 | Mori et al. | 88.0 | 3 | 60.0 | 8.0 | 12.0 | |
| 7 | Mpotsaris A, et al. | 41 | TIMI | 85.0 | 3 | 36.6 | 22.0 | 7.3 | |
| 8 | Costalat V, et al. | 50 | TICI | 88.0 | 3 | 54.0 | 12.0 | 2.0 | |
| 9 | Roth C, et al. | 40 | TICI | 95.0 | 3 | 60.0 | 12.5 | 10.0 | 2.5 |
| 10 | Cohen JE, et al. | 31 | TIMI | 90.0 | 3 | 77.0 | 10.0 | 23.0 | 3.2 |
| 11 | Machi P, et al. | 56 | TICI | 89.0 | 0 | 46.0 | 7.1 | 5.4 | 1.7 |
| 12 | Mpotsaris A, et al. | 26 | TIMI | 88.0 | 3 | 38.4 | 7.7 | ||
| 13 | Sanak D, et al. | 50 | TICI | 94.0 | 3 | 60.0 | 14.0 | 28.0 | 6.0 |
| 14 | Nogueira RG, et al. | 88 | TICI | 86.0 | 3 | 40.0 | 34.1 | 41.0 | 4.0 |
| 15 | Saver JL, et al. | 58 | TIMI | 61.0 | 3 | 58.0 | 17.0 | 17.0 | 2.0 |
| 16 | Yoon YH, et al. | 26 | TICI | 77.0 | 3 | 39.0 | 8.0 | 12.0 | 0.0 |
| 17 | Mokin M, et al. | 101 | TIMI | 88.0 | 1 | 38.0 | 26.0 | 23.0 | 15.0 |
| 18 | Castano C, et al. | 20 | TICI | 90.0 | 3 | 45.0 | 20.0 | 30.0 | 10.0 |
| 19 | Leker RR, et al. | 22 | TICI | 95.0 | 3 | 60.0 | 20.0 | 14.0 | 5.0 |
| 20 | Roman LS, et al. | 60 | TICI | 73.3 | 3 | 45.0 | 28.3 | 11.7 |
Functional outcomes
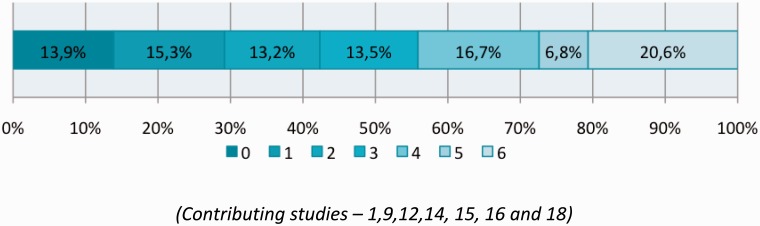
Overall independent functional outcome (mRS≤2) was achieved in 51.2% (19 comparisons; 95%CI 0.461, 0.562; p = 0.653) (Figure 4) and the mortality rate was 16.8% (19 comparisons; 95%CI 0.132, 0.211; p < 0.001). A breakdown of mRS was only provided by seven studies (Miteff et al., 2011; Roth et al., 2013; Mpotsaris et al., 2012; Nogueira et al., 2012;14 Saver et al., 2012;13 Yoon et al., 2013; Castano et al., 2010) and the combined 90 day mRS score from these studies is shown in Figure 5.
Figure 5.
90-day modified Rankin Scores (mRS). (Contributing studies – 1, 9, 12, 14, 15, 16 and 18).
Of patients, 22.0% (14 comparisons; 95%CI 0.169, 0.281; p < 0.001) suffered an ICH, of which only 7.6% (17 comparisons; 95%CI 0.056, 0.102; p < 0.001) were symptomatic (sICH).
Comparison with previous studies
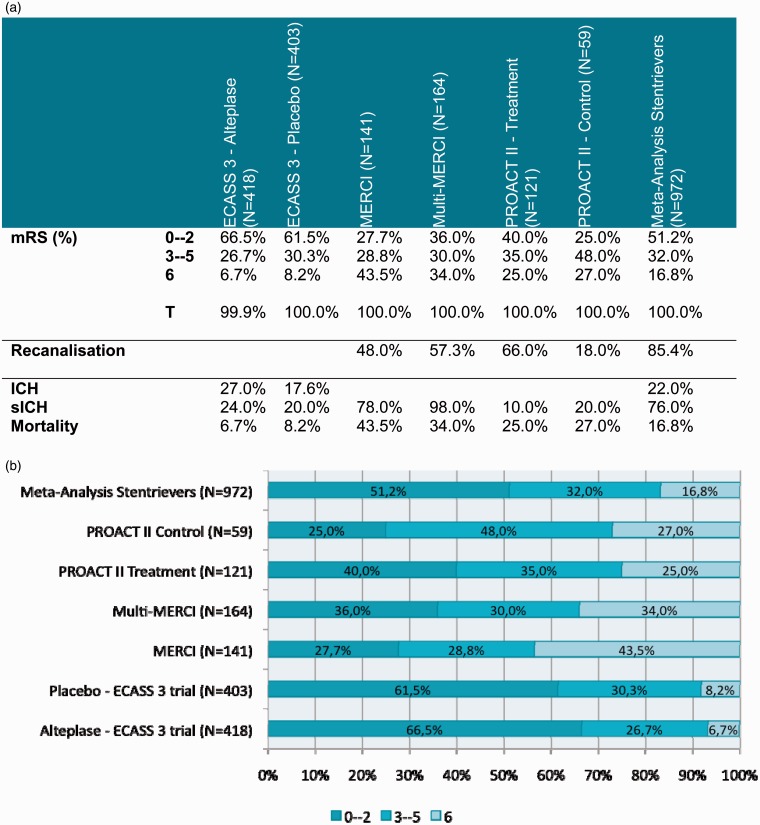
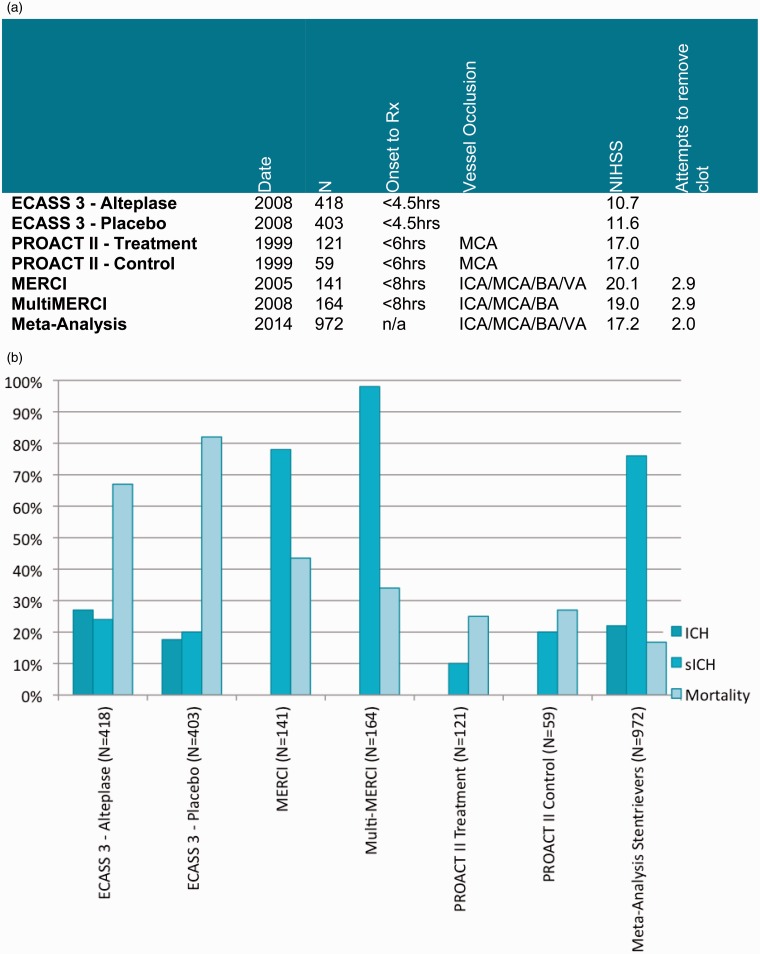
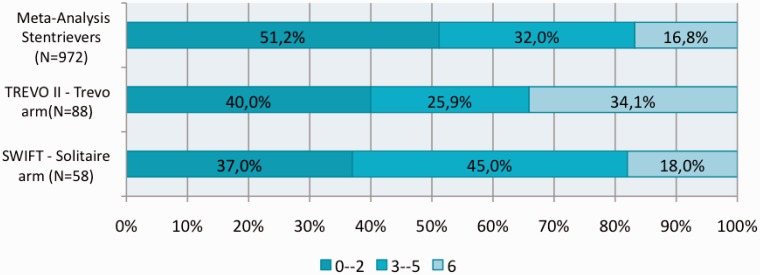
The primary and secondary outcomes of stent retrievers derived from our meta-analysis were then compared with previous trials of stroke therapy (Figures 6 and 7) and more recent RCTs of Solitaire (ev3/Covidien, Irvine, California, USA) and Trevo (Stryker, Kalamazoo, Michigan, USA) devices (Figure 8).
Figure 6.
Comparison with previous studies. (a) Outcomes. (b) mRS.
Figure 7.
Comparison with previous studies. (a) Study Characteristics. (b) Safety outcomes.
Figure 8.
Comparison with SWIFT and TREVO II trials.
Discussion
Following the results of the National Institute of Neurological Disorders and Stroke (NINDS) trial,21 the US FDA licensed intravenous rt-PA for the treatment of acute ischaemic stroke. NINDS showed improved clinical outcome in acute stroke patients who received intravenous rt-PA as opposed to placebo.
The initial 3-hour window was then revised and extended to 4.5 hours following the ECASS 3 trial,5 which also prompted the European Stroke Organization to recommend a 4.5-hour window for intravenous thrombolysis in its 2009 Stroke Guidelines.
Despite the wider time window for rt-PA administration, intravenous thrombolysis still poses numerous challenges. A large number of patients still present outside the 4.5-hour time window,6 and the treatment efficacy of rt-PA decreases with time post ictus. The rate of recanalization of intravenous rt-PA is only 30–50%10,22 and re-occlusions occur frequently.23,24 Intravenous rt-PA was also shown to perform poorly in cases of large-vessel occlusions.25
The main goal of intra-arterial stroke therapy is to recanalize the primary AOL in order to restore flow to the ischaemic territory and therefore allow reperfusion of the arterial bed.7 Timely recanalization in acute ischaemic stroke was shown to correlate with improved long-term functional outcomes.8,9
Over the past decade intra-arterial strategies, which started as microwire disruption (or maceration) of the intraluminal clot and clot aspiration (thrombo-aspiration), have developed quickly. Angioplasty and stenting were also utilized in an attempt to restore cerebral flow. The benefit of administering prourokinase via a catheter-based approach directly into the clot was clearly demonstrated in the PROACT II trial.10
Two single-arm studies of the MERCI Device (Concentric Medical, Mountain View, California, USA), namely the MERCI trial11 and the Multi MERCI trial,12 demonstrated a higher rate of successful large-vessel recanalization compared with the control arm of PROACT II.10 An independent direct association between vessel recanalization and improved functional outcomes was demonstrated in both MERCI and Multi MERCI trials.
The second generation of endovascular devices moved towards a retrievable stent design. The Solitaire FR Revascularization Device (Covidien) was shown to achieve better recanalization rates and improved clinical outcomes compared with the MERCI retriever (a first-generation device) in the SWIFT trial,13 a small RCT which was published in The Lancet. Similarly TREVO 214 – a multi-centre RCT – proved that Trevo Pro Retriever (Stryker Corp.) was safer and more efficient compared with the MERCI device. These two studies prompted the clearance of the two stent retriever devices by the FDA in 2012.
Three RCTs published in the New England Journal of Medicine in March 2013, namely Mechanical Retrieval and Recanalization of Stroke Clots Using Embolectomy (MR RESCUE),15 SYNTHESIS Expansion16 and Interventional Management of Stroke III Trial (IMS III),17 failed to prove that endovascular stroke therapy improved long-term functional outcomes as compared with intravenous therapy. These trials utilized predominantly ‘first-generation’ thrombectomy devices and intra-arterial thrombolysis. In addition, large-vessel occlusion was not proven in these studies.
MR RESCUE was a multi-centre RCT with randomization of acute stroke patients into the endovascular treatment or ‘standard medical care’ groups stratified by the penumbral pattern on preliminary imaging. Mechanical embolectomy was performed with either the MERCI device (Concentric Medical, Mountain View, California, USA) or the Penumbra System (Penumbra, Alameda, California, USA). The relatively low rate of independent functional outcome (compared with IMS 3 and SYNTHESIS Expansion trials) may be secondary to a very long symptom onset to arterial puncture time (mean of 6.3 hours). The generalizability of MR RESCUE may also have been hampered by the limited use of intravenous therapy, and the heterogeneity of imaging and site expertise throughout the study.
In SYNTHESIS Expansion, patients treated with intra-arterial therapy did not receive intravenous rt-PA before or during the procedure, and this does not reflect the practice in most neuro-interventional centres where intravenous thrombolysis is administered uniformly unless contraindicated.
IMS III sought to enrol 900 patients from more than 50 centres in the US to assess whether a combined intravenous and intra-arterial approach in stroke treatment was superior to standard intravenous rt-PA. NINDS stopped enrolment into the study because preliminary analysis (n = 587) met the threshold for ‘futility’. A stent retriever device was, however, used in a very small percentage of the 656 patients that had been enrolled.
Though the three trials may have had potential design flaws and utilized mainly first-generation devices, they have introduced clinical equipoise surrounding the perceived effectiveness of intra-arterial thrombectomy devices.
We identified two previous meta-analysis that evaluated stent retriever devices in acute ischaemic stroke treatment. A meta-analysis published in 201326 included five RCTs to compare endovascular therapy with intravenous thrombolysis in acute stroke, and showed that the two approaches had similar safety outcomes and functional independence. Three out of the five (60%) included studies, however, utilized intra-arterial thrombolysis as the sole endovascular treatment. The meta-analysis by Almekhlafi et al.27 included 16 studies of intra-arterial stroke therapy. However, only four (25%) of the included studies utilized stent retriever technology.
We sought to collect all the available data on the use of second-generation devices (stent retrievers) in acute stroke treatment by performing a systematic review and meta-analysis. To our knowledge our meta-analysis is the first one to exclusively include stent retrievers as the sole mechanical therapy under investigation. The 20 studies identified included 972 patients who presented with a mean NIHSS of 17.2.
Successful recanalization was achieved in 85.4% of acute stroke patients treated with stent retrievers. This is a significant improvement compared with the rates of recanalization achieved by both intra-arterial thrombolysis (66.0% in PROACT II) and first-generation thrombectomy devices (48.0 % and 57.3% in the MERCI and Multi MERCI trials, respectively).
Functional independence (as defined by a mRS ≤2 at 90 days) was achieved in 51.2% of patients in our meta-analysis, which compares favourably with PROACT II, MERCI and Multi MERCI trials (Figure 6). mRS ≤ 2 was achieved in 66.5% of patients in the Alteplase arm of ECASS 3 trial. However, one should interpret this result with caution. A very high rate of functional independence (61.5%) was also achieved in the placebo arm of the same trial. The reasons why such favourable outcomes were achieved are probably two-fold. Primarily, the trial included patients presenting within 4.5 hours of acute stroke treatment as opposed to the 6-hour window in PROACT II and 8-hour windows in both MERCI and Multi MERCI trials. Secondly, the presenting NIHSS was 10.7 in the Alteplase arm of the ECASS 3 trial, which was considerably lower than those included in PROACT II, MERCI and Multi MERCI trials and in our meta-analysis. Both time-to-treatment17 and stoke severity28 (as assessed by NIHSS at presentation) have been shown to be strong predictors of functional outcomes. Patients included in the PROACT II, MERCI and Multi MERCI trials and in our meta-analysis were shown to have a large-vessel (ICA, MCA, BA or VA) occlusion. Large-vessel occlusion was not demonstrated in patients included in ECASS 3, and one could postulate that it was not present in a large number of patients since the mean presenting NIHSS score was just 10.7. The presence of large-vessel occlusion was shown to be associated with a 4.5-fold increased odds of death compared with patients with a normal CT angiogram.29
Our meta-analysis demonstrated favourable rates of independent functional outcome and mortality as previously demonstrated by SWIFT and TREVO 2 trials. The mRS score from these studies and the meta-analysis are summarized in Figure 8. Though the SWIFT trial claimed a 58% ‘good clinical outcome’, the actual percentage of patients who achieved a mRS of ≤2 was in fact 37.0%. Our meta-analysis not only demonstrates better functional outcomes than those that emerged from the SWIFT and TREVO 2 trials, but also adds more weight to the expected outcomes by combining a total of 972 patients from 20 different studies.
Stent retrievers were also shown to have a good safety profile. Mortality at 90 days was 16.8% as compared with 25.0%, 43.5% and 34.0% in the treatment arm of PROACT II, MERCI and Multi MERCI trials, respectively. The rate of sICH (defined as parenchymal haemorrhage with a neurological decline of NIHSS ≥4 occurring within 36 hour of treatment) in patients treated with stent retrievers was 7.6%, which is considerably lower compared with trials utilizing intra-arterial thrombolysis and first-generation thrombectomy devices. Finally the overall rate of ICH in patients treated with stent retrievers also compared well with the rates from ECASS 3 trial. The safety outcomes of the different trials are summarized in Figure 7.
Our meta-analysis also showed that the mean number of stent retriever passes during thrombectomy was significantly lower than the mean number of attempts in MERCI and Multi MERCI trials (2.0 vs. 2.9), which may account for the reduction in procedure time.
The major limitation of our meta-analysis was the study design of most of the included studies. Only two (10%) studies were RCTs, with the rest comprising observational (n = 16) and a non-randomized controlled design (n = 2). We also failed to retrieve five papers despite searching different resources.
The mechanical devices mostly used in MR RESCUE, SYNTHESIS Expansion and IMS III were first-generation thrombectomy devices, and although these studies failed to demonstrate the superiority of mechanical embolectomy compared with standard treatment, one would expect stent retrievers to have the potential to achieve better results. These results should be further validated by randomized controlled studies utilizing stent retriever devices which are already underway.
We conclude that stent retriever devices are safe and able to achieve a high rate of recanalization and functional independence. They may not only be an alternative in acute stroke patients who are ineligible for intravenous rt-PA, but may also be crucial in the MMRT approach in stoke.
Appendix 1
Database: Embase < 1980 to 2013 Week 41>
Search Performed: 15/10/2013
Search Strategy: --------------------------------------------------
exp cerebrovascular accident/ (71392)
exp stroke/ (71392)
exp brain ischemia/ (99749)
exp brain infarction/ (47822)
exp brain hypoxia/ (9473)
exp occlusive cerebrovascular disease/ (24155)
(cerebrovasc$ accident or cerebrovasc$ event).tw. (5003)
((brain or cerebr$) adj5 (ischaemi$ or ischemi$ or infarct$ or hypoxia$)).tw. (75220)
((MCA or middle cerebral artery or ACA or anterior cerebral artery or ICA or internal carotid artery or vertebrobasilar or basilar artery) adj5 (insufficiency or ischaemi$ or ischemi$ or embol$ or thromb$)).tw. (7226)
((acute or sudden or wake-up) adj5 (neuro$ defici$ or hemipare$ or aphasi$ or NIHSS or national institute of neurological disorders)).tw. (1959)
((occlu$ or block$) adj5 (carotid-T or carotid or middle cerebral or MCA or anterior cerebral or ACA or vertebral or basilar or vertebrobasilar)).tw. (25502)
((penumbra or core or perfusion) adj5 (brain or cerebr$)).tw. (15798)
AIS.tw. (6895)
CVA.tw. (3266)
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 (252690)
exp percutaneous thrombectomy/ or exp thrombectomy/ or exp mechanical thrombectomy/ (9582)
exp embolectomy/ (3007)
exp cerebral revascularization/ (1226)
((recanalis$ or recanaliz$ or revascularis$ or revasculariz$) adj5 (brain or cerebr$)).tw. (1400)
((retriev$ or device or stent or extraction or stentriever) adj5 (brain or cerebr$)).tw. (3168)
((treatment or intervention or mechanical or proced$) adj5 (brain or cerebr$)).tw. (35616)
((intra arterial or intra-arterial or IA) adj5 (brain or cerebr$)).tw. (509)
((thromboembolect$ or thromboaspirat$) adj5 (brain or cerebr$)).tw. (7)
((Trevo or Solitaire or Penumbra or Merci or Revive or ERIC or Catch or Capture or 3D Separator or Aperio or Preset) adj5 (brain or cerebr$)).tw. (781)
thrombectomy.tw. (6159)
embolectomy.tw. (2622)
16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 (55621)
15 and 27 (12727)
limit 28 to human (8877)
limit 29 to (adult < 18 to 64 years > or aged < 65 + years>) (3917)
limit 30 to “review” (139)
30 not 31 (3778)
limit 32 to yr = “2007 -Current” (1961)
************************************************************************************************
Appendix 2
Database: Ovid MEDLINE(R) < 1946 to March Week 1 2013>
Search Performed: 15/10/2013
Search Strategy: --------------------------------------------------
exp Stroke/ (83232)
exp Brain Ischemia/ (80833)
exp Cerebral Infarction/ or exp Brain Infarction/ (28959)
exp Hypoxia, Brain/ (10127)
exp Infarction, Middle Cerebral Artery/ or exp Infarction, Posterior Cerebral Artery/ or exp Infarction, Anterior Cerebral Artery/ (5632)
(cerebrovasc$ accident or cerebrovasc$ event).tw. (3441)
((brain or cerebr$) adj5 (ischaemi$ or ischemi$ or infarct$ or hypoxia$)).tw. (56684)
((MCA or middle cerebral artery or ACA or anterior cerebral artery or ICA or internal carotid artery or vertebrobasilar or basilar artery) adj5 (insufficiency or ischaemi$ or ischemi$ or embol$ or thromb$)).tw. (5245)
((acute or sudden or wake-up) adj5 (neuro$ defici$ or hemipare$ or aphasi$ or NIHSS or national institute of neurological disorders)).tw. (1262)
((occlu$ or block$) adj5 (carotid-T or carotid or middle cerebral or MCA or anterior cerebral or ACA or vertebral or basilar or vertebrobasilar)).tw. (19615)
((penumbra or core or perfusion) adj5 (brain or cerebr$)).tw. (12207)
AIS.tw. (4237)
CVA.tw. (1702)
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 (170725)
exp Thrombectomy/ (3291)
exp Embolectomy/ (1001)
((recanalis$ or recanaliz$ or revascularis$ or revasculariz$) adj5 (brain or cerebr$)).tw. (979)
((retriev$ or device or stent or extraction or stentriever) adj5 (brain or cerebr$)).tw. (2425)
((treatment or intervention or mechanical or proced$) adj5 (brain or cerebr$)).tw. (26106)
((intra arterial or intra-arterial or IA) adj5 (brain or cerebr$)).tw. (396)
((thromboembolect$ or thromboaspirat$) adj5 (brain or cerebr$)).tw. (3)
((Trevo or Solitaire or Penumbra or Merci or Revive or ERIC or Catch or Capture or 3D Separator or Aperio or Preset) adj5 (brain or cerebr$)).tw. (578)
thrombectomy.tw. (4019)
embolectomy.tw. (2272)
15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 (37776)
14 and 25 (7171)
limit 26 to human (5189)
limit 27 to yr="2007 -Current” (2139)
limit 28 to “review articles” (511)
28 not 29 (1628)
limit 30 to “all child (0 to 18 years)” (218)
30 not 31 (1410)
************************************************************************************************
Appendix 3
Search Name: COCHRANE
Date Run: 15/10/2013 10:55:12.142
Description:
ID Search
#1 MeSH descriptor: [Stroke] explode all trees
#2 MeSH descriptor: [Brain Ischemia] explode all trees
#3 MeSH descriptor: [Cerebral Infarction] explode all trees
#4 MeSH descriptor: [Brain Infarction] explode all trees
#5 MeSH descriptor: [Hypoxia, Brain] explode all trees
#6 MeSH descriptor: [Infarction, Anterior Cerebral Artery] explode all trees
#7 MeSH descriptor: [Infarction, Middle Cerebral Artery] explode all trees
#8 MeSH descriptor: [Infarction, Posterior Cerebral Artery] explode all trees
#9 cerebrovasc* next (accident or event):ti,ab,kw (Word variations have been searched)
#10 (brain or cerebral) near/5 (ischemi* or infarct* or hypoxi*):ti,ab,kw (Word variations have been searched)
#11 (MCA or middle cerebral artery or ACA or anterior cerebral artery or ICA or internal carotid artery or vertebrobasilar or basilar artery) near/5 (insufficiency or ischemi* or embol* or thromb*):ti,ab,kw (Word variations have been searched)
#12 (acute or sudden or wake-up) near/5 (neuro* defici* or hemipare* or aphasi* or NIHSS or national institute of neurological disorders):ti,ab,kw (Word variations have been searched)
#13 (occlu* or block*) near/5 (carotid-T or carotid or middle cerebral or MCA or anterior cerebral or ACA or vertebral or basilar or vertebrobasilar):ti,ab,kw (Word variations have been searched)
#14 (penumbra or core or perfusion) near/5 (brain or cerebr*) (Word variations have been searched)
#15 cva:ti,ab,kw (Word variations have been searched)
#16 ais:ti,ab,kw (Word variations have been searched)
#17 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16
#18 MeSH descriptor: [Thrombectomy] explode all trees
#19 MeSH descriptor: [Embolectomy] explode all trees
#20 MeSH descriptor: [Thrombolytic Therapy] explode all trees
#21 MeSH descriptor: [Tissue Plasminogen Activator] explode all trees
#22 (recanalis* or recanaliz* or revascularis* or revasculariz*) near/5 (brain or cerebr*):ti,ab,kw (Word variations have been searched)
#23 (retriev* or device or stent or extraction or stentriever) near/5 (brain or cerebr*):ti,ab,kw (Word variations have been searched)
#24 (treatment or intervention or mechanical or proced*) near/5 (brain or cerebr*):ti,ab,kw (Word variations have been searched)
#25 (intra arterial or intra-arterial or IA) near/5 (brain or cerebr*):ti,ab,kw (Word variations have been searched)
#26 (thromboembolect* or thromboaspirat*) near/5 (brain or cerebr*):ti,ab,kw (Word variations have been searched)
#27 (Trevo or Solitaire or Penumbra or Merci or Revive or ERIC or Catch or Capture or 3D Separator or Aperio or Preset) near/5 (brain or cerebr*):ti,ab,kw
#28 thrombectomy:ti,ab,kw (Word variations have been searched)
#29 embolectomy:ti,ab,kw (Word variations have been searched)
#30 #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29
#31 #17 and #30
#32 #31 Publication Date from 2007 to 2013 (470)
************************************************************************************************
Appendix 4: Evaluation sheet
| Study ID: | |
|---|---|
| Paper Reference: | |
| Authors: | |
| Year of Publication: | |
| Stand-alone trial? | Y / N / Unclear |
| If ‘No’, specify: | |
| Checklist completed by: | |
| Checklist verified by: | |
| Evaluation date: | |
| Revision date: | |
| Report ID: | |
| Paper included or excluded? | Y / N |
| If ‘No’, specify reasons for exclusion. |
1. STUDY ELIGIBILITY
Mandatory requirements
If any of these questions can be answered with a ‘No’, discard the paper
| 1.1 | Did the patients present with an acute ischaemic stroke? | Y / N / Unclear |
| 1.2 | Did they receive thrombolysis (with rt-PA within 4.5hours of symptom onset) or mechanical thrombectomy (with a Stentriever) in the acute setting? | Y / N / Unclear |
| 1.3 | Does the study provide enough information regarding recanalisation rates/functional outcome? | Y / N / Unclear |
| 1.4 | Have all exclusion criteria been met? | Y / N / Unclear |
| Desirable study features If any of these questions can be answered with a ‘No’, put aside the paper | ||
| 1.5 | Y / N / Unclear | Y / N / Unclear |
| 1.6 | Is the therapeutic procedure described in enough detail? | Y / N / Unclear |
| 1.7 | Have treatment drop-outs/exclusions been described in enough detail? | Y / N / Unclear |
| 1.8 | Was the data collected prospectively? | Y / N / Unclear |
2. STUDY QUALITY
| 2.1 | Does the study describe its aims or research questions? | Y / N / Unclear |
| 2.2 | Which study design was used? | RCT / Non-randomised control study / Observational study / Other |
| 2.3 | How was the data collected? | Prospectively / Retrospectively / Unclear |
| 2.4 | Does the study describe its inclusion and exclusion criteria and the setting where the data was collected? | Y / N / Unclear |
| 2.5 | Does the study specify where the patients were referred from? | Y / N / Unclear |
| 2.6 | Did the sample include an appropriate spectrum of participants (representative of the target population)? | Y / N / Unclear |
| 2.7 | Does the study describe the recruitment method well? | Y / N / Unclear |
| 2.8 | Does the patient group consist of a consecutive series or people randomly sampled from a larger population? | |
| 2.9 | Does the study describe the clinical and demographic characteristics of the patients in sufficient detail (age range, gender ratio, NIHSS, time from symptom onset? | Y / N / Unclear |
| 2.10 | Does the study report the number of patients who dropped out or other protocol violations? | Y / N / Unclear |
| 2.11 | Were intermediate or dubious results reported? | Y / N / Unclear |
| 2.12 | Does the study describe the training or expertise of the clinician who assessed the results/performed the intervention (e.g. Neurologist, Neuroradiologist) | Y / N / Unclear |
| 2.13 | Are tables and/or graphs adequately labelled and understandable? | Y / N / Unclear |
| 2.14 | Are you confident with the author's choice and use of statistical methods, if employed? | Y / N / Unclear |
| CONTROL GROUP (if present) | ||
| 2.15 | Was an appropriate control group utilised (having similar baseline characteristics)? | Y / N / Unclear |
| 2.16 | How comparable are the cases and controls with respect to potential confounding factors? | |
| 2.17 | Was the allocation sequence adequately generated? | Y / N / Unclear |
| 2.18 | Was the allocation sequence adequately concealed? | Y / N / Unclear |
| 2.19 | Were the controls randomly selected from the same population as the cases? | Y / N / Unclear |
| 2.20 | Were the assessors and/or clinicians blind to the different groups? | Y / N / Unclear |
| 2.21 | Were recanalisation rates and outcomes assessed similarly in the two groups? | Y / N / Unclear |
| 2.22 | Were incomplete outcome data adequately addressed? | Y / N / Unclear |
3. DATA EXTRACTION
| Section 3A – Study characteristics | ||
| 3.1 | When was the study published? | |
| 3.2 | In which journal was the study published? | |
| 3.3 | When were the patients recruited? | |
| 3.4 | What is the sample size (n)? | |
| 3.5 | Is the study a single institution or multi-centre study? | |
| Section 3B – Baseline Characteristics of Patients | ||
| 3.6 | What is the age range and the average age of the cohort? | |
| 3.7 | Was an age limit (eg. 80years) used to exclude/ include patients in the study? | Y / N / Unclear |
| If ‘Yes’, specify: | ||
| 3.8 | What is the male: female ratio? | |
| 3.9 | What was the stroke severity (average NIHSS at presentation)? | |
| 3.10 | What was the time interval from symptom onset to presentation? | |
| 3.11 | What was the mean pre-morbid mRS? | |
| 3.12 | Did the patient have an anterior or posterior circulation stroke? | |
| 3.13 | Was the site of occlusion specified? | Y / N / Unclear |
| If ‘Yes’, specify: | ICA / Carotid T / Carotid L / M1 / M2 / A1 | |
| 3.14 | Was a score used to determine severity of occlusion? | Y / N / Unclear |
| If ‘Yes’, specify: | TICI / MIBI / Other | |
| 3.15 | If you answered ‘Yes’ to 3.14, what was the mean score of occlusion? | |
| 3.16 | What imaging was utilised at presentation? | |
| Section 3C – Procedural Characteristics (Fill Section ‘3C’ twice if the study had a control group) | ||
| 3.17 | Was the patient treated with: | |
| a) Intra-venous thrombolysis with rt-PA (alteplase) within 4.5hours of symptom onset? (Go to 3.18-3.21) | Y / N / Unclear | |
| b) Mechanical thrombectomy with a stentriever? | Y / N / Unclear | |
| c) Intra-venous thrombolysis and mechanical thrombectomy with a stentriever? (Go to 3.18-3.30) | Y / N / Unclear | |
| d) Other treatments? | If ‘Yes’, exclude paper | |
| IV Thrombolysis | ||
| 3.18 | What was the mean time interval from symptom onset to bolus administration? | |
| 3.19 | Was there a time limit to rt-PA administration? | Y / N / Unclear |
| If ‘Yes’, specify: | <3hours / < 4.5hours / 4.5-6hours / other | |
| 3.20 | What dose of rt-PA was administered? | |
| 3.21 | Was rt-PA given as a bolus, continuous infusion or both? | |
| IA Thrombectomy | ||
| 3.22 | Was the procedure performed under local or general anaesthetic? | Local / General |
| 3.23 | What was the mean time from stroke onset to arterial puncture? | |
| 3.24 | Which device(s) was/were used? | |
| 3.25 | What was the mean number of device passes? | |
| 3.26 | What was the mean procedure time? | |
| 3.27 | Was i.v. thrombolysis also administered before or during the procedure? (If ‘Yes’, fill sections 3.15-3.18) | Y / N / Unclear |
| 3.28 | Was intra-arterial thrombolysis administered? | Y / N / Unclear |
| If ‘Yes’, specify: | ||
| 3.29 | Were any procedural complications recorded? | Y / N / Unclear |
| If ‘Yes’, specify: | ||
| 3.30 | What was the device-related complication rate? | Y / N / Unclear |
| If ‘Yes’, specify: | ||
| Section 3D – Outcomes | ||
| 3.31 | What modality was used to determine post treatment / intervention recanalisation? | US / CT / MR / Angiography |
| 3.32 | Was a score used to determine the final recanalisation rate? | Y / N / Unclear |
| If ‘Yes’, specify: | ||
| 3.33 | If you answered ‘Yes’ to 3.29, what was the mean final score? | |
| 3.34 | Was successful recanalisation achieved? | Y / N / Unclear |
| 3.35 | What was the duration of patient follow-up? | |
| 3.36 | Which score was used to determine the functional outcome? | |
| 3.37 | What was the mean mRS score at 90 days? | |
| 3.38 | Was the % improvement in mRS recorded for the cohort? | |
| 3.39 | What percentage of the cohort achieved functional independence at 90 days? | |
| 3.40 | What percentage of patients had died (mRS = 6) at 90 days? | |
| 3.41 | What percentage of patients suffered intra-cranial haemorrhage (ICH) post treatment/intervention? | |
| 3.42 | What percentage of patients suffered symptomatic intra-cranial haemorrhage (sICH) post treatment/intervention? | |
| 3.43 | Is any cost-information provided? | |
Notes:
Functional independence = mRS 0-2
Successful recanalisation = TIMI grade 2 or 3; TICI grade 2b and 3
Dr_Grech_NRJ-D-14-00168
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Mukherjee D, Patil CG. Epidemiology and the global burden of stroke. World Neurosurg 2011; 76(6 Suppl): S85–S90. [DOI] [PubMed] [Google Scholar]
- 2.Saver JL. Time is brain-quantified. Stroke 2006; 37: 263–266. [DOI] [PubMed] [Google Scholar]
- 3.Jauch EC, Saver JL, Adams HP, Jr, et al. American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Peripheral Vascular Disease; Council on Clinical Cardiology. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013; 44: 870–947. [DOI] [PubMed] [Google Scholar]
- 4.National Institute for Health and Care Excellence 2008. Diagnosis and initial management of acute stroke and transient ischaemic attack (TIA). CG68, London: National Institute for Health and Care Excellence, 2008. [Google Scholar]
- 5.Hacke W, Kaste M, Bluhmki E, et al. ECASS Investigators. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008; 359: 1317–1329. [DOI] [PubMed] [Google Scholar]
- 6.Kleindorfer D, Lindsell CJ, Brass L, et al. National US estimates of recombinant tissue plasminogen activator use: ICD-9 codes substantially underestimate. Stroke 2008; 39: 924–928. [DOI] [PubMed] [Google Scholar]
- 7.Tomsick T. TIMI, TIBI, TICI: I came, I saw, I got confused. Am J Neuroradiol 2007; 28: 382–384. [PMC free article] [PubMed] [Google Scholar]
- 8.Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: A meta-analysis. Stroke 2007; 38: 967–973. [DOI] [PubMed] [Google Scholar]
- 9.Nogueira RG, Yoo AJ, Buonanno FS, et al. Endovascular approaches to acute stroke, part 2: A comprehensive review of studies and trials. Am J Neuroradiol 2009; 30: 859–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furlan A, Higashida R, Wechsler L, et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: A randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. JAMA 1999; 282: 2003–2011. [DOI] [PubMed] [Google Scholar]
- 11.Smith WS, Sung G, Starkman S, et al. MERCI Trial Investigators. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: Results of the MERCI trial. Stroke 2005; 36: 1432–1438. [DOI] [PubMed] [Google Scholar]
- 12.Smith WS, Sung G, Saver J, et al. Mechanical thrombectomy for acute ischemic stroke: Final results of the Multi MERCI trial. Stroke 2008; 39: 1205–1212. [DOI] [PubMed] [Google Scholar]
- 13.Saver JL, Jahan R, Levy EI, et al. The SWIFT Trialists. SOLITAIRE™ with the intention for thrombectomy (SWIFT) trial: Design of a randomized, controlled, multicenter study comparing the SOLITAIRE™ Flow Restoration device and the MERCI Retriever in acute ischaemic stroke. Int J Stroke 2014; 9(5): 658–668. [DOI] [PubMed] [Google Scholar]
- 14.Nogueira RG, Lutsep HL, Gupta R, et al. TREVO 2 Trialists. Trevo versus Merci retrievers for thrombectomy revascularisation of large vessel occlusions in acute ischaemic stroke (TREVO 2): A randomised trial. Lancet 2012; 380: 1231–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kidwell CS, Jahan R, Gornbein J, et al. MR RESCUE Investigators. A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med 2013; 368: 914–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ciccone A, Valvassori L, Nichelatti M, et al. SYNTHESIS Expansion Investigators. Endovascular treatment for acute ischemic stroke. N Engl J Med 2013; 368: 904–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Broderick JP, Palesch YY, Demchuk AM, et al. Interventional Management of Stroke (IMS) III Investigators. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med 2013; 368: 893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2, The Cochrane Collaboration, 2009. [Google Scholar]
- 19.Schardt C, Adams MB, Owens T, et al. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak 2007; 7: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Viswanathan M, Berkman N. Development of the RTI item bank on risk of bias and precision of observational studies. J Clin Epidemiol 2012; 2: 163. [DOI] [PubMed] [Google Scholar]
- 21.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995; 333: 1581–1587. [DOI] [PubMed] [Google Scholar]
- 22.Lee KY, Han SW, Kim SH, et al. Early recanalization after intravenous administration of recombinant tissue plasminogen activator as assessed by pre- and post-thrombolytic angiography in acute ischemic stroke patients. Stroke 2007; 38: 192–193. [DOI] [PubMed] [Google Scholar]
- 23.Alexandrov AV, Grotta JC. Arterial reocclusion in stroke patients treated with intravenous tissue plasminogen activator. Neurology 2002; 59: 862–867. [DOI] [PubMed] [Google Scholar]
- 24.Grotta JC, Welch KM, Fagan SC, et al. Clinical deterioration following improvement in the NINDS rt-PA Stroke Trial. Stroke 2001; 32: 661–668. [DOI] [PubMed] [Google Scholar]
- 25.Saqqur M, et al. CLOTBUST Investigators. Clinical deterioration after intravenous recombinant tissue plasminogen activator treatment: A multicenter transcranial Doppler study. Stroke 2007; 38: 69–74. [DOI] [PubMed] [Google Scholar]
- 26.Lin C, Li N, Zhao X, et al. Efficacy and safety of endovascular treatment versus intravenous thrombolysis for acute ischemic stroke: A meta-analysis of randomized controlled trials. PLoS One 2013; 8: e77849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Almekhlafi MA, Menon BK, Freiheit EA, et al. A meta-analysis of observational intra-arterial stroke therapy studies using the Merci device Penumbra system, and retrievable stents. Am J Neuroradiol 2013; 34: 140–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fonarow GC, Saver JL, Smith EE, et al. Relationship of national institutes of health stroke scale to 30-day mortality in medicare beneficiaries with acute ischemic stroke. J Am Heart Assoc 2012; 1: 42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith WS. Significance of large vessel intracranial occlusion causing acute ischemic stroke and TIA. Stroke 2009; 40: 3834–3840. [DOI] [PMC free article] [PubMed] [Google Scholar]