Abstract
Introduction
Kennedy’s disease (KD) is a progressive degenerative disorder affecting lower motor neurons. We investigated the correlation between disease severity and whole brain white matter microstructure, including upper motor neuron tracts, by using diffusion-tensor imaging (DTI) in eight patients with KD in whom disease severity was evaluated using the Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS).
Methods
From DTI acquisitions we obtained maps of fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (L1) and radial diffusivities (L2, L3). We then employed tract-based spatial statistics (TBSS) to investigate within-patient correlations of DTI invariants with ALSFRS and disease duration (DD).
Results
We found a significant correlation between low ALSFRS and 1) low FA values in association commissural and projection fibers, and 2) high L3 values in commissural tracts and fronto-parietal white matter. Additionally, we found a significant association between longer DD and 1) low FA in the genu and body of corpus callosum, association fibers and midbrain and 2) high L1 in projection and association tracts.
Conclusions
The associations between clinical variables and white matter microstructural changes in areas thought to be spared by the disease process support the hypothesis of a multisystem involvement in the complex pathogenic mechanisms responsible for the clinical disability of these patients.
Keywords: Kennedy’s disease, fractional anisotropy, mean diffusivity, DTI, TBSS, ALSFRS, disease duration
Background
Spino-bulbar muscular atrophy (SBMA), or Kennedy’s disease (KD), is a rare adult-onset, progressive neurodegenerative disease, caused by the expansion of a polyglutamine (CAG) triplet repeat in the androgen receptor (AR) gene, transmitted with an X-linked recessive modality. The prevalence is low (of the order of 1/400,000) and affects exclusively males, whereas female carriers are usually asymptomatic.1 The clinical features of this disease include progressive weakness with muscular atrophy localized in hips and shoulders, which gradually extends to muscles with bulbar innervation, resulting in interference with routine physical activities such as walking, climbing up and down stairs as well as difficulties swallowing, chewing and articulating speech.2
Electrophysiological studies suggest that muscle atrophy in KD is caused by a progressive loss of motor axons and cell bodies, resulting in denervation atrophy. Almost always a loss of sensory fibers—especially of type IA, which inform the motoneuron about the state of muscular tension—is present and responsible for a reduction of deep tendon reflexes.2 Also, histopathological studies have documented the loss of motor fibers with depletion of cell bodies of motor neurons at both the spinal and bulbar level, resulting in a reduced volume of the ventral horns of the spinal cord and in muscle fiber atrophy and degeneration.2 The amount of brain involvement in KD has not yet been clarified. Although upper motor neurons (UMN) are not clinically affected, several studies suggest their subclinical involvement.3–6 Transcranial magnetic stimulation7,8 findings have been controversial, with some authors suggesting normal corticomotoneuronal function9 while others imply involvement of multiple nervous system levels.10 Moreover, disease-related cognitive and autonomic changes have been reported in KD patients.
Diffusion-tensor imaging (DTI) is an established magnetic resonance imaging (MRI) method that allows the investigation of white matter (WM) integrity in a noninvasive manner. Under the assumption that a reduction in fractional anisotropy (FA) and or an increase in mean diffusivity (MD) are markers of WM degeneration, DTI has been largely successful in detecting subtle microstructural WM damage in a number of diseases.11–20 To date, only few ultrastructural imaging studies have investigated WM in KD, revealing widespread changes in the brainstem and central WM tracts of KD patients when compared to controls.21,22 The hypothesis of a possible within-disease correlation between disability rating as quantified by the Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS) and the degree of WM alteration has not been tested before. The purpose of this cross-sectional study was to explore associations between WM integrity, as quantified by DTI metrics, and clinical severity of symptoms in patients with KD.
Materials and methods
Patient recruitment and selection
The study was approved by the local ethics committee and written informed consent was obtained from all participants. The population under study included 10 patients with KD recruited from the Department of Neuroscience of our University Hospital. Out of these 10 patients, two refused to undergo MR examination, resulting in a total of eight patients enrolled in this imaging study. Mean age was 54 ± 14 years (range 33–70), and all patients were male. In agreement with the genetic characteristics of the disease, two patients were brothers, while two other patients were first cousins. The average age at disease onset was 44 ± 11 years (range 28–64), with an average disease duration (DD) of 9 ± 8 years (range 3–24). The length of the expanded CAG repeat in the AR gene ranged between 43 and 49. Since a clinical rating scale specific for KD is not available, evaluation of the severity of the disease was performed within a month of MR examination using the ALSFRS, with a mean score of 32 ± 4.6 (range 26–39). None of the patients had symptoms/signs of corticospinal tract disorder. The dosage of creatine kinase (CK), a serological marker of muscle damage, amounted to 1888 ± 1595 IU/l (range 560–4396). A summary of clinical data is shown in Table 1.
Table 1.
Patients’ clinical and laboratory features.
| Pat 1 | Pat 2 | Pat 3 c | Pat 4 c | Pat 5 | Pat 6 | Pat 7 d | Pat 8 d | |
|---|---|---|---|---|---|---|---|---|
| Age at examination | 39 | 48 | 68 | 62 | 48 | 32 | 65 | 59 |
| Age at onset | 35 | 45 | 64 | 57 | 43 | 28 | 42 | 41 |
| Dysarthria/dysphagia | a | b | a | b | a | b | a | b |
| Upper limb weakness | a | a | a | a | a | a | a | b |
| Lower limb weakness | a | a | a | a | a | b | a | b |
| Facial muscle weakness | b | a | a | a | a | b | a | a |
| Sensory symptoms | b | b | a | b | b | b | b | b |
| Pyramidal signs | b | b | b | b | b | b | b | b |
| CKe (I/U) | 4396 | 622 | 655 | 1500 | 560 | 624 | 3860 | 2890 |
| ALSFRS (normal score = 40) | 34 | 37 | 27 | 31 | 33 | 39 | 26 | 29 |
| Disease duration | 3 | 3 | 6 | 6 | 5 | 5 | 22 | 24 |
| CAG repeat length (n) | 49 | 43 | 43 | 43 | 46 | 48 | 46 | 47 |
Present. bAbsent. cMaternal cousins. dSiblings. eSerum level of creatine kinase. CK: creatine kinase; ALSFRS: Amyotrophic Lateral Sclerosis Functional Rating Scale; CAG: polyglutamine.
MRI
MR scanning was performed on a 3-Tesla scanner (Achieva 3 T Intera, Philips Healthcare, The Netherlands) equipped with gradients of maximum amplitude and rise time of 80 mT/m and 200 mT/m/ms, respectively. A dedicated eight-element head coil was used for transmission and reception of the radiofrequency impulses. The imaging protocol included an axial T2-weighted turbo spin echo (TSE) sequence (repetition time (TR) 3000 ms, echo time (TE) 80 ms, thickness/gap 3 mm/1), axial T2-fluid attenuated inversion recovery (FLAIR) (TR 11000 ms, TE 120 ms, thickness/gap 3 mm/0), sagittal T1-weighted TSE sequence (TR 2000 ms, TE 10 ms, thickness/gap 3 mm/1), and a T1-three-dimensional (3D) fast field echo (FFE) sequence (field of view (FOV) 224 × 224, TR 25 ms and TE 50 ms), which were employed by an expert neuroradiologist for ruling out visible abnormalities. For DTI we used a spin-echo (SE) echo-planar (EPI) single shot sequence with interleaved slice acquisition and the following parameters: TE 59 ms, TR 4805 ms, slice thickness 3 mm, 42 slices, no gap, FOV 224 × 224, matrix 256 × 256 voxel, sensitivity encoding (SENSE) reduction factor R = 2.4, partial Fourier encoding 60%. Diffusion weighting with a single b value of 800 s/mm2 was applied in 32 noncollinear directions. One additional non-diffusion-weighted reference image (b0 image) was also acquired. Total scan time per patient was approximately 25 minutes.
Diffusion-weighted image processing and tract-based spatial statistics (TBSS)
Diffusion-weighted images were corrected for head motion and eddy current distortions using Tortoise DTI (Tolerably Obsessive Registration and Tensor Optimization Indolent Software Ensemble 1.4.023), after which brain tissue was segmented using BET, part of FSL 5.0.2.24 The b matrix was reoriented by applying the rotational part of the affine transformation employed in the eddy- and motion correction step. We then confirmed that average whole-brain voxel displacement computed in this step did not significantly correlate with clinical variables (i.e. DD and/or ALSFRS score). A tensor model was then fitted to the raw data using a constrained nonlinear least squares procedure implemented in the software package CAMINO,25 and residual non-positive definite tensors (in regions where the nonlinear algorithm failed to converge) were removed by tensor interpolation in the log-Euclidean domain.26 The following tensor invariants were then computed from the estimated tensor field using the software package DTI-TK:27 FA, MD and single eigenvalues of the diffusion tensor (MD, L1/2/3, respectively). The primary eigenvalue (L1) represents axial diffusivity, while the second and third eigenvalues (L2 and L3) represent radial diffusivities (RDs). Voxelwise statistical analysis of all tensor invariant data was carried out using TBSS,28 also part of FSL, which involves a) nonlinear registration of every FA image to every other one; b) identification of the “most representative image” as the one that requires the least warping to align every other image to it; c) affine alignment of this target image into Montreal Neurological Institute (MNI)152 space; d) transformation of every other image into MNI space by combining the single nonlinear transforms into the target image with the affine transform into MNI space; e) creation of a mean FA image; and f) thinning to create a mean FA skeleton that represents the centers of all tracts common to the group. Each individual’s aligned FA data were then projected onto this skeleton, and previously computed warps were successively applied to MD, L1, L2 and L3 images, thereby transforming all images into MNI space for subsequent skeletonization as above. Resulting data were then fed into voxelwise inter- as well as intrasubject statistics. The latter included full correction for multiple comparisons over space using permutation-based nonparametric inference within the framework of the general linear model (10,000 permutations), which included age (orthogonalized with respect to the clinical variable under investigation) as a nuisance covariate. p values were calculated and corrected for multiple comparisons using the “two-dimensional (2D)” parameter settings with threshold-free cluster enhancement (TFCE), thereby avoiding the use of an arbitrary threshold for the initial cluster formation.29 Specifically, we investigated within-patient correlation of DTI invariants with clinical variables. This optimized processing pipeline has been previously employed in several clinical research studies.
Results
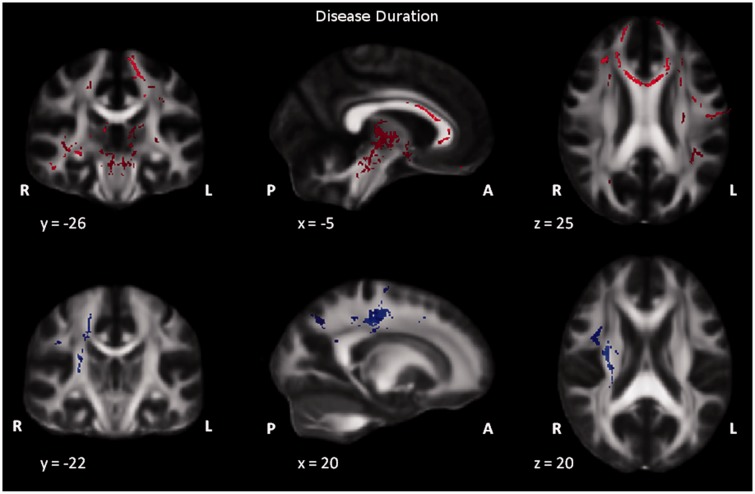
We found a significant (p ≤ 0.05, Spearman Rank Correlation R = –0.8) correlation between ALSFRS and disease duration. No other significant pairwise correlations were found between ALSFRS, disease duration, CAG repeat length and CK. When investigating possible associations between clinical variables and DTI parameters, TBSS analysis revealed significant correlation of DTI metrics both with ALSFRS rating and DD. Specifically, a low-value ALSFRS was seen to be significantly correlated with low FA values in association, commissural and projection fibers, as well as in the midbrain. Also, low-value ALSFRS was seen to be significantly correlated with a high diffusivity in the radial direction (as represented by L3) in commissural tracts and fronto-parietal WM with relative sparing of occipital regions (Figure 1). Additionally, a long disease duration was seen to be significantly correlated with low FA values in the genu and body of corpus callosum (anterior segment), association fibers and midbrain with an increase in axial diffusivity (L1) in projection and association tracts (Figure 2). No other significant correlations between DTI invariants and clinical variables were found.
Figure 1.
Results of voxel-based analysis of FA and L3 maps in patient group by using TBSS. Upper panel: Red shows regions where FA correlates significantly with ALSFRS score: in highlighted areas, FA is significantly lower (p < 0.05, TFCE) when ALFSRS is lower. Lower panel: Blue shows regions where L3 correlates significantly with ALSFRS score: in highlighted areas, L3 is significantly higher (p < 0.05, TFCE) when ALSFRS is lower. For each brain section Montreal Neurological Institute (MNI) coordinates are displayed. FA: fractional anisotropy; TBSS: tract-based spatial statistics; ALSFRS: Amyotrophic Lateral Sclerosis Functional Rating Scale; L3: radial diffusivity; TFCE: threshold-free cluster enhancement.
Figure 2.
Results of voxel-based analysis of FA and L1 maps in patient group by using TBSS. Upper panel: Red shows regions where FA correlates significantly with disease duration in highlighted areas, FA is significantly lower (p < 0.05, TFCE) when disease duration is higher. Lower panel: Blue shows regions where L1 correlates significantly with disease duration in highlighted areas, L1 is significantly lower (p < 0.05, TFCE) when disease duration is higher. For each brain section Montreal Neurological Institute (MNI) coordinates are displayed. FA: fractional anisotropy; TBSS: tract-based spatial statistics; L1: axial diffusivity.
Discussion
In this cross-sectional DTI study, we demonstrated a significant correlation between microstructural integrity (as quantified through DTI) and both DD and clinical disability in KD patients. Among other regions, such correlations were localized in the supratentorial WM, which represents the main novelty of our findings. Previous DTI studies found widespread FA changes in central WM tracts when comparing KD patients to controls, supporting the hypothesis that brain involvement in KD might be significantly larger than previously hypothesized.22 On the other hand, the same study22 did not show statistically significant correlations between FA and DD in KD. Conflicting findings about correlations between DTI indexes and clinical disability scores have also been reported in other diseases that display upper motor neuron (UMN) involvement such as Amyotrophic Lateral Sclerosis (ALS). While some studies demonstrated significant correlations between UMN involvement and ALSFRS,30,31 others reported no correlation between DTI invariants and disability ratings.32 A large share of these differences may result from the wide range of acquisition techniques and processing strategies currently employed in DTI and the impact they can have on final analysis results33—for instance, a number of previous studies have been performed using manual region of interest (ROI) placement, which might have introduced an operator-dependent bias in the quantification of DTI indexes. However, it is important to note the vast majority of studies published to date confirm the potential of DTI as a marker of degenerative processes in both ALS and KD.
Our findings should be interpreted in light of the pathophysiological processes involved in KD that may potentially result in regional axonal degeneration with increased extracellular WM volume. In KD, the compound action potentials of motor and sensory nerves are reduced, possibly indicating that peripheral axonal degeneration is the primary pathogenic process, determined by defective AR accumulation in nuclei and cytoplasm of neurons and resulting in disruption of cellular function, degeneration and loss. Interestingly, the mutant AR also has been proved to be widespread in the brain.34 By using multimodal evoked potentials (EP), Lai et al. observed several abnormal findings (e.g. visual EPs showed prolonged peaks) suggesting that KD involves multiple functional levels of the central nervous system (CNS).10 Also, evidence for subtle involvement of corticospinal tracts has been reported: using transcranial magnetic stimulation,7,8 Pachatz et al.6 demonstrated subclinical involvement of UMN in two patients with KD. Moreover, Karitzky et al.4 reported changes in UMN in a magnetic resonance spectroscopy (MRS) study, and Shaw et al.3 showed UMN degeneration at autopsy in two cases. Additional evidence of brain involvement comes from the latter study, which demonstrated neural depletion and gliosis in the hippocampus and subcortical gliosis in the prefrontal region in a KD patient with cognitive deficit, and from Kessler et al.35 and Soragna et al.,36 who reported two KD cases, one with dementia and frontal lobe atrophy, and another one with parkinsonism.
Our finding of low FA values and high RD values in a number of regions in patients with more severe clinical impairment likely reflect WM tract degeneration following cell death of lower motor neurons. A significant correlation between DTI indexes and clinical data was also found in the internal capsula, demonstrating the involvement of cortico-spinal tracts and therefore supporting the usefulness of DTI metrics as a surrogate marker of UMN involvement in KD. Also, the relationship between FA and ALSFRS (i.e. higher FA values in less-disabled patients) we observed both in the normal-appearing subcortical WM and in the corpus callosum may reflect complex anterograde degeneration/axonal loss with involvement of projection WM tracts to/from bulbar regions. Our DTI results are also consistent with pathologic findings that have been reported in KD.37 While we did not compare our patient population to a healthy control group, it is important to note that our patient study is focused on elucidating within-disease mechanisms by exploring which regions may drive disease worsening and/or may exhibit progressively severe damage as the disease persists. In this context, correlation-based patient studies between MRI and clinical parameters/scales have been widely useful in a variety of contexts such as grading gliomas,38 predicting hand motor outcome in stroke,39 understanding cognitive damage in traumatic brain injury40 and quantifying damage in glaucoma.41 Since the present study was carried out on a relatively small number of patients, future studies on larger samples will be necessary to confirm these hypotheses.
Conclusion
This study demonstrates widespread associations between clinical variables indicative of disease severity and microstructural WM changes in areas previously thought to be spared by the disease process, supporting the hypothesis of a complex-multisystem CNS involvement in KD.
Acknowledgments
Authors’ contributions are as follows:
Guarantor of integrity of the entire study: RF, MG, RM, GS.
Study concepts: RF, FG, RM, NT.
Study design: FG, GM, NT.
Definition of intellectual content: RF, FG, MG, NT.
Literature research: FD, FG, AM, SL, NT.
Clinical studies: GM, RM.
Experimental studies: FG, RM, GM, SM.
Data acquisition: SM, AM, FD, FG.
Data analysis: SL, NT.
Statistical analysis: SL, NT.
Manuscript preparation: FD, FG, MG, SL, RM, NT.
Manuscript editing: FG, SL, SM, RM, NT.
Manuscript review: MG, RF, GS.
Abbreviations: KD: Kennedy’s disease; DTI: diffusion tensor imaging; ALSFRS: Amyotrophic Lateral Sclerosis Functional Rating Scale; FA: fractional anisotropy, MD: mean diffusivity; L1: axial diffusivity; L2 and L3 radial diffusivities; DD: disease duration; AR: androgen receptor; UMN: upper motor neurons; MRI: magnetic resonance imaging; NAWM: normal appearing white matter; WM: white matter; EP: evoked potentials; CNS: central nervous system.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declare that they have no conflicts of interests. No financial, consulting, and personal relationships with other people or organizations influenced this work.
References
- 1.Katsuno M, Banno H, Suzuki K, et al. Clinical features and molecular mechanisms of spinal and bulbar muscular atrophy (SBMA). Adv Exp Med Biol 2010; 685: 64–74. [DOI] [PubMed] [Google Scholar]
- 2.Harding AE, Thomas PK, Baraitser M, et al. X-linked recessive bulbospinal neuronopathy: A report of ten cases. J Neurol Neurosurg Psychiatry 1982; 45: 1012–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaw PJ, Thagesen H, Tomkins J, et al. Kennedy’s disease: Unusual molecular pathologic and clinical features. Neurology 1998; 51: 252–255. [DOI] [PubMed] [Google Scholar]
- 4.Karitzky J, Block W, Mellies JK, et al. Proton magnetic resonance spectroscopy in Kennedy syndrome. Arch Neurol 1999; 56: 1465–1471. [DOI] [PubMed] [Google Scholar]
- 5.Mader I, Karitzky J, Klose U, et al. Proton MRS in Kennedy disease: Absolute metabolite and macromolecular concentrations. J Magn Reson Imaging 2002; 16: 160–167. [DOI] [PubMed] [Google Scholar]
- 6.Pachatz C, Terracciano C, Desiato MT, et al. Upper motor neuron involvement in X-linked recessive bulbospinal muscular atrophy. Clin Neurophysiol 2007; 118: 262–268. [DOI] [PubMed] [Google Scholar]
- 7.Toschi N, Welt T, Guerrisi M, et al. Transcranial magnetic stimulation in heterogeneous brain tissue: Clinical impact on focality, reproducibility and true sham stimulation. J Psychiatr Res 2009; 43: 255–264. [DOI] [PubMed] [Google Scholar]
- 8.Toschi N, Welt T, Guerrisi M, et al. A reconstruction of the conductive phenomena elicited by transcranial magnetic stimulation in heterogeneous brain tissue. Phys Med 2008; 24: 80–86. [DOI] [PubMed] [Google Scholar]
- 9.Vucic S, Kiernan MC. Clarifying variability of corticomotoneuronal function in Kennedy disease. Muscle Nerve 2011; 44: 197–201. [DOI] [PubMed] [Google Scholar]
- 10.Lai TH, Soong BW, Chen JT, et al. Multimodal evoked potentials of Kennedy’s disease. Can J Neurol Sci 2007; 34: 328–332. [DOI] [PubMed] [Google Scholar]
- 11.Garaci FG, Floris R, Bozzao A, et al. Increased brain apparent diffusion coefficient in tuberous sclerosis. Radiology 2004; 232: 461–465. [DOI] [PubMed] [Google Scholar]
- 12.Garaci FG, Colangelo V, Ludovici A, et al. A diffusion longitudinal MR imaging study in normal-appearing white matter in untreated relapsing–remitting multiple sclerosis. AJNR Am J Neuroradiol 2007; 28: 475–478. [PMC free article] [PubMed] [Google Scholar]
- 13.Watson R, Blamire AM, Colloby SJ, et al. Characterizing dementia with Lewy bodies by means of diffusion tensor imaging. Neurology 2012; 79: 906–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhuang L, Sachdev PS, Trollor JN, et al. Microstructural white matter changes, not hippocampal atrophy, detect early amnestic mild cognitive impairment. PloS One 2013; 8: e58887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conte A, Brancati F, Garaci F, et al. Kinematic and diffusion tensor imaging definition of familial Marcus Gunn jaw-winking synkinesis. PloS One 2012; 7: e51749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pardini M, Garaci FG, Bonzano L, et al. White matter reduced streamline coherence in young men with autism and mental retardation. Eur J Neurol 2009; 16: 1185–1190. [DOI] [PubMed] [Google Scholar]
- 17.Giannelli M, Toschi N, Passamonti L, et al. Diffusion kurtosis and diffusion-tensor MR imaging in Parkinson disease. Radiology 2012; 265: 645–646; author reply 646–647. [DOI] [PubMed] [Google Scholar]
- 18.Diciotti S, Ginestroni A, Bessi V, et al. Identification of mild Alzheimer’s disease through automated classification of structural MRI features. Conf Proc IEEE Eng Med Biol Soc 2012; 2012: 428–431. [DOI] [PubMed] [Google Scholar]
- 19.Mascalchi M, Ginestroni A, Toschi N, et al. The burden of microstructural damage modulates cortical activation in elderly subjects with MCI and leuko-araiosis. A DTI and fMRI study. Hum Brain Mapp 2014; 35: 819–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giannelli M, Belmonte G, Toschi N, et al. Technical note: DTI measurements of fractional anisotropy and mean diffusivity at 1.5 T: Comparison of two radiofrequency head coils with different functional designs and sensitivities. Med Phys 2011; 38: 3205–3211. [DOI] [PubMed] [Google Scholar]
- 21.Unrath A, Muller HP, Riecker A, et al. Whole brain-based analysis of regional white matter tract alterations in rare motor neuron diseases by diffusion tensor imaging. Hum Brain Mapp 2010; 31: 1727–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pieper CC, Konrad C, Sommer J, et al. Structural changes of central white matter tracts in Kennedy’s disease—a diffusion tensor imaging and voxel-based morphometry study. Acta Neurol Scand 2013; 127: 323–328. [DOI] [PubMed] [Google Scholar]
- 23.Pierpaoli C, Walker L, Irfanoglu MO, et al. TORTOISE: An integrated software package for processing of diffusion MRI data. International Society for Magnetic Resonance in Medicine (ISMRM) 18th annual meeting, Stockholm, Sweden, 2010.
- 24.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004; 23(Suppl 1): S208–S219. [DOI] [PubMed] [Google Scholar]
- 25.Cook PA, Bai Y, Nedjati-Gilani S, et al. Camino: Open-source diffusion-MRI reconstruction and processing. 14th Scientific Meeting of the International Society for Magnetic Resonance in Medicine, Seattle, WA, USA. May 2006, p.2759.
- 26.Arsigny V, Fillard P, Pennec X, et al. Log-Euclidean metrics for fast and simple calculus on diffusion tensors. Magn Reson Med 2006; 56: 411–421. [DOI] [PubMed] [Google Scholar]
- 27.Zhang H, Avants BB, Yushkevich PA, et al. High-dimensional spatial normalization of diffusion tensor images improves the detection of white matter differences: An example study using amyotrophic lateral sclerosis. IEEE Trans Med Imaging 2007; 26: 1585–1597. [DOI] [PubMed] [Google Scholar]
- 28.Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage 2006; 31: 1487–1505. [DOI] [PubMed] [Google Scholar]
- 29.Smith SM, Nichols TE. Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 2009; 44: 83–98. [DOI] [PubMed] [Google Scholar]
- 30.Woo JH, Wang S, Melhem ER, et al. Linear associations between clinically assessed upper motor neuron disease and diffusion tensor imaging metrics in amyotrophic lateral sclerosis. PloS One 2014; 9: e105753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El Mendili MM, Cohen-Adad J, Pelegrini-Issac M, et al. Multi-parametric spinal cord MRI as potential progression marker in amyotrophic lateral sclerosis. PloS One 2014; 9: e95516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu C, Jiang R, Yi X, et al. Role of diffusion tensor imaging or magnetic resonance spectroscopy in the diagnosis and disability assessment of amyotrophic lateral sclerosis. J Neurol Sci 2015; 348: 206–210. [DOI] [PubMed] [Google Scholar]
- 33.Jones DK, Cercignani M. Twenty-five pitfalls in the analysis of diffusion MRI data. NMR Biomed 2010; 23: 803–820. [DOI] [PubMed] [Google Scholar]
- 34.Adachi H, Katsuno M, Minamiyama M, et al. Widespread nuclear and cytoplasmic accumulation of mutant androgen receptor in SBMA patients. Brain 2005; 128(Pt 3): 659–670. [DOI] [PubMed] [Google Scholar]
- 35.Kessler H, Prudlo J, Kraft S, et al. Dementia of frontal lobe type in Kennedy’s disease. Amyotroph Lateral Scler Other Motor Neuron Disord 2005; 6: 250–253. [DOI] [PubMed] [Google Scholar]
- 36.Soragna D, Messa C, Mochi M, et al. Dopaminergic pathways involvement in Kennedy’s disease: Neurophysiological and. J Neurol 2001; 248: 710–712. [DOI] [PubMed] [Google Scholar]
- 37.Finsterer J. Bulbar and spinal muscular atrophy (Kennedy’s disease): A review. Eur J Neurol 2009; 16: 556–561. [DOI] [PubMed] [Google Scholar]
- 38.Server A, Graff BA, Josefsen R, et al. Analysis of diffusion tensor imaging metrics for gliomas grading at 3 T. Eur J Radiol 2014; 83: e156–e165. [DOI] [PubMed] [Google Scholar]
- 39.Song F, Zhang F, Yin DZ, et al. Diffusion tensor imaging for predicting hand motor outcome in chronic stroke patients. J Int Med Res 2012; 40: 126–133. [DOI] [PubMed] [Google Scholar]
- 40.Baek SO, Kim OL, Kim SH, et al. Relation between cingulum injury and cognition in chronic patients with traumatic brain injury; diffusion tensor tractography study. NeuroRehabilitation 2013; 33: 465–471. [DOI] [PubMed] [Google Scholar]
- 41.Omodaka K, Murata T, Sato S, et al. Correlation of magnetic resonance imaging optic nerve parameters to optical coherence tomography and the visual field in glaucoma. Clin Experiment Ophthalmol 2014; 42: 360–368. [DOI] [PubMed] [Google Scholar]