Abstract
We sought to report a central T2 hypointensity within the optic nerve on 3 T MRI studies obtained as part of the NASA Flight Medicine Visual Impairment Intracranial Pressure Protocol that had not been described previously. Twenty-one astronauts, who had undergone MRI of both orbits with direct coronal T2 sequences between 2010 and 2012, were retrospectively included. Two of the astronauts did not have previous exposure to microgravity at the time of their scans. A central T2 hypointensity was observed in 100% of both right and left eyes. It was completely visualized throughout the nerve course in 15 right eyes (71.4%) and in 19 left eyes (90.5%).We describe a new finding seen in all study participants: a central T2 hypointensity in the epicenter of the optic nerve. We speculate that this T2 hypointensity may represent flow voids caused by the central retinal vessels.
Keywords: optic nerve, T2 hypointensity, 3 Tesla, MR imaging
Introduction
Using high-resolution 3 Tesla magnetic resonance imaging (MRI) of the eye and orbits, acquired as part of a National Aeronautics and Space Administration (NASA)-initiated protocol for occupational surveillance of visual impairment and structural changes of the eyes and optic nerves in astronauts, we unexpectedly observed a central T2 hypointensity in the center of the optic nerve that has not been previously described (Figures 1 and 2). The improved signal-to-noise ratio of 3 Tesla MRI over 1.5 Tesla MRI allows visualization of physiologic variants and pathology affecting small anatomical structures such as the optic nerve.
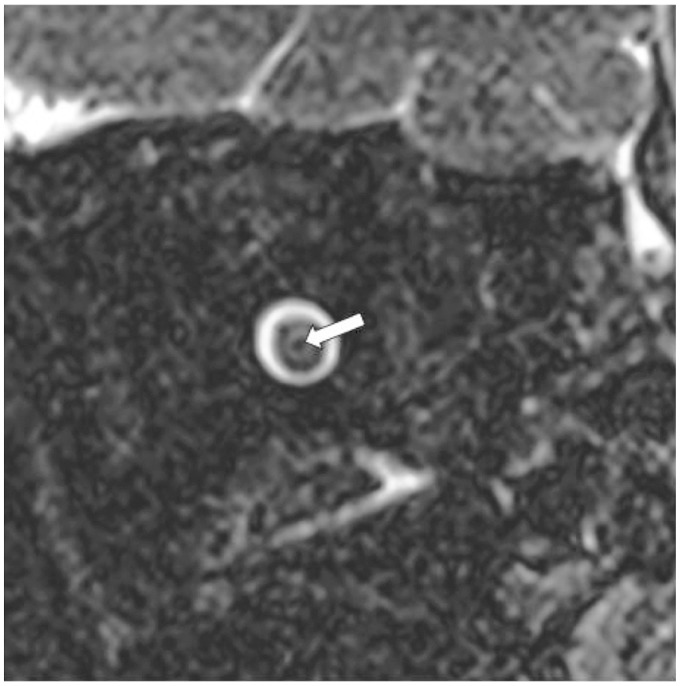
Figure 1.
Optic nerve central T2 hypointensity on coronal view. Isovolumetric T2 fat-saturated coronal images of the left optic nerve demonstrating central T2 hypointensity (white arrow) surrounded by three discrete concentric structures.
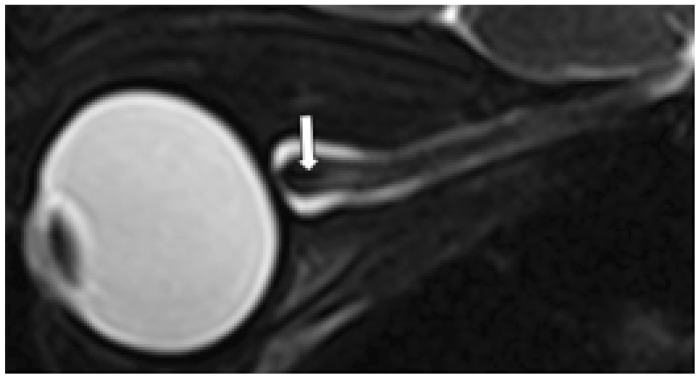
Figure 2.
Optic nerve central T2 hypointensity on sagittal view. Isovolumetric T2 fat-saturated sagittal images of the optic nerve demonstrating central T2 hypointensity (white arrow) surrounded by three discrete concentric structures.
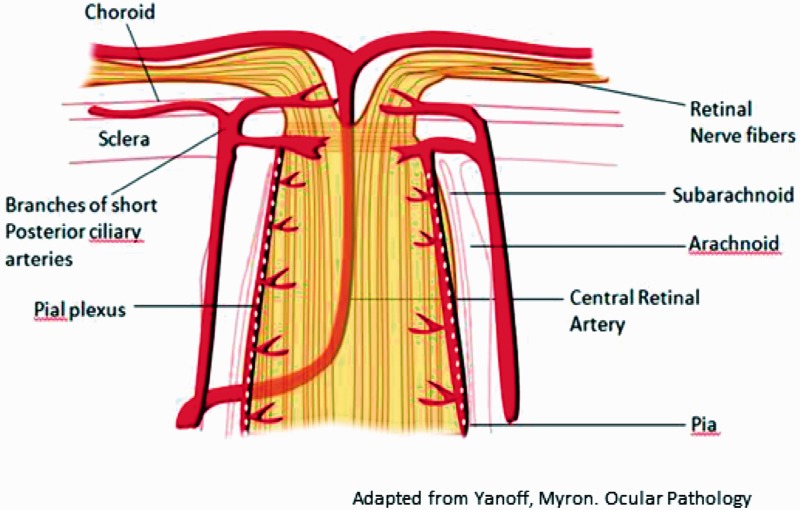
The optic nerve, beginning at the optic disc and ending at the optic chiasm, consists of the efferent axons of the retinal ganglion cells.1 Within the orbit, the optic nerve receives blood supply from three anastomosing vascular networks: a longitudinal system including the central retinal artery and the short posterior ciliary arteries and a centripetal system comprised of the pial plexus, all of which originate from the ophthalmic artery (Figure 3).2,3
Figure 3.
Graphic illustration of blood supply to the optic nerve in sagittal section.3
Previous literature has described subarachnoid cerebrospinal fluid (CSF) signal within the optic nerve sheath enveloping a concentric T2-hypointense rim surrounding an intermediate-signal T2 hyperintensity thought to represent darker myelinated fibers surrounding non-myelinated fibers and interstitial space, respectively (Figures 1 and 2).4
Case series
This study was approved by the University of Texas Medical Branch’s Institutional Review Board and by the NASA Institutional Review Board, and was compliant with the Health Insurance Portability and Accountability Act.
Twenty-one subjects who had undergone MRI of the orbits with 3 Tesla 3D isovolumetric T2-weighted, fat-saturated, fast spin echo (3D T2W FS FSE) pulse sequences between 2010 and 2012 were retrospectively included. The MRI sequences used were part of the NASA Flight Medicine Visual Impairment/Intracranial Pressure Protocol, done for occupational surveillance of astronauts, and are not routine clinical MR sequences (Table 1). In total, 42 eyes were evaluated. Of note, two subjects did not have previous exposure to microgravity at the time of their scans. A novel, central T2 hypointensity was observed at some point along the optic nerve in 100% of both right and left eyes. This hypointensity was completely visualized throughout the distal third of the nerve’s course in 15 right eyes (71.4%) and in 19 left eyes (90.5%). The two astronauts not yet exposed to microgravity also show the central T2 hypointensity throughout the distal third of the optic nerves bilaterally.
Table 1.
Imaging parameters.
| Parameter | Axial T2 3D TSE with Fat Saturation | Sagittal T2 3D TSE with Fat Saturation |
|---|---|---|
| Repetition time (msec) | 750 | 750 |
| Echo time (msec) | 118 | 116 |
| Field of view (mm) | 65 | 8 |
| Slice thickness (mm) | 0.6 | 0.6 |
| Slice oversampling (%) | 25.0 | 23.1 |
| Matrix | 128 × 128 | 128 × 128 |
| Number of averages | 1 | 1 |
| Gaze | Neutral | Neutral |
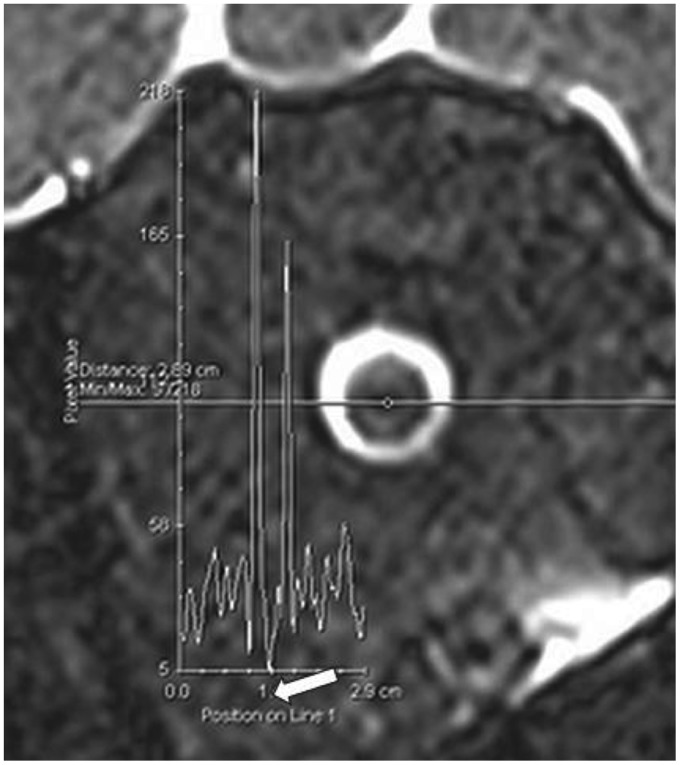
Of note, the T2 signal of the orbital fat surrounding the optic nerve is not completely homogeneous. We used the orbital fat as a reference for noise. The plots of the signal across the optic nerve in Figure 4 show that the central T2 hypointensity is below the variation range of the orbital fat signal. Therefore, this structure is unlikely to be a partial volume artifact.
Figure 4.
Plots of the orbital T2 signal. Plots of the measured signal along the indicated lines on T2 fat-saturated coronal images of the optic nerve are illustrated. The central T2 hypointensity corresponds to the plunge pointed by white arrow.
Discussion
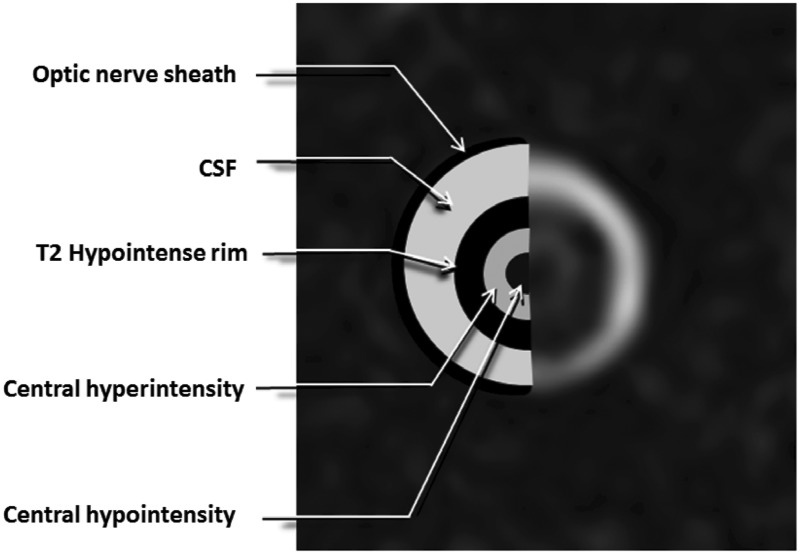
We speculate that the central T2 hypointensity represents a flow void artifact, to which T2W FS FSE pulse sequences are particularly susceptible, due to the central retinal artery (Figure 5). The cylindrical T2-weighted intermediate-signal intensity (referred to in previous literature as the central hyperintensity) represents the fibers of the optic nerve, and the thin surrounding hypointense rim may represent darker myelinated fibers or could represent a hypointense flow artifact from the surrounding pial plexus. Incomplete visualization of the central T2 hypointensity in our study can be attributed to motion artifact and other image quality issues.
Figure 5.
T2 intensities of the optic nerve. A peripheral hypointensity represents the optic nerve sheath. The outer hyperintensity represents CSF signal within the optic nerve sheath enveloping a concentric T2-hypointense rim (which may represent peripheral myelinated fibers of the optic nerve or flow void from the pial vasculature. The central T2 intermediate intensity is thought to represent the non-myelinated fibers of the optic nerve. In the center of this region, a central hypointensity is present which could be due to flow void artifacts corresponding to the central retinal artery.
Our direct coronal sequences did not extend posterior to the orbit and thus the central T2 hypointensity could not be traced proximally to the ophthalmic artery, but future studies could evaluate this relationship.
Given the absence of known optic nerve pathologies in our subjects and the observation of the central T2 hypointensity in the two subjects not exposed to prolonged microgravity, we believe these findings are unlikely to represent pathology. However, further studies involving a larger control group are necessary to evaluate this finding in a normal population.
The diagnosis of pathologies affecting the optic nerve, including optic neuritis, optic nerve compression by pituitary macroadenomas, and Leber’s hereditary optic neuropathy, are facilitated by MRI.5–8 The transition from 1.5 to 3 Tesla enables higher-resolution imaging via improvement in the signal-to-noise ratio, which allows detection of new anatomical findings not previously described. Further research is necessary to determine if changes regarding the described central T2 hypointensity of the optic nerve could potentially be seen in certain disease states affecting the central nervous system, including the optic nerve.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Hayreh SS. The central artery of the retina. Its role in the blood supply of the optic nerve. Br J Ophthalmol 1963; 47: 651–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chapter II The optic disc and the peripapillary choroid. Doc Ophthalmol 1978; 45: 34–54.
- 3.Yanoff M and Sassani JW. Ocular Pathology. Edinburgh: Mosby/Elsevier, 2009. Available from: http://site.ebrary.com/id/10430630.
- 4.Kramer LA, Sargsyan AE, Hasan KM, et al. Orbital and intracranial effects of microgravity: findings at 3-T MR imaging. Radiology 2012; 263: 819–827. [DOI] [PubMed] [Google Scholar]
- 5.Gass A, Moseley IF, Barker GJ, et al. Lesion discrimination in optic neuritis using high-resolution fat-suppressed fast spin-echo MRI. Neuroradiology 1996; 38: 317–321. [DOI] [PubMed] [Google Scholar]
- 6.Tokumaru AM, Sakata I, Terada H, et al. Optic nerve hyperintensity on T2-weighted images among patients with pituitary macroadenoma: Correlation with visual impairment. Am J Neuroradiol 2006; 27: 250–254. [PMC free article] [PubMed] [Google Scholar]
- 7.Lamirel C, Cassereau J, Cochereau I, et al. Papilloedema and MRI enhancement of the prechiasmal optic nerve at the acute stage of Leber hereditary optic neuropathy. J Neurol Neurosurg Psychiatry 2010; 81: 578–580. [DOI] [PubMed] [Google Scholar]
- 8.Boretius S, Gadjanski I, Demmer I, et al. MRI of optic neuritis in a rat model. Neuroimage 2008; 41: 323–334. [DOI] [PubMed] [Google Scholar]