Abstract
Neuroeconomics employs neuroscience techniques to explain decision-making behaviours. Prospect theory, a prominent model of decision-making, features a value function with parameters for risk and loss aversion. Recent work with normal participants identified activation related to loss aversion in brain regions including the amygdala, ventral striatum, and ventromedial prefrontal cortex. However, the brain network for loss aversion in pathologies such as depression has yet to be identified. The aim of the current study is to employ the value function from prospect theory to examine behavioural and neural manifestations of loss aversion in depressed and healthy individuals to identify the neurobiological markers of loss aversion in economic behaviour. We acquired behavioural data and fMRI scans while healthy controls and patients with depression performed an economic decision-making task. Behavioural loss aversion was higher in patients with depression than in healthy controls. fMRI results revealed that the two groups shared a brain network for value function including right ventral striatum, ventromedial prefrontal cortex, and right amygdala. However, the neural loss aversion results revealed greater activations in the right dorsal striatum and the right anterior insula for controls compared with patients with depression, and higher activations in the midbrain region ventral tegmental area for patients with depression compared with controls. These results suggest that while the brain network for loss aversion is shared between depressed and healthy individuals, some differences exist with respect to differential activation of additional areas. Our findings are relevant to identifying neurobiological markers for altered decision-making in the depressed.
Keywords: prospect theory, loss aversion, neural loss aversion, dopaminergic system, depression
Introduction
Neuroeconomics utilises the methods of neuroscience, such as functional brain imaging, to understand economic behaviour in humans. Prospect theory, developed by Daniel Kahneman and Amos Tversky,1 is one of the most influential theories of human decision-making. It is descriptive in nature, encompassing the computation of value and probability weighting. The value function computation involves gain and loss parameters as independent variables, and the probability weighting function computation transforms objective information on odds into subjective estimates using nonlinear weighting.
The value function of prospect theory suggests that, for participants to accept a mixed-prospect gamble with even odds (+x, .50; –y, .50), the gain must be substantially larger than the loss. (Typically, x must be ≥2y.) This phenomenon is loss aversion: people are more sensitive to losses than to gains. The loss aversion coefficient is a measure of behavioural loss aversion; its mean value was reported to be around 2 in healthy individuals.2 Functional magnetic resonance imaging (fMRI) literature on neural loss aversion suggests the involvement of sub-cortical and cortical brain regions such as amygdala, ventral striatum, and ventromedial prefrontal cortex.2–4 However, we still do not understand the extent of loss aversion in psychopathologies such as depression, nor its specific neural correlates. Deeper understanding of the neural substrates of loss aversion in patients with depression in contrast to healthy individuals may enable the identification of the neurobiological markers of the disease.
Tom and colleagues2 suggested that future studies integrating methods from behavioural economics and cognitive neuroscience may provide greater insight into the nature of these pathologies. The aim of this functional MRI investigation was to extend our understanding of loss aversion in patients with depression using an empirical approach.
Earlier literature related to reward and decision-making in depression suggested that depressed individuals show reduced neural activity to rewards, undervalue rewarding stimuli, and are less able to learn to avoid risky options than healthy individuals.5–7 Neurobiological research on depression suggested dysfunction in frontostriatal areas during some stages of reward processing,8–11 probably involving projections of the dopaminergic system from the ventral tegmental area (VTA) to the frontal cortex.12 Recent research on the VTA further highlights its involvement in aversion and reward processing,13 and suggests that its abnormal functioning could be implicated in neuropsychiatric disorders such as depression. The dopaminergic system is known to be integral to reward processing and decision-making in healthy individuals.
The aim of this fMRI study was to quantify differences between depressed and healthy individuals using the experimental paradigm of loss aversion,2 in order to identify neurobiological markers of depression. We hypothesise a shared network of neural loss aversion comprising the amygdala, ventral striatum, and ventromedial frontal cortex as reported elsewhere.2–4 We further hypothesise that the observed differences in neural loss aversion between the two groups reflect altered activation patterns of dopaminergic brain regions including the VTA.
Materials and methods
Participants
For this study 20 volunteers were recruited: 10 healthy volunteers (females = 4) and 10 patients with depression (females = 2). The two groups performed an economic decision-making task while lying supine inside a 1.5 Tesla Siemens Avanto whole-body fMRI scanner. Participants were matched for age (healthy volunteers: 27.5 ± 2.41; depressed patients: 31.9 ± 7.5), were assessed as right-handed via the Edinburgh Handedness Inventory, and educated up to the 10th class in the Indian secondary education system. Informed consent was obtained from each participant. This study was approved by the Institutional Ethics Committee of Sree Chitra Tirunal Institute for Medical Sciences & Technology, Trivandrum, India.
Patients with depression were selected based on references from a psychiatrist. The screening of patients with depression relied on the Hospital Anxiety and Depression Scale (HADS), and the screening of healthy volunteers was based on the Mini International Neuropsychiatric Interview (MINI).14 All participants were given INR 100 as an initial endowment before the scanning session. Participants were informed that they would receive further compensation at the end of the scanning session, with payoffs based on the outcomes of four or five randomly selected gambles. They were thus encouraged to focus on the task. Participants earned INR 100 and INR 200 in this manner.
Task procedures
The two groups of participants performed the following decision-making task, adapted from Tom et al.2 The experiment consisted of 64 trials presented serially. Each trial offered a 50/50 monetary gamble, representing a 50% chance of obtaining some amount of loss, and a 50% chance of obtaining some amount of gain. We used the reduced version of monetary gambles with the decision phase alone, that is, an 8 × 8 loss × gain matrix (in contrast to in Tom et al.,2 who used 16 × 16 gambles). In our experiment, loss values ranged from INR 50 to INR 200 incremented in steps of INR 20, and the gain values ranging from INR 100 to INR 400 incremented in steps of INR 40. Participants were given INR 100 as an initial endowment with which to fund gambles. Participants were informed that each trial was independent of other trials in the experiment, and that their task in each trial was to either accept or reject the monetary gamble with reference to their initial endowment amount. They were also instructed that accepting the 50/50 gamble would yield either the loss or the gain amount presented. In the example trial shown in Figure 1, the loss and gain values are –50 and +140, respectively. Rejecting the gamble caused no change in current wealth. There was no time limit set for accepting or rejecting a gamble. Participants were also instructed that the decision-making in each trial (i.e. either accepting or rejecting a monetary gamble) should be made with reference to the current wealth (i.e. endowment amount received at the beginning of the experiment), and the final amount (i.e. at the end of the experiment) as a consequence of experimental performance would be computed based on four or five randomly selected trial outcomes.
Figure 1.
The experimental time line.
Functional imaging
A standard EPI sequence 1.5 Tesla Siemens Avanto whole-body scanner with the following parameters was used at a TR = 3580 ms, TE = 50 ms, Flip angle = 90°, FOV = 256 mm × 256 mm, matrix size = 64 × 64, number slices = 36, slice thickness = 3 mm. In addition, a T1-weighted structural scan was acquired from each participant (flash3D, TR = 5000 ms, TE = 418 ms, flip angle = 90°, number of slices = 176, slice thickness = 1 mm, matrix size = 222 × 256). Functional images were analysed using the General Linear Model (GLM) using the Statistical Parametric Mapping software package (SPM8).
Behavioural parameters and analysis
On each experimental trial, the participant’s decision (accept or reject) and his/her reaction time (RT, time from stimulus onset to decision entry) were recorded. Based on each participant’s decisions and RT, we computed parameters related to prospect theory such as loss aversion coefficient (LAC), risk factor (alpha), and decision times for each pair of loss–gain across participants within each group.2,15 The LAC parameter indicates the steepness of the value function over losses, and the risk factor (alpha) refers to the curvature of the value function. In prospect theory, the risk factor or alpha value is close to 1, indicating risk neutrality.
Statistical analysis of functional images
The imaging analysis was performed in SPM8 using a standard 2-level random-effects model. The imaging data was first subjected to standard preprocessing procedures of SPM, which consist of realignment and unwarping followed by coregistration, normalisation and smoothing (FWHM of 8 × 8 × 8 mm) of functional images. The first-level fMRI analysis involved constructing a GLM with appropriate regressors for each participant from the two groups. The first-level model for each participant was constructed for the decision phase, that is from the onset of gamble presentation to the response (keypress related to either accept or reject) of every trial. As the decision-making process in each experimental trial involved the independent variables, namely loss and gain values, and the dependent variable, choice (accept or reject), we included three parameters (loss, gain, and choice) as trial-wise parametric modulators. Using this first-level model we analysed brain activations responding positively to gain values and negatively to loss values (following the prospect theory value function, gain > loss) and brain regions related to neural loss (NLA, as suggested by Tom et al.2). The NLA corresponded to brain regions responding to the decreasing activity for increasing loss values compared with the increased activity for increasing gain values of gambles presented to the participants. The second-level model involved constructing two separate (gain > loss and NLA) 1 × 2 factorial designs (two factors: contrasts from the first-level models related to comparisons between controls and patients). First, we evaluated the shared activations between the two groups for the contrast gain > loss, to understand the brain substrates of the gain–loss function based on the prospect theory. Next, we assessed the difference in neural loss aversion using the NLA model, to understand the differences in brain activations related to loss aversion in patients in contrast to the control group. Finally, we performed region of interest (ROI) analysis on brain activation (resolution: spherical volume of 6 mm3), and correlated activation with behavioural loss aversion values (LAC) across participants. These brain–behaviour correlations (BBC) offered more insights into the relationship between behaviour and brain activations in the two groups.
Results
Behavioural results
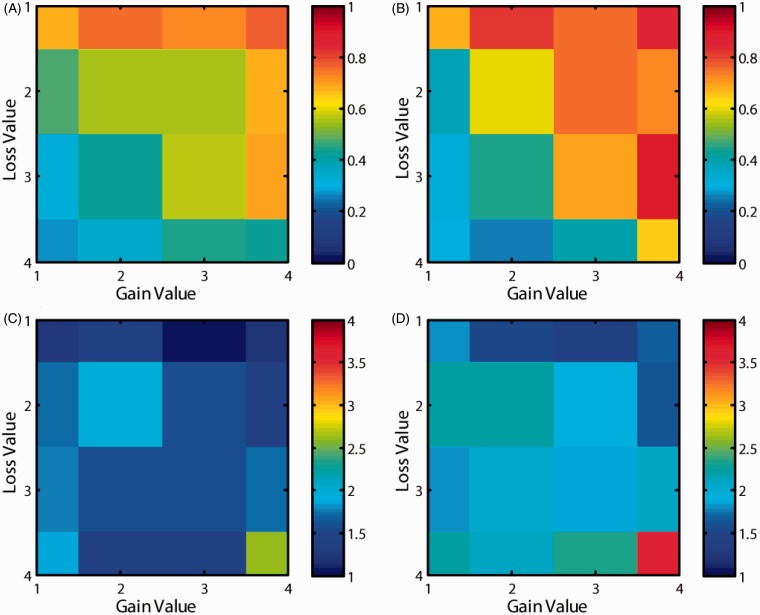
Analysis of participants’ decisions (accept or reject) revealed that patients with depression were more likely than controls to accept gambles with high gain and low loss values (Figure 2(a) and(b)). However, there were no differences in RTs between the two groups (Figure 2(c) and (d)) except in the very high gain and low loss condition (right bottom grid location).
Figure 2.
Probability and decision time. The probability of acceptance of gamble in (A) healthy volunteers and (B) patients with depression. The decision time (from the onset of gamble till response) in (C) healthy volunteers and (D) patients with depression.
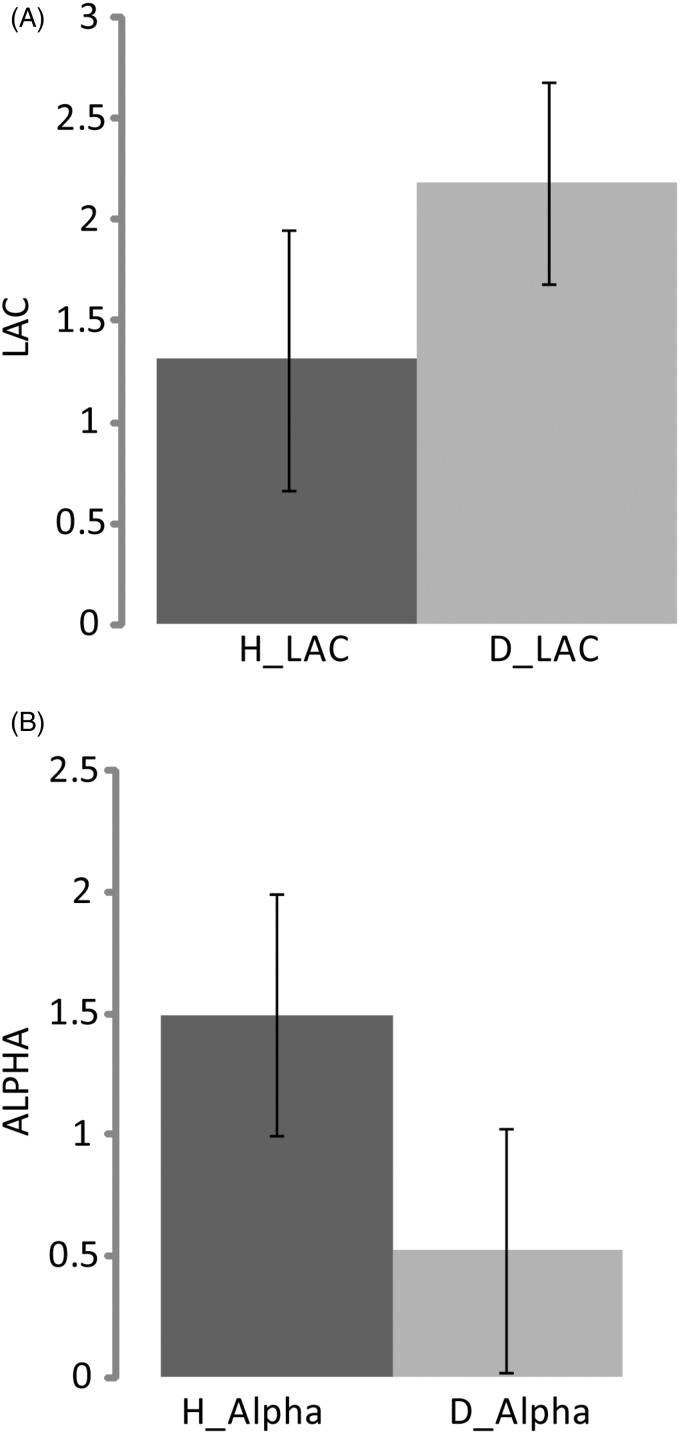
Evaluation of the behavioural loss aversion coefficient (LAC, lambda) and risk factor (alpha) showed differences between healthy individuals and patients with depression (Figure 3). The analysis of the behavioural data of loss aversion showed higher LAC for depressed patients (mean = 2.18 ± 0.64, median = 1.93) than for controls (mean = 1.31 ± 0.64, median = 1.82). However, the risk factor (alpha) values for controls (mean = 1.49 ± 0.13, median = 1.56) were higher than values for patients (mean = 0.52 ± 1.00, median = 1.20).
Figure 3.
The behavioural results. (A) The behavioural loss aversion coefficients (lambda) values healthy (H_LAC) and depressed (D_LAC) individuals. (B) The behavioural risk preference (alpha) values for healthy (H_Alpha) and depressed individuals (D_Alpha).
Functional imaging results
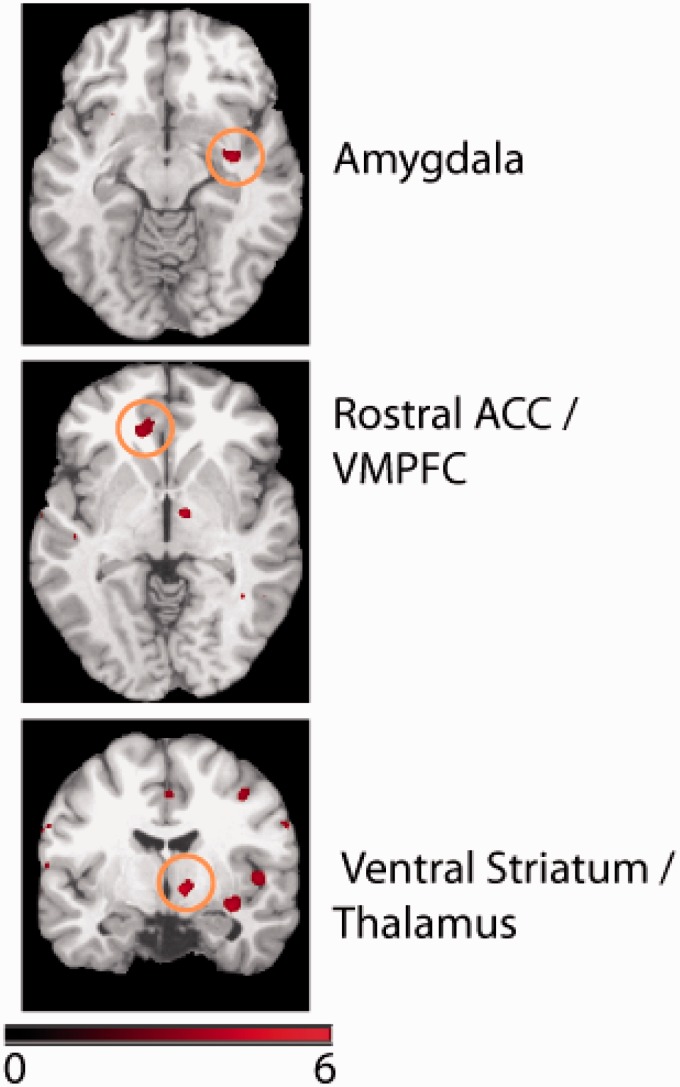
Application of the gain > loss contrast showed shared areas of brain activation among healthy and depressed participants in the left superior temporal cortex, right ventral striatum/thalamus, left rostral anterior cingulate cortex (ACC)/ventromedial prefrontal cortex, right superior temporal cortex, right superior medial frontal cortex, right paracentral lobule, right premotor (dorsal), right amygdala, right middle temporal cortex, right dorsolateral prefrontal cotex, right posterior insula, and left inferior frontal cortex (Table 1). Brain activation in the right ventral striatum/thalamus, left rostral ACC/ventromedial prefrontal cortex, and right amygdala, subserving the value function, is shown in Figure 4. In addition to these brain areas conjointly activated in the two groups, we also observed the following differences in activation between the two groups in the gain>loss contrast. Patients showed greater activation than controls in right middle temporal cortex (BA 37/21, MNI coordinate: (56,58,-4), p < 0.001 uncorrected). However, the same contrast showed no significant activations (p < 0.001 uncorrected) in controls compared with patients.
Table 1.
Shared (common) brain areas. The brain activations commonly responded positively to gain values and negatively to loss values across the two groups of individuals (healthy and depression). Stereotaxic MNI coordinates of significant BOLD signals were obtained with uncorrected p < 0.001, number of voxels ≥ 10.
| Brain area | BA | Coordinates (mm) | T Score (number of voxels) | |||
|---|---|---|---|---|---|---|
| Superior temporal cortex | L | 41/21 | −40 | −34 | 8 | 5.04 (18) |
| Ventral striatum / pallidum / thalamus | R | 10 | −8 | −4 | 4.71 (11) | |
| Ventromedial prefrontal cortex / rostral anterior cingulate cortex | L | 11 | −14 | 36 | −2 | 4.54 (28) |
| Superior temporal cortex | R | 48/42 | 48 | −24 | 12 | 4.38 (42) |
| Superior medial frontal cortex / middle cingulate cortex | R | 9/32 | 12 | 48 | 38 | 4.35 (13) |
| Paracentral lobule | R | 5/4 | 8 | −38 | 60 | 4.22 (13) |
| Premotor (dorsal) | R | 6 | 40 | −10 | 48 | 4.22 (16) |
| Amygdala | R | 20/34 | 34 | −8 | −12 | 4.21 (15) |
| Middle temporal cortex | R | 37 | 50 | −52 | 0 | 4.15 (14) |
| Dorsolateral prefrontal cotex | R | 46 | 36 | 20 | 42 | 4.14 (11) |
| Posterior insula / superior temporal cortex | R | 48 | 50 | −6 | 4 | 4.11 (15) |
| Inferior frontal cortex | L | 45/47 | −44 | 20 | 38 | 3.96 (10) |
Figure 4.
Shared (Common) network of activations conjointly activated for both gains and losses in both the groups.
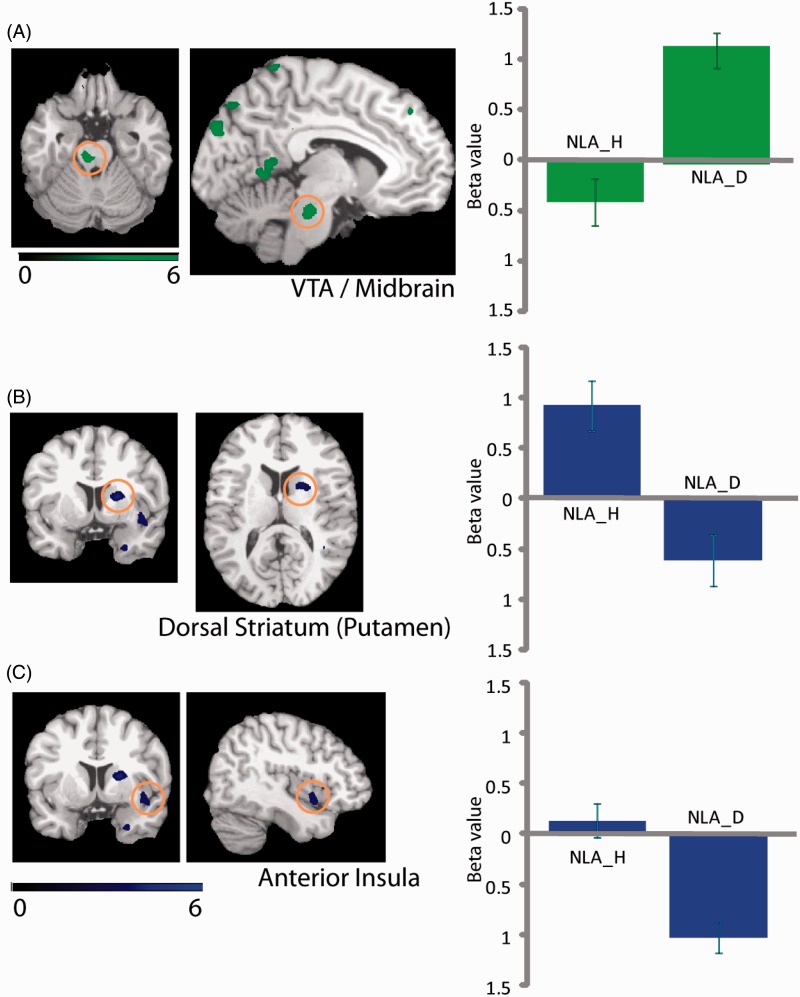
Differences were found in activation related to NLA between controls and patients. The depressed patients compared with healthy participants showed activation differences in the left cuneus, right lingual gyrus, right middle occipital cortex, left VTA/midbrain, left lingual gyrus, bilateral posterior cerebellum, left middle occipital cortex, right inferior occipital cortex, right lingual gyrus, right precuneus, right posterior cingulate cortex, and right precuneus. Another set of regions was activated in controls but not in patients, including right anterior insula, right dorsal striatum (putamen), and right parahippocampal cortex. These results suggest that a distinct, only partly overlapping set of brain regions subserves loss aversion in the healthy and the depressed populations (Table 2 and Figure 5).
Table 2.
Neural loss aversion differences. The brain activations related to neural loss aversion in depression > healthy and healthy > depression contrasts. Stereotaxic MNI coordinates of significant BOLD signals were obtained with uncorrected p < 0.001, number of voxels ≥ 10.
| Brain area | BA | Coordinates (mm) | T Score (number of voxels) | |||
|---|---|---|---|---|---|---|
| Depression > Healthy | ||||||
| Cuneus | L | 18/19 | −8 | −88 | 30 | 5.94 (55) |
| Lingual gyrus | R | 17/18 | 10 | −54 | 2 | 5.93 (339) |
| Middle occipital cortex | R | 19/18 | 28 | −80 | 16 | 5.00 (77) |
| Ventral tagmental area (vta)/ midbrain | L | −6 | −28 | −26 | 4.83 (50) | |
| Lingual gyrus | L | 18/19 | −22 | −68 | −6 | 4.63 (36) |
| Posterior cerebellum | R | 28 | −80 | −50 | 4.59 (15) | |
| Middle occipital cortex | L | 39/19 | −34 | −68 | 22 | 4.58 (45) |
| Inferior occipital cortex | R | 19 | 38 | −72 | −12 | 4.44 (111) |
| Lingual gyrus | R | 18 | 24 | −82 | 0 | 4.37 (36) |
| Precuneus | R | 23 | 8 | −50 | 26 | 4.24 (39) |
| Posterior cerebellum | L | −16 | −74 | −42 | 4.22 (15) | |
| Posterior cingulate cortex | R | 29/26 | 6 | −34 | 16 | 4.12 (10) |
| Precuneus | R | 23 | 6 | −68 | 26 | 3.97 (15) |
| Healthy > Depression | ||||||
| Anterior insula | R | 48/47 | 36 | 12 | −14 | 4.82 (29) |
| Dorsal striatum (putamen) | R | 48 | 22 | 6 | 12 | 4.20 (23) |
| Parahippocampal cortex | R | 20/36 | 26 | 0 | −36 | 3.82 (12) |
Figure 5.
The neural loss aversion differences between patients with depression and healthy individuals. The NLA_H and NLA_D refer to the neural loss aversion values in healthy individuals and patients with depression, respectively.
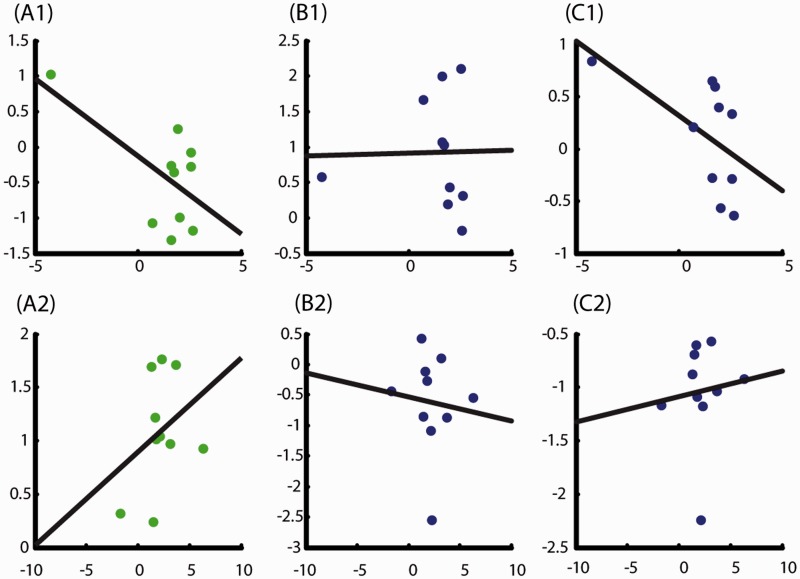
Brain–behaviour correlations were computed based on differential activations (Figure 5) related to neural loss coefficients (LACs) (Figure 6). BBCs for controls were as follows: left VTA/midbrain (negative correlation, R = –0.6), right dorsal striatum (no correlation, R = 0.02), and right anterior insula (negative correlation, R = –0.55), with the VTA and right anterior insula showing a similar trend. In patients, BBCs were as follows: left VTA/midbrain (positive correlation, R = 0.33), right dorsal striatum (no correlation, R = –0.09), and right anterior insula (positive correlation, R = 0.10).
Figure 6.
The brain-behavioural correlations (BBC). A1, B1, C1 indicate BBCs for healthy participants and A2, B2, C2 indicate BBCs for patients with depression. The left-hand panel (A) is for VTA, middle panel (B) for dorsal striatum and right-hand panel (C) for anterior insula. The x-axis represents behavioural loss aversion and the Y-axis represents neural loss aversion across the participants.
Discussion
The aim of this study was to identify neurobiological markers of economic decision-making, dissociating healthy individuals from patients with depression in risk-based decision-making.1 The behavioural and neural changes related to loss aversion observed in the two groups16 were used to identify neurobiological markers. The neuroeconomics literature suggests that loss aversion is a critical aspect of risk-based decision-making, and is an important form of aversive behaviour in addition to risk aversion.17 It is to be noted that when empirically computing the value function of prospect theory, two factors18 are important: the loss aversion coefficient (LAC or lambda) and the risk factor (alpha). These two parameters of the value function15 represent the steepness (lambda computation) and curvature (alpha computation) of the value function of prospect theory. The higher the value of LAC as measured by lambda, the higher is the aversion to losses. The risk parameter alpha <1 represents behavioural risk-seeking. The dopamine system is implicated in subserving loss aversion;19 the parameter of loss aversion computed from behavioural and neural data was used to contrast the two participant groups based on differences in reward perception. Our research suggests that differential brain activation related to loss aversion may be one component of a neurobiological marker of altered decision-making in depression.
The behavioural results of this study revealed that the LAC and the risk factor (alpha) computation differ between controls and patients with depression. Depressed participants showed higher LAC values than controls, and controls showed higher alpha values than patients. Also note that the variability of alpha values was greater in the depression group (larger standard error). The mean and median of alpha values (alpha > 1) indicated risk aversion in both groups. Since the aim of this study was to use the loss aversion parameter as a marker to dissociate the two groups, the occurrence of higher LAC values in depressed patients than controls is an important finding of our study.
Our fMRI results revealed that the value function over gains and losses is the basis for loss aversion behaviour and is subserved by the ventral striatum/thalamus, rostral ACC/ventromedial prefrontal cortex, and amygdala. Activations in these areas correlated to computing the value function reported previously in the literature.2–4 However, our comparison of NLA activation between patients with depression and controls showed activation mainly in the VTA, which is known to be involved in reward processing and value computation.20 Our results confirm recent research suggesting that abnormal functioning of the VTA and ventral striatum, part of the dopaminergic system involved in economic decision-making, may be implicated in neuropsychiatric disorders such as depression.13,20,21
Activation of the ventral striatum was common across the two groups, suggesting that it is involved in computing the value function across these two populations. The differential activation of the VTA suggests its role in characterising patients with depression. Thus, differential activity in the VTA could be used to differentiate patients with depression, and could possibly could serve as neurobiological marker of this disease.
The results of our BBC analysis revealed negative correlations of the VTA and anterior insula in healthy participants, meaning that the NLA activity of these areas decreased with respect to behavioural coefficient. Conversely, activation in the VTA and anterior insula showed positive correlations for patients with depression. These correlations imply that the VTA, an important area in the mesolimbic dopaminergic system, is being recruited more strongly in patients with depression to compensate for the decreased sensitivity during reward processing.6 VTA activation in this study was related to loss aversion and is implicated during an aversive behaviour in patients with depression, whereas the ventral striatum and ventromedial prefrontal cortex were found to be part of the general reward circuitry (irrespective of population type). Thus our study adds an additional dimension to the role of the VTA in aversive behaviours in patients with depression,13 and indicates the recruitment of additional areas for reward processing.
The above results also indicate the role of insula in loss aversion behaviour in patients with depression. The anterior insula is known to play a prominent role in emotion processing, specifically in the explicit appraisal of emotional and body-visceral stimuli.22–24 Wager et al.23 observed increased left insula activity during tasks associated with negative or withdrawal-related emotions. We propose that aversion to losing is an emotional response during economic decision-making, and is potentially enhanced in patients experiencing above-normal negative emotions. This may be reflected in increased inhibitory neural activity. The insula is crucial in anticipatory anxiety induced by exposure to aversive stimuli,25 and is proposed to be involved in a number of psychiatric disorders, including major depressive disorder, anxiety, addiction, and autism. A meta-analysis of neuroimaging studies suggested a common increased engagement of fear circuitry, including amygdala and insula hyperactivity, in anxiety disorders.26 Studies on anxiety-prone subjects reported increased amygdala and insula activation during both anticipation and observation of aversive pictures.27 In light of the above, it is crucial to ask whether there a way in which activation levels in the insula can be modulated to alleviate some of the adverse behavioural effects of affective disorders. A series of studies28–33 performed by one of the authors, using real-time fMRI Brain–Computer Interface (BCI), showed that learned control of activation in the anterior insula influences the behavioural response to emotional stimuli.
In summary, our results show that, in addition to the shared base network for value function computation, additional brain areas are recruited differentially in depressed as compared with healthy individuals. Comparing activation in these areas to baseline during an economic decision-making task may constitute a neurobiological marker of depression.
Acknowledgements
We thank Amita Basu for helpful comments on the manuscript.
Conflict of interest
The authors declare no conflict of interest.
Funding
This study was supported by the grants from Department of Biotechnology (No: BT / PR14364 / MED / 30 / 521 / 2010), Government of India.
References
- 1.Kahneman D, Tversky A. Prospect theory: An analysis of decision under risk. Econometrica 1979; 47: 263–291. [Google Scholar]
- 2.Tom SM, Fox CR, Trepel C, et al. The neural basis of loss aversion in decision-making under risk. Science 2007; 315: 515–518. [DOI] [PubMed] [Google Scholar]
- 3.De-Martino B, Kumaran D, Seymour B, et al. Frames, biases, and rational decision- making in the human brain. Science 2006; 313: 684–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De-Martino B, Camereer C, Adolphs R. Amygdala damage eliminates monetary loss aversion. Proc Natl Acad Sci U S A 2010; 107: 3788–3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth ed Washington, DC: APA, 1994. [Google Scholar]
- 6.Engelmann JB, Maciuba B, Vaughan C, et al. Posttraumatic stress disorder increases sensitivity to long term losses among patients with major depressive disorder. PLoS ONE 2013; 8: e78292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paulus MP, Yu AJ. Emotion and decision-making: Affect-driven belief systems in anxiety and depression. Trends Cogn Sci 2012; 16: 476–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smoski MJ, Rittenberg A, Dichter GS. Major depressive disorder is characterized by greater reward network activation to monetary than pleasant image rewards. Psychiatry Res 2011; 194: 263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knutson B, Bhanji JP, Cooney RE, et al. Neural responses to monetary incentives in major depression. Biol Psychiatry 2008; 63: 686–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forbes EE, Christopher May J, Siegle GJ, et al. Reward-related decision-making in pediatric major depressive disorder: An fMRI study. J Child Psychol Psychiatry 2006; 47: 1031–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henriques JB, Davidson RJ. Decreased responsiveness to reward in depression. Emot Cogn 2010; 14: 711–724. [Google Scholar]
- 12.Depue RA, Iacono WG. Neurobehavioral aspects of affective disorders. In: Rosenzweig MR, Porter LW. (eds). Annual Review of Psychology, Palo Alto, CA: Annual Reviews, 1989, pp. 457–492. [DOI] [PubMed] [Google Scholar]
- 13.Lammel S, Lim BK, Malenka RC. Reward and aversion in a heterogeneous midbrain dopamine system. Neuropharmacology 2014; 76: 351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lecrubier Y, Sheehan DV, Weiller E, et al. The Mini International Neuropsychiatric Interview (MINI). A short diagnostic structured interview: Reliability and validity according to the CIDI. Eur Psychiatry 1997; 12: 224–231. [Google Scholar]
- 15.Brooks AM, Pammi VSC, Noussair C, et al. From bad to worse: Striatal coding of the relative value of painful decisions. Front Neurosci 2010; 176: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tversky A and Kahneman D. Loss aversion in riskless choice: A reference-dependent model. Quarterly Journal of Economics 1991; 106: 1039–1061.
- 17.Camerer C. Three cheers—psychological, theoretical, empirical—for loss aversion. J Market Res 2005; XLII: 129–133. [Google Scholar]
- 18.Trepel C, Fox CR, Poldrack RA. Prospect theory on the brain? Toward a cognitive neuroscience of decision under risk. Brain Res Cogn Brain Res 2005; 23: 34–50. [DOI] [PubMed] [Google Scholar]
- 19.Dreher J-C. Sensitivity of the brain to loss aversion during risky gambles. Trends Cogn Sci 2007; 111: 270–272. [DOI] [PubMed] [Google Scholar]
- 20.Smith DV, Huettel SA. Decision neuroscience: Neuroeconomics. Wiley Interdiscip Rev Cogn Sci 2010; 1: 854–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyapuram KP, Pammi VSC. Understanding decision neuroscience - A multidisciplinary perspective and neural substrates. Prog Brain Res 2013; 202: 239–266. [DOI] [PubMed] [Google Scholar]
- 22.Phan KL, Wager TD, Taylor SF, et al. Functional neuroanatomy of emotion: A meta-analysis of emotion activation studies in PET and fMRI. Neuroimage 2002; 16: 331–348. [DOI] [PubMed] [Google Scholar]
- 23.Wager TD, Phan KL, Liberzon I, et al. Valence, gender, and lateralization of functional brain anatomy in emotion: A meta-analysis of findings from neuroimaging. Neuroimage 2003; 19: 513–531. [DOI] [PubMed] [Google Scholar]
- 24.Craig AD. How do you feel? Interoception: The sense of the physiological condition of the body. Nat Rev Neurosci 2002; 3: 655–666. [DOI] [PubMed] [Google Scholar]
- 25.Chua P, Krans M, Toni I, et al. A functional anatomy of anticipatory anxiety. Neuroimage 1999; 9: 563–571. [DOI] [PubMed] [Google Scholar]
- 26.Etkin A, Wager TD. Functional neuroimaging of anxiety: A meta- analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry 2007; 164: 1476–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simmons A, Strigo I, Matthews SC, et al. Anticipation of aversive visual stimuli is associated with increased insula activation in anxiety-prone subjects. Biol Psychiatry 2006; 60: 402–409. [DOI] [PubMed] [Google Scholar]
- 28.Sitaram R, Caria A, Veit R, et al. Volitional control of the anterior insula in criminal psychopaths using real-time fMRI neurofeedback: A pilot study. Front Behav Neurosci 2014; 8: 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruiz S, Buyukturkoglu K, Rana M, et al. Real-time fMRI brain computer interfaces: Self-regulation of single brain regions to networks. Biol Psychol 2014; 95: 4–20. [DOI] [PubMed] [Google Scholar]
- 30.Ruiz S, Birbaumer N, Sitaram R. Abnormal neural connectivity in schizophrenia and fMRI-brain-computer interface as a potential therapeutic approach. Front Psychiatry 2013; 4: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caria A, Sitaram R, Veit R, et al. Volitional control of anterior insula activity modulates the response to aversive stimuli. A real-time fMRI study. Biol Psychiatry 2010; 68: 425–432. [DOI] [PubMed] [Google Scholar]
- 32.Veit R, Singh V, Sitaram R, et al. Using real-time fMRI to learn voluntary regulation of the anterior insula in the presence of threat-related stimuli. Soc Cogn Affect Neurosci 2012; 7: 623–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caria A, Veit R, Sitaram R, et al. Regulation of anterior insular cortex activity using real-time fMRI. Neuroimage 2007; 35: 1238–1246. [DOI] [PubMed] [Google Scholar]