Abstract
Bevacizumab (BEV) is increasingly used to treat recurrent glioblastoma (GBM) with some reported improvement in neurocognitive function despite potential neurotoxicities. We examined the effects of BEV on cerebral blood flow (CBF) within recurrent GBM tumor and in the contralateral middle cerebral artery (MCA) territory.
Post-chemoradiation patients with histologically confirmed GBM were treated with BEV and underwent routine, serial tumor imaging with additional pseudocontinuous arterial spin labeling (pcASL) following informed consent. Circular regions-of-interest were placed on pcASL images directly over the recurrent tumor and in the contralateral MCA territory. CBF changes before and during BEV treatment were evaluated in tumor and normal tissue. Linear mixed models were used to assess statistical significance.
Fifty-three pcASL studies in 18 patients were acquired. Evaluation yielded lower mean tumoral CBF during BEV treatment compared with pre-treatment (45 ± 27 vs. 65 ± 27 ml/100 g/min, p = 0.002), and in the contralateral MCA territory during, compared with pre-BEV treatment (35 ± 8.4 vs. 41 ± 8.4 ml/100 g/min, p = 0.03). The decrease in mean CBF tended to be greater in the tumoral region than in the contralateral MCA, though the difference did not reach statistical significance (31% vs. 13%; p = 0.082).
Conclusions
BEV administration results in statistically significant global CBF decrease with a potentially preferential decrease in tumor perfusion compared with normal brain tissue.
Keywords: glioblastoma, bevacizumab, arterial spin labeling, cerebral blood flow, magnetic resonance imaging
Introduction
Glioblastoma multiforme (GBM), a World Health Organization grade IV tumor with a dismal prognosis,1 is often associated with extensive angiogenesis due to tumor secretion of, and the local effects exerted by vascular endothelial growth factor (VEGF), a major regulator of angiogenesis, and other cytokines.2,3 Over the past two decades, various anti-angiogenic agents have been developed in an attempt to inhibit VEGF, and have shown promise in treating various solid tumors.4–7 Bevacizumab (BEV), a humanized monoclonal antibody, has been increasingly used for the treatment of GBM, alone or in conjunction with other chemotherapeutic agents and radiation therapy, with reports of mild prolongation of life expectancy via the proposed mechanism of VEGF receptor-A antagonism.1,8
BEV has been reportedly associated with improved neurocognitive function (NCF), exemplified by a recent study reporting a majority of patients with recurrent GBM treated with BEV alone demonstrated stable or improved NCF during the first six weeks of therapy and overall improvement in quality of life.9,10 However, BEV administration has also been associated with several potentially severe side-effects in adults including thromboembolic phenomena,11–14 cognitive disturbances, dizziness, nausea, mild confusion and headache,15 and more recently a decrease in quality of life,16 suggesting that the reduction of circulating VEGF may have effects on CBF within both tumor and normal brain. Therefore, we evaluated the effect of BEV therapy on the CBF of both the recurrent GBM itself as well as brain tissue distant to the tumor, specifically in the contralateral gray matter of the MCA territory, using pseudocontinuous arterial spin labeling (pcASL), a non-contrast, quantitative, reproducible17,18 MRI-based CBF technique that has previously been shown to favorably compare with CBF values obtained using dynamic susceptibility contrast perfusion weighted imaging19–21 and Xenon-CT.20,22
Methods
Patient Population and Study Design
This was a single-center, retrospective study approved by the Institutional Review Board, and was Health Insurance Portability and Accountability Act compliant. Informed consent was obtained from all subjects, who were clinical oncology patients seeking treatment for GBM throughout the study period.
Inclusion criteria consisted of the pathological diagnosis of GBM with subsequent tumor recurrence, treatment with BEV and pcASL MRI sequence acquisition performed before and/or during BEV treatment. All subjects underwent standard of care clinical work-up consisting of resection and/or biopsy to establish a pathological diagnosis of GBM. An initial Stupp protocol was followed for all subjects consisting of radiotherapy (XRT) administered in conjunction with temozolamide (TMZ). Additional administered chemotherapy routinely included TMZ and/or lomustine (CCNU), and may have potentially included the following additional agents: verubulin, erlotinib, carboplatin, MEDI-575, carmustine (BCNU), etirinotecan pegol (NKTR-102) prior to, or concurrent with BEV therapy. In some, radiation therapy was performed in conjunction with TMZ. Some subjects also received human corticotropin-releasing factor and/or imatinib mesylate during therapy. All subjects included in this study were diagnosed with recurrent GBM based upon clinical and/or conventional radiological data interpreted by a neuro-oncologist, based upon current RANO criteria.23 Pseudoprogression was not observed in any of the included cases. While specific BEV dosing regimens varied slightly from patient to patient dependent upon specific side-effects and patient tolerance, the typical dosing scheme was comprised of 7.5–10 mg/kg every two to three weeks, considered standard dosing used in current GBM therapy.
Imaging Protocol
The study period lasted from October 2008 to January 2012. Subjects with pathologically proven GBM underwent routine tumor MR imaging with additional pcASL sequence at 1.5T and 3 T. Two outpatient 3 T (Discovery 750; GE Healthcare, Milwaukee, WI, USA) and two 1.5 T (Signa; GE Healthcare, Milwaukee, WI, USA; one inpatient, one outpatient) MR scanners were used. All subjects received standard tumor imaging, which included: a three-plane single-shot FSE T2-weighted localizer, sagittal T1-weighted spin-echo (TR/TE, 500/22 ms), axial diffusion-weighted (TR/TE, 6000/70 ms; b = 0 and 1000 s/mm2), axial gradient-echo (TR/TE, 600/30 ms), T2-weighted FSE (TR/TE, 4717/85 ms), axial fluid-attenuated inversion recovery (TR/TE/TI, 8802/110/2200 ms), and three-plane T1 post-contrast (TR/TE 600/15 ms) images following injection of 0.1 mmol/kg of a gadolinium based contrast agent. All anatomic imaging was performed with 5-mm section thickness, 0–1.5-mm skip, and 24-cm FOV. In a select few patients, gradient-echo bolus dynamic susceptibility contrast (DSC) perfusion weighted images were acquired at the same imaging session, prior to the acquisition of the three-plane T1-weighted post-contrast images, using single-shot echo planar imaging (TR/TE 2000/60 ms) and a single dose (0.1 mmol/kg) of a gadolinium-based contrast agent injected via a power-injector at a rate of 4 mL/s as previously described24. DSC perfusion parameters and parametric maps were obtained using the automated RAPID post-processing toolbox.24,25
pcASL imaging without vessel suppression was serially performed with a 3D background-suppressed fast-spin-echo stack-of-spirals readout module with eight in-plane spiral interleaves, 512 data points per arm, and three NEX, as previously described and quantified with identical DSC parameters.20,24,26–28 A labeling period of 1500 ms and a post-label delay of 2000 ms were employed, with the labeling plane at the level of the foramen magnum, and in-plane and through-plane resolution of 3 and 4 mm, respectively, acquired in under five minutes. CBF quantification (in mL/100 g/min) was performed following M0 map incorporation as previously described, using an automated script.22
ROI Placement
pcASL images were co-registered to three-plane post-contrast T1 weighted and axial T2 FLAIR images in OsiriX,29 with subsequent ROI placement performed without knowledge of BEV administration, patient disposition, treatment status, or additional clinical parameters. Two uniform, 5-mm-diameter circular ROIs, similar to,30 were drawn on pcASL images over definable tumor regions (placed over enhancing tumor in the case of unresectable tumors, and over new enhancing peritumoral regions immediately adjacent to the resection cavity in the case of prior tumor resection), and manually maneuvered over pcASL images to obtain the highest maximal tumoral CBF value. Both maximal and mean CBF values for each ROI were recorded. Two additional, uniform, 5-mm-diameter circular ROIs were then placed in the gray matter of the contralateral MCA territory on an imaging slice 40 mm below the vertex, using the co-registered FLAIR and T1-weighted images as a guide. Mean CBF values for ROIs were then averaged for each location and recorded. Care was taken to avoid placement of ROIs over cortical and large intracranial vessels.
Quantitative Analysis
Continuous variables were summarized as mean ± standard deviation (SD). Linear mixed models (LMMs) were used to estimate the mean CBF before and during BEV treatment and to test CBF changes between the before and during BEV period. Tumoral and contralateral MCA CBF were modeled separately. This method is able to efficiently incorporate the variable number of scans of each subject, even for subjects who only had imaging either before or during BEV (non-paired).31 A random intercept term was included to account for the correlation between repeated measurements. Based on these models, the change in CBF was tested using Wald tests. The non-parametric bootstrap was used to test for differences in mean CBF between the tumoral and contralateral MCA models. Per-patient resampling was performed to retain the dependence between repeated measurements.32
All statistical calculations were conducted with the statistical computing language R (version 2.14.1; R Foundation for Statistical Computing, Vienna, Austria). Throughout, two-tailed tests were used with p<0.05 defined as statistical significant.
Results
Eighteen subjects (mean age: 57.3 ± 12.2 yrs; range: 29 – 80 yrs; F:M = 8:10) met the inclusion criteria and collectively received 53 MRI examinations with pcASL sequence acquisition. pcASL was performed before (n = 15 exams) and during BEV therapy (n = 15 exams) in eight subjects, only before BEV therapy (n = 1 exam) in one subject, and only during BEV therapy (n = 22 exams) in nine subjects. Subject demographic data are presented in Table 1.
Table 1.
Subject demographics.
| Subject | Age (yrs.) at presentation | Gender | Diagnosis | Location | Resection | Chemo (TMZ) | XRT | BEV | Disposition at study conclusion |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 52 | F | GBM | L frontal | STR | Y | Y | Y | Deceased |
| 2 | 63 | M | GBM | L thalamus | N | Y | Y | Y | Deceased |
| 3 | 63 | F | GBM | L frontal/motor cortex | N | Y | Y | Y | Deceased |
| 4 | 29 | M | GBM | frontal | STR | Y | Y | Y | Deceased |
| 5 | 69 | M | GBM | L parieto-occipital | GTR | Y | Y | y | Deceased |
| 6 | 64 | M | GBM | L frontoparietal | GTR | Y | Y | Y | Lost to follow-up outside country |
| 7 | 48 | F | GBM | R frontoparietal | STR | Y | Y | y | Initial response to BEV, subsequent progression |
| 8 | 35 | M | GBM | R occipital | STR | Y | Y | Y | Deceased |
| 9 | 59 | M | GBM | L temporal | GTR | Y | Y | Y | Deceased |
| 10 | 58 | F | GBM | R frontal | STR | Y | Y | Y | Deceased |
| 11 | 54 | M | GBM | L temporal | GTR | Y | Y | Y | Deceased |
| 12 | 46 | M | GBM | L temporal | GTR | Y | Y | Y | Alive, failed BEV, placed on NKTR-102 |
| 13 | 62 | F | GBM | R frontal | N | Y | Y | Y | Alive, Responded to BEV |
| 14 | 55 | F | GBM | L temporal | STR | Y | Y | Y | Deceased |
| 15 | 80 | M | GBM | R frontal | STR | Y | Y | Y | Deceased |
| 16 | 63 | F | GBM | R parietal | STR | Y | Y | Y | Deceased |
| 17 | 67 | M | GBM | R frontal | GTR | Y | Y | Y | Deceased |
| 18 | 65 | F | GBM | L frontal | GTR | Y | Y | Y | Deceased |
| Total | Average: 57.3 | M: 10 (55.6%) | GTR: 7 (38.9%) | 100% | 100% | 100% | |||
| Median: 60.5 | F: 8 (44.4%) | STR: 8 (44.4%) | |||||||
| N: 3 (16.7%) |
Yrs, years; Chemo, chemotherapy; F, female; M, male; STR, sub-total resection; GTR, gross total resection; N, none/biopsy; Y, yes.
An example of acquired subject imaging in this group is provided in Figure 1. Mean CBF before and during BEV administration is summarized in Table 2. Before BEV, mean CBF was higher within the tumoral region than in the contralateral MCA (65 ± 27 vs. 41 ± 8.4 mL/100g/min; p = 0.002). The difference in mean CBF between the tumoral region and contralateral MCA was no longer statistically significant during BEV administration (45 ± 27 vs. 35 ± 8.4 mL/100g/min; p = 0.11).
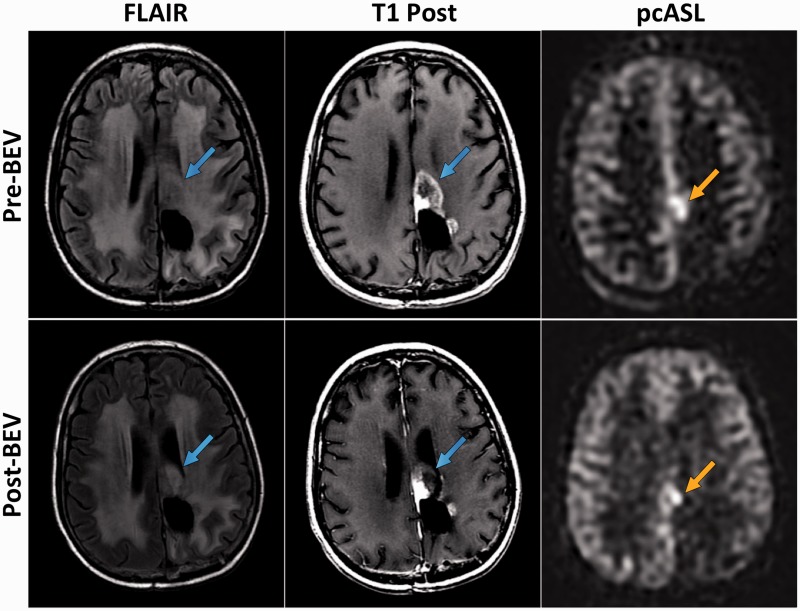
Figure 1.
MRI images of Subject 6 obtained prior to initial BEV treatment (Pre- BEV) and following 2 cycles of BEV therapy (Post-BEV). Mass-like, enhancing recurrent GBM tumor (blue arrows) is identified immediately anterior to the left parietal lobe resection cavity on axial FLAIR and axial T1 post-contrast (T1 Post) images. There is corresponding, focally elevated CBF (orange arrow) identified within the tumor on Pre-BEV pcASL image. The Post-BEV pcASL image reveals interval decrease in size of focally elevated CBF, still with residual elevated tumoral perfusion (orange arrow).
Table 2.
Mean CBF in the tumoral region and contralateral MCA before and during BEV treatment based on linear mixed models (LMM) utilizing all available subjects.
| ROI | Before BEV (N = 16) | During BEV (N = 37) | Mean Change (%*) | P-value† |
|---|---|---|---|---|
| Tumoral | 65 ± 27 | 45 ± 27 | −20 (−31%) | 0.002 |
| Contralateral MCA | 41 ± 8.4 | 35 ± 8.4 | −5.5 (−13%) | 0.030 |
| Difference | 24 | 9.3 | −15 | |
| P-Value‡ | 0.002 | 0.11 | 0.082 |
Values are mean or mean ± SD; all units are mL/100 g/min;
Mean change of CBF expressed as percent of the mean CBF before BEV;
Wald test from LMM comparing CBF before and during BEV;
Bootstrap test comparing CBF or change in CBF in tumoral and contralateral MCA regions.
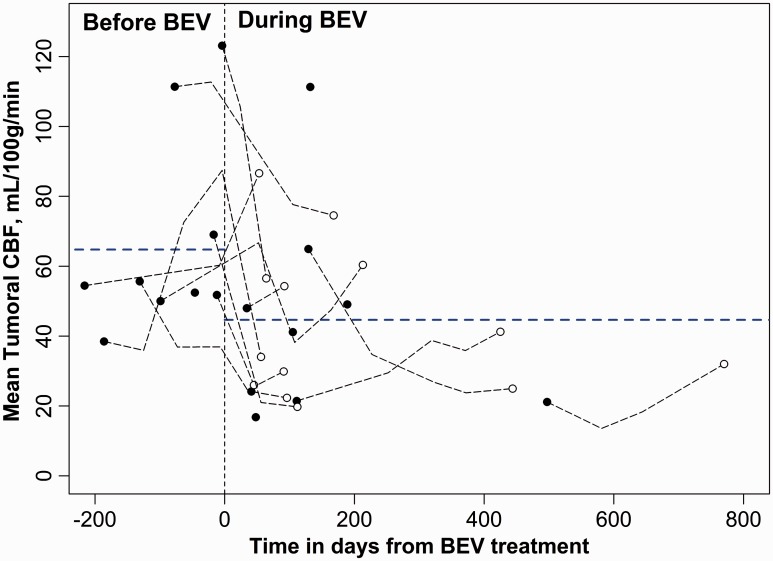
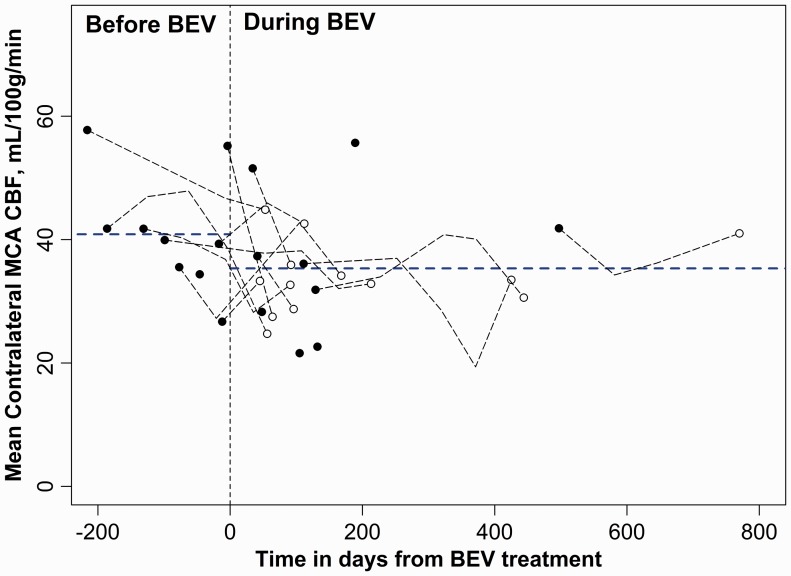
The mean CBF in the tumoral region decreased 31% during BEV treatment compared to the pre-BEV period (p = 0.002). Mean CBF also decreased significantly in the contralateral MCA region during BEV treatment (p = 0.03). The drop in mean CBF tended to be larger in the tumoral region than the contralateral MCA but did not reach statistical significance (31% vs. 13%; p=0.08). Graphical plots of ROI-derived mean CBF values obtained in the GBM tumoral region and in the contralateral MCA territory gray matter relative to time from commencement of BEV treatment for all eighteen subjects are presented in Figures 2 and 3, respectively.
Figure 2.
Graphical plot of ROI-derived mean CBF values obtained in the GBM tumoral region in all eighteen subjects relative to time from commencement of BEV treatment (time = 0). The horizontal dotted blue lines are the mean CBF estimates before and during BEV treatment, derived from the LMM analysis. Individual points with black fill represent the first measured mean CBF values for each subject and points with white fill represent the final mean CBF values obtained for each subject, relative to BEV administration. Black dotted lines connecting the latter two points (those with black fill and those with white fill, respectively) represent the trajectory of mean CBF changes with time. Subjects for which only one MRI scan was obtained are depicted with a single point with black fill. The X-axis depicts time in days from when BEV treatment was begun, where 0 (vertical dashed line) indicates the start time and negative values are relative days before BEV administration.
Figure 3.
Graphical plot of ROI-derived mean CBF values obtained in the MCA territory contralateral to the recurrent GBM tumor in all eighteen subjects relative to time from commencement of BEV treatment (time = 0). The horizontal dotted blue lines are the mean CBF estimates before and during BEV treatment, derived from the LMM analysis. The X-axis depicts time in days from when BEV treatment was begun, where 0 (vertical dashed line) indicates the start time and negative values are relative days before BEV administration.
Discussion
Glioblastoma multiforme (GBM) is the most common and fatal form of primary brain malignancy in adults, and similar to other solid tumors, has a strong angiogenic component which serves both as a key patho-diagnostic marker and a major contributor to its aggressive phenotype. Among the many cytokines mediating angiogenic proliferation, VEGF is a well studied, well-known angiogenic factor and a common target of anti-angiogenic inhibitors, including BEV.8
The tumoricidal component of BEV therapy is thought to affect target tissue through its function as an inhibitor of blood vessel formation and subsequent reduction in tumor perfusion. However, it is unclear how targeted a therapy BEV really is. Despite its use in treating patients with glioma, little is known about the effects of BEV on tumor perfusion, and more importantly, on cerebral perfusion in areas of normal brain. This latter point is relevant to the potential toxicities of BEV that have been reported, such as thromboembolic disease,11–14 lightheadedness, dizziness, and cognitive decline.15,33
Arterial spin labeling (ASL) provides a robust and reliable measure of CBF without the need for an exogenous, intravenous contrast agent, is easily repeatable, and relatively rapid, requiring less than five minutes of clinical scan time. Relative to gadolinium-based dynamic susceptibility contrast perfusion imaging, the pcASL technique is less affected by damage to the blood brain barrier either prior to, or after tumor resection, thus potentially offering improvements in CBF quantification, and in evaluating GBM.34
A review of the literature reveals several recent publications describing generally reduced CBF in the tumoral and peritumoral regions prior to BEV treatment, which subsequently increases to approach the CBF of the contralateral, presumably normal brain following BEV treatment.35,36 This observation has been termed “vascular normalization”.35–38 However, in our study, we observed generally elevated peritumoral CBF prior to initial BEV treatment, which subsequently decreases following treatment with BEV. One explanation for this discrepancy is that, unlike the current study, these prior studies utilized an ASL sequence with crusher gradients meant to suppress ASL signal from large vessels. It is worth noting that other sources have similarly reported decreased intratumoral perfusion upon successful treatment with VEGF-blocking therapies.39–41 Given the generally unknown and probably larger vessel size in GBM,42–47 non-vessel suppressed pcASL enables evaluation of both the macro- and micro-vascular CBF components, and could be argued to represent a more accurate measure of true CBF given that blood in large vessels surrounding the tumor is ultimately destined to perfuse the tumor itself.
The intra-tumoral effects of BEV on CBF have been recently documented using arterial spin labeling in a mouse xenograft tumor model41 with reported initial decrease in tumoral perfusion observed upon BEV administration similar to the findings observed in the current study, purportedly related to alteration in tumor vascularity. However, the effects that BEV may exert on overall CBF have not been evaluated.
This study was limited by a relatively small sample size and the retrospective study design, which in particular led to heterogeneity in frequency and availability of imaging relative to BEV treatment and required the use of complex statistical modeling. Proximal and concurrent chemo- and radiotherapies may have confounded our results, and a prospective study evaluating the initial effects of chemoradiation and other therapies on CBF in the contralateral cerebral hemisphere would be of significant benefit. In addition, CBF may vary substantially due to any number of individual and short-term factors, such as caffeine intake, which were not controlled in this study. Finally, we were not able to correlate NCF with ASL-derived CBF in this study. Given these limitations, the results suggest that BEV preferentially reduces CBF in recurrent GBM tumor, but appears to have a smaller but significant effect on the CBF of normal brain tissue.
Conclusion
Our results suggest that a statistically significant reduction in CBF is observed both within the recurrent GBM tumor and within the unaffected, presumably normal cerebral hemisphere during treatment with BEV. In addition, the magnitude of the decrease in the latter appeared to be smaller than in the recurrent tumor during the same time period, although this difference approached, but did not reach, statistical significance, suggesting that BEV may preferentially reduce CBF in GBM relative to normal brain. The effects of BEV on normal brain may account for some of its reported toxicities. Larger, prospective studies are needed to better assess how precisely targeted are the therapeutic effects of BEV.
Acknowledgements
This work was performed at, and in collaboration with the Radiology Department at Stanford University Medical Center. The authors are grateful to Reena Thomas, MD, and Abdullah Feroze, MD for assistance with the patient data collection.
Funding
Sources of support NIH (5R01EB002711, 5R01EB008706, 5R01EB006526, 5R21EB006860, 5R01NS047607, 2P41RR009784), Lucas Foundation, Oak Foundation.
Presentation
This research was presented in part as an oral presentation at the American Society of Neuroradiology 51st Annual Meeting, San Diego, CA, May, 2013.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas: a clinical review. JAMA. 2013; 310(17): 1842–1850. doi: 10.1001/jama.2013.280319. [DOI] [PubMed] [Google Scholar]
- 2.Lopes MB. Angiogenesis in brain tumors. Microsc Res Tech 2003; 60(2): 225–230. doi: 10.1002/jemt.10260. [DOI] [PubMed] [Google Scholar]
- 3.Reiss Y, Machein MR, Plate KH. The role of angiopoietins during angiogenesis in gliomas. Brain Pathol 2005; 15(4): 311–317. doi: 10.1111/j.1750–3639.2005.tb00116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kieran MW, Kalluri R, Cho YJ. The VEGF pathway in cancer and disease: responses, resistance, and the path forward. Cold Spring Harb Perspect Med 2012; 2(12): a006593 doi: 10.1101/cshperspect.a006593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kowanetz M, Ferrara N. Vascular endothelial growth factor signaling pathways: therapeutic perspective. Clin Cancer Res 2006; 12(17): 5018–5022. doi: 10.1158/1078–0432.CCR–06–1520. [DOI] [PubMed] [Google Scholar]
- 6.Ho QT, Kuo CJ. Vascular endothelial growth factor: biology and therapeutic applications. Int J Biochem Cell Biol 2007; 39(7–8): 1349–1357. doi: 10.1016/j.biocel.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Los M, Roodhart JM, Voest EE. Target practice: lessons from phase III trials with bevacizumab and vatalanib in the treatment of advanced colorectal cancer. Oncologist 2007; 12(4): 443–450. doi: 10.1634/theoncologist.12–4–443. [DOI] [PubMed] [Google Scholar]
- 8.Chinot OL. Bevacizumab-based therapy in relapsed glioblastoma: rationale and clinical experience to date. Expert Rev Anticancer Ther 2012; 12(11): 1413–1427. doi: 10.1586/era.12.128. [DOI] [PubMed] [Google Scholar]
- 9.Wefel JS, Cloughesy T, Zazzali JL, et al. Neurocognitive function in patients with recurrent glioblastoma treated with bevacizumab. Neuro Oncol 2011; 13(6): 660–668. doi: 10.1093/neuonc/nor024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henriksson R, Asklund T, Poulsen HS. Impact of therapy on quality of life, neurocognitive function and their correlates in glioblastoma multiforme: a review. J Neurooncol 2011; 104(3): 639–646. doi: 10.1007/s11060–011–0565–x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kindler HL, Karrison TG, Gandara DR, et al. Multicenter, double-blind, placebo-controlled, randomized phase II trial of gemcitabine/cisplatin plus bevacizumab or placebo in patients with malignant mesothelioma. J Clin Oncol 2012; 30(20): 2509–2515. doi: 10.1200/JCO.2011.41.5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009; 27(5): 740–745. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armstrong TS, Wen PY, Gilbert MR, et al. Management of treatment-associated toxicites of anti-angiogenic therapy in patients with brain tumors. Neuro Oncol 2012; 14(10): – 1203–1214. doi: 10.1093/neuonc/nor223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gressett SM, Shah SR. Intricacies of bevacizumab-induced toxicities and their management. Ann Pharmacother 2009; 43(3): 490–501. doi: 10.1345/aph.1L426. [DOI] [PubMed] [Google Scholar]
- 15.Cabrera AR, Cuneo KC, Desjardins A, et al. Concurrent stereotactic radiosurgery and bevacizumab in recurrent malignant gliomas: a prospective trial. Int J Radiat Oncol Biol Phys 2013; 86(5): 873–879. doi: 10.1016/j.ijrobp.2013.04.029. [DOI] [PubMed] [Google Scholar]
- 16.Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med 2014; 370(8): 699–708. doi: 10.1056/NEJMoa1308573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sousa I, Vilela P, Figueiredo P. Reproducibility of the quantification of arterial and tissue contributions in multiple postlabeling delay arterial spin labeling. J Magn Reson Imaging 2014; 40(6): 1453–1462. doi: 10.1002/jmri.24493. [DOI] [PubMed] [Google Scholar]
- 18.Wu B, Lou X, Wu X, et al. Intra- and interscanner reliability and reproducibility of 3D whole-brain pseudo-continuous arterial spin-labeling MR perfusion at 3T. J Magn Reson Imaging 2014; 39(2): 402–409. doi: 10.1002/jmri.24175. [DOI] [PubMed] [Google Scholar]
- 19.Lindgren E, Wirestam R, Markenroth Bloch K, et al. Absolute quantification of perfusion by dynamic susceptibility contrast MRI using Bookend and VASO steady-state CBV calibration: a comparison with pseudo-continuous ASL. MAGMA 2014; 27(6): 487–499. doi: 10.1007/s10334–014–0431–x. [DOI] [PubMed] [Google Scholar]
- 20.Zaharchuk G, Straka M, Marks MP, et al. Combined arterial spin label and dynamic susceptibility contrast measurement of cerebral blood flow. Magn Reson Med 2010; 63(6): 1548–1556. doi: 10.1002/mrm.22329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mirasol RV, Bokkers RP, Hernandez DA, et al. Assessing reperfusion with whole-brain arterial spin labeling: a noninvasive alternative to gadolinium. Stroke 2014; 45(2): 456–461. doi: 10.1161/STROKEAHA.113.004001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qiu D, Straka M, Zun Z, et al. CBF measurements using multidelay pseudocontinuous and velocity-selective arterial spin labeling in patients with long arterial transit delays: comparison with xenon CT CBF. J Magn Reson Imaging 2012; 36(1): 110–119. doi: 10.1002/jmri.23613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010; 28(11): 1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 24.Zaharchuk G, El Mogy IS, Fischbein NJ, et al. Comparison of arterial spin labeling and bolus perfusion-weighted imaging for detecting mismatch in acute stroke. Stroke 2012; 43(7): 1843–1848. doi: 10.1161/STROKEAHA.111.639773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Straka M, Albers GW, Bammer R. Real-time diffusion-perfusion mismatch analysis in acute stroke. J Magn Reson Imaging 2010; 32(5): 1024–1037. doi: 10.1002/jmri.22338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zaharchuk G, Olivot JM, Fischbein NJ, et al. Arterial spin labeling imaging findings in transient ischemic attack patients: comparison with diffusion- and bolus perfusion-weighted imaging. Cerebrovasc Dis 2012; 34(3): 221–228. doi: 10.1159/000339682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iv M, Fischbein NJ, Zaharchuk G. Association of Developmental Venous Anomalies with Perfusion Abnormalities on Arterial Spin Labeling and Bolus Perfusion-Weighted Imaging. J Neuroimaging. 2014. doi: 10.1111111/jon.12119. [DOI] [PubMed]
- 28.Zaharchuk G, Bammer R, Straka M, et al. Arterial spin-label imaging in patients with normal bolus perfusion-weighted MR imaging findings: pilot identification of the borderzone sign. Radiology 2009; 252(3): 797–807. doi: 10.1148/radiol.2523082018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosset A, Spadola L, Ratib O, Osiri X. an open-source software for navigating in multidimensional DICOM images. J Digit Imaging. 2004; 17(3): 205–216. doi: 10.1007/s10278–004–1014–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andre JB, Lu S, Spearman K, et al. Peritumoral apparent diffusion coefficient as a metric of response in patients with recurrent glioblastoma multiforme treated with bevacizumab and irinotecan. Neuroradiol J 2008; 21(3): 350–361. [DOI] [PubMed] [Google Scholar]
- 31.Pinheiro JC, Bates DM. Mixed-effects models in S and S-PLUS, New York: Springer, 2000. doi: 10.1007/978–1–4419–0318–1. [Google Scholar]
- 32.Davison AC, Hinkley DV. Bootstrap methods and their application. Cambridge, New York, NY, USA: Cambridge University Press, 1997. doi: 10.1017/CBO9780511802843. [Google Scholar]
- 33.Lai A, Filka E, McGibbon B, et al. Phase II pilot study of bevacizumab in combination with temozolomide and regional radiation therapy for up-front treatment of patients with newly diagnosed glioblastoma multiforme: interim analysis of safety and tolerability. Int J Radiat Oncol Biol Phys. 2008; 71(5): 1372–1380. doi: 10.1016/j.ijrobp.2007.11.068. [DOI] [PubMed] [Google Scholar]
- 34.White CM, Pope WB, Zaw T, et al. Regional and Voxel-Wise Comparisons of Blood Flow Measurements Between Dynamic Susceptibility Contrast Magnetic Resonance Imaging (DSC-MRI) and Arterial Spin Labeling (ASL) in Brain Tumors. J Neuroimaging. 2014; 24(1): 23–30. doi: 10.1111/j.1552–6569.2012.00703.x. [DOI] [PubMed] [Google Scholar]
- 35.Sorensen AG, Emblem KE, Polaskova P, et al. Increased survival of glioblastoma patients who respond to antiangiogenic therapy with elevated blood perfusion. Cancer Res 2012; 72(2): 402–407. doi: 10.1158/0008–5472.CAN–11–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jain RK. Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. J Clin Oncol 2013; 31(17): 2205–2218. doi: 10.1200/JCO.2012.46.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sorensen AG, Batchelor TT, Zhang WT, et al. A “vascular normalization index” as potential mechanistic biomarker to predict survival after a single dose of cediranib in recurrent glioblastoma patients. Cancer Res 2009; 69(13): 5296–5300. doi: 10.1158/0008–5472.CAN–09–0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med 2001; 7(9): 987–989. doi: 10.1038/nm0901–987. [DOI] [PubMed] [Google Scholar]
- 39.Thomsen H, Steffensen E, Larsson EM. Perfusion MRI (dynamic susceptibility contrast imaging) with different measurement approaches for the evaluation of blood flow and blood volume in human gliomas. Acta Radiol 2012; 53(1): 95–101. doi: 10.1258/ar.2011.110242. [DOI] [PubMed] [Google Scholar]
- 40.Wong ET, Brem S. Antiangiogenesis treatment for glioblastoma multiforme: challenges and opportunities. J Natl Compr Canc Netw. 2008; 6(5): 515–522. [DOI] [PubMed] [Google Scholar]
- 41.Rajendran R, Huang W, Tang AM, et al. Early detection of antiangiogenic treatment responses in a mouse xenograft tumor model using quantitative perfusion MRI. Cancer Med 2014; 3(1): 47–60. doi: 10.1002/cam4.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nagy JA, Chang SH, Shih SC, et al. Heterogeneity of the tumor vasculature. Semin Thromb Hemost 2010; 36(3): 321–331. doi: 10.1055/s–0030–1253454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nagy JA, Dvorak AM, Dvorak HF. VEGF-A and the induction of pathological angiogenesis. Annu Rev Pathol 2007; 2: 251–275. doi: 10.1146/annurev.pathol.2.010506.134925. [DOI] [PubMed] [Google Scholar]
- 44.Nagy JA, Vasile E, Feng D, et al. VEGF-A induces angiogenesis, arteriogenesis, lymphangiogenesis, and vascular malformations. Cold Spring Harb Symp Quant Biol 2002; 67(–): 227–237. doi: 10.1101/sqb.2002.67.227. [DOI] [PubMed] [Google Scholar]
- 45.Pettersson A, Nagy JA, Brown LF, et al. Heterogeneity of the angiogenic response induced in different normal adult tissues by vascular permeability factor/vascular endothelial growth factor. Lab Invest 2000; 80(1): 99–115. doi: 10.1038/labinvest.3780013. [DOI] [PubMed] [Google Scholar]
- 46.Nagy JA, Feng D, Vasile E, et al. Permeability properties of tumor surrogate blood vessels induced by VEGF-A. Lab Invest 2006; 86(8): 767–780. [DOI] [PubMed] [Google Scholar]
- 47.Dvorak HF. Rous-Whipple Award Lecture. How tumors make bad blood vessels and stroma. Am J Pathol. Jun 2003; 162(6): 1747–1757. doi: 10.1016/S0002–9440(10)64309–X. [DOI] [PMC free article] [PubMed] [Google Scholar]