Abstract
The lack of safe and reliable methods to sample vascular tissue in situ limits discovery of the underlying genetic and pathophysiological mechanisms of many vascular disorders, including aneurysms. We investigated the feasibility and comparable efficacy of in vivo vascular endothelial cell sampling using a spectrum of endovascular devices.
Using the rabbit elastase carotid aneurysm model we evaluated the performance of existing aneurysmal coils, intracranial stents, and stent-like devices to collect vascular endothelial cells. Additionally, we modified a subset of devices to assess the effects of alterations to coil pitch, coil wire contour, and stent surface finishing. Device performance was evaluated by (1) the number of viable endothelial cells harvested, (2) the degree of vascular wall damage analyzed using digital subtraction angiography and histopathological analysis, and (3) the ease of device navigability and retrieval. Isolated cells underwent immunohistochemical analysis to confirm cell type and viability. Coil and stent specifications, technique, and endothelial cell counts were tabulated and statistical analysis performed.
Using conventional detachable-type and modified aneurysm coils 11 of 14 (78.6%) harvested endothelial cells with a mean of 7.93 (±8.33) cells/coil, while 15 of 15 (100%) conventional stents, stent-like devices and modified stents harvested endothelial cells with a mean of 831.33 (±887.73) cells/device. Coil stiffness was significantly associated with endothelial cell count in univariate analysis (p = 0.044). For stents and stent-like devices univariate analysis demonstrated stent-to-aorta diameter ratios (p = 0.001), stent length (p = 0.049), and the use of a pulling retrieval technique (p = 0.019) significantly predictive of endothelial cell counts, though a multivariate model using these variables demonstrated only the stent-to-aorta diameter ratio (p = 0.029) predictive of endothelial cell counts. Modified devices did not significantly impact harvesting.
The efficacy and safety of existing aneurysm coils, intracranial stents and stent-like devices in collecting viable endothelial cells was confirmed. The technique is reproducible and the quantity and quality of collected endothelial cells is adequate for targeted genetic analysis.
Keywords: Stroke, endothelial cell, aneurysm, coil, stent
Introduction
Stroke related to intracranial aneurysmal rupture affects in excess of 30,000 patients per year in the United States, while a larger percentage of the population (1--6%) harbor clinically silent aneurysms.1–3 There are studies detailing familial, epidemiological, and imaging (aneurysm size, location, and morphology) risk factors related to aneurysm growth and rupture, though there are no true molecular prognosticators.4–6 Tissue analysis of aneurysm domes harvested during open surgical treatment has shed light on the differential gene expression of inflammatory and reparative pathways.7 These studies are, however, limited in number due to the larger aneurysms needed to provide adequate tissue, as well as the risks related to removing aneurysm domes.8 There are a multitude of animal studies looking at aneurysm molecular pathogenesis, though these experimental aneurysms do not routinely progress to rupture and are limited to saccular, arterial bifurcation-type aneurysm morphologies.7,9–18 Thus, we still lack reliable molecular markers to assess aneurysm growth and rupture risk. Given the approximately 5--10% morbidity and mortality associated with treatment of non-ruptured aneurysms using open or endovascular techniques, surgeons must weigh these elements carefully when deciding whether or not to treat, particularly for aneurysms less than 7.0 mm in diameter.
A major obstacle to solving this problem is the lack of a safe and reliable means to sample vascular tissue in vivo. Portions of vessels within and surrounding the brain can be collected during open operative intervention, (e.g., aneurysm dome or AVM nidus) and undergo genetic analysis. Tissue samples such as these, however, are collected in the setting of treatment, providing only a single molecular snapshot of the disease process. Furthermore, the removal of non-pathological intracranial pial arteries as “normal” control material is usually contraindicated given the risk of causing stroke. As such, dural or extracranial arteries, such as the superficial temporal artery, are typically used as control samples, though the cellular architecture of such vessels is fundamentally different from the CNS vasculature.7–9 A method to collect cells from similar sites of pathology over multiple time points without changing their natural history would, however, provide vital longitudinal cellular and molecular information to correlate with clinical and radiographic parameters.
Feng et al. described a technique for harvesting endothelial cells using a standard guidewire whereby in the expected usage of the wire, its non-specific contact with the vessel wall allows non-circulating endothelial cells to bind to the wire.8 We replicated this technique using embolic coils in the peripheral vasculature of pigs.19 As expected, secondary coil diameter proved a significant predicator of endothelial cell yield with larger coils producing higher endothelial cell counts. For every 1 mm increase in coil diameter, we estimated a 53% increase in mean endothelial cell count (95% CI: 6--123%) with the largest diameter coils generating endothelial cell yields (225.1 ± 123.4) comparable to values published by Feng et al. (262 ± 45 cells per wire).20 This may stem from increased force exerted by the coil - oversized in relation to the vessel lumen diameter - as it attempts to assume its defined diameter within a confined space.
The purpose of this study was to further characterize the ability of neurointerventional devices to collect vascular endothelial cells. We assessed the efficacy of commercially available embolic coils, intracranial stents and stent-like devices as compared to custom-modified devices in collecting viable endothelial cells using a rabbit aneurysm model. Novel devices were made by altering their surfaces by either leaving their surfaces unpolished (stent-based devices) or using flat instead of round wire (coil-based devices). Device performance was determined by: (1) the number of viable endothelial cells harvested,2 the degree of vascular wall damage analyzed using digital subtraction angiography and histopathological analysis, and3 the ease of device navigability and retrieval. We hypothesized that custom surface-modified (flat wire, micro-abrasive) devices would gather larger numbers of cells than their non-altered counterparts.
Materials and Methods
Animal preparation
All animal care procedures were in accordance with the Guide for the Care and Use of Laboratory Animals and were conducted within an AAALAC accredited facility. All protocols were approved by the IACUC at the University of California San Francisco. Animals were divided into three groups: controls (n = 7) where aneurysm surgeries performed, though no sampling undertaken, coils (n = 14) where animals underwent aneurysm cell sampling using conventional and modified coils, and stent/stent-like device (n = 15), where animals underwent aortic cell sampling using conventional and modified stent/stent-like devices.
The rabbit aneurysm model was created by means of unilateral occlusion of the right common carotid artery and intraluminal elastase injection that induces degeneration of the elastic laminae as described by Kallmes et al.21 Thirty-six male New Zealand rabbits were brought into the facility a minimum of 72 hours prior to procedure to acclimate to housing and feeding. Anesthesia was induced by intramuscular injection of buprenorphine (0.03 mg/kg) followed approximately 30 min later by a ketamine (25--35 mg/kg) and xylazine (3 mg/kg) mixture. Anesthesia was then maintained with isoflurane in oxygen as needed, delivered via endotracheal tube. The neck region of each animal was shaved and prepped and draped in sterile fashion. The right carotid artery was surgically exposed and accessed via a cut down. Heparin was administered (100 iu/kg) prior to the advancement of the sheath. The vessel was isolated using silk suture and a 5 F sheath was placed and secured into the artery. The anatomy was assessed via contrast media injection prior to continuing with the procedure. A 3 F Fogarty balloon was advanced into the right brachiocephalic artery, inflated, and pulled back to occlude the ostia of carotid artery. Fifty units of porcine type I pancreatic elastase (Sigma Chemical, St Louis, MO, USA) were infused into the lumen of the artery above the balloon and left in place for 30 minutes. Following the 30 min time-point the remaining elastase was withdrawn from the arterial stump, the balloon was deflated and the catheter system was removed. The vessel was then ligated, and a local block was placed following closure of the subcutaneous tissues with absorbable suture. The skin was closed with absorbable suture.
Animals were placed on oral ASA and Plavix daily for the duration of the study. After three weeks the animals were brought back to the angiography lab and anesthetized as previously described. The femoral area was shaved, prepped and draped in sterile fashion. The superficial femoral artery was surgically exposed and accessed via cut down. A 4 F sheath was placed into the femoral artery. Heparin was administered prior to the advancement of the guide-wire and devices. Over a 0.035 in guidewire (J-wire; Cook, Bloomington, IN, USA) and under fluoroscopic guidance, a 4 F UCSF3 catheter (Cordis Inc., Miami Lakes, FL, USA) was advanced into the aortic arch. Diagnostic angiography was performed of the aneurysm, contralateral carotid artery, and aortic arch. This catheter was removed. A PX Slim microcatheter (Penumbra Inc., Alameda, CA, USA) was advanced over a 0.014 in. Transcend (Stryker Inc., Fremont CA, USA) microwire into the target vessel (aneurysm for coils; aortic arch for stents and stent-like devices). The device was then deployed into the target and left in position for 30 seconds. The device was then recaptured using standard neurointerventional practice and the microcatheter removed. The device was pushed out of the microcatheter, cut, and placed into dissociation buffer. For a subset of stents a pulling technique was used to replicate the practice employed during stent-based embolectomy retrieval during acute stroke intervention. In these cases, the stent was not recaptured with the microcatheter and instead pulled through the aorta and femoral vasculature.
Following the harvesting procedures, animals were euthanized by intravenous injection of saturated KCl while still at a surgical anesthetic plane. At postmortem, the aortic arch, carotid and brachiocephalic arteries were excised and fixed with 10% formalin. These blood vessels were stained with hematoxylin/eosin and Masson trichrome stains for characterizing vascular changes and scar tissue, respectively.
Devices
Coils, stents and stent-like devices are listed along with their specifications in Tables 1 and 3. To better understand which design elements improve endovascular cell collection while maintaining safety and ease of use we altered existing coils and stents. The engineers at Penumbra Inc. (Alameda, CA, USA) modified a traditional coil design increasing the pitch between coil winds from 10 um to 50 um (approximately .0004 inches to .002 inches). These coils were also constructed using rectangular “flat” wire instead of traditional round wire for the outer wound wire. The engineers at Penumbra Inc. also modified the Liberty stent. They modified the device by not polishing the stent, leaving a micro-abraded surface instead (the commercial device has a smooth electro-polished finish). The strut dimensions of the polished and unpolished stents were similar to remove any dimensional bias between the two devices.
Cell isolation and quantification
After removal from the animal, a device was cut from its delivery wire and put in a tube that contained 10 ml of endothelial cell dissociation buffer (Gibco, Grand Island, NY, USA). After vortex agitation of about ten seconds, the device was discarded and the dissociation buffer with dislodged cells centrifuged at 1500 RPM for ten minutes at 4°C. The cell pellet was re-suspended in 1 ml ACK Lysing Buffer (Gibco, Grand Island, NY, USA) for five minutes and then rinsed with 4 mL PBS. Then the nucleated cells were re-suspended in PBS and counted with hemocytometer. The cells then were seeded into Chamber Slide (BD Bioscience, San Jose, CA, USA) which was coated with Poly-L-Lysine (Sigma, St. Louis, MO, USA) in advance. After immunofluorescent staining of CD31 and DAPI, endothelial cells were identified by morphology and positive CD31 signal on cell surface, and counted under immunofluorescence microscopy (Lecia Microsystems Inc, Chicago, IL, USA).
Histomorphology and immunohistochemistry
Tissue segments of aneurysm, aortic arch and left carotid artery were cut and embedded in paraffin, then sliced by 5 µm and stained with standard hematoxylin and eosin and Masson's trichrome procedure. Morphology were scrutinized and images were taken with a Leica MZFL III microscope (Leica Microsystems Inc, Chicago, IL, USA) with Spot Insight Software (Diagnostic Instruments, Inc., Sterling Heights, MI, USA).
Dislodged cells in chamber slides were fixed with 2% PFA for 10 min, washed with PBS and blocked by 2.5% goat serum for one hour. The cells were incubated with primary mouse anti-rabbit CD31 (Abcam, Cambridge, MA, USA) antibody diluted in PBS with 2.5% goat serum overnight at 4°C. Then the cells were incubated in Alexa Fluor 488-conjugated goat anti-mouse IgG (Molecular probes, Eugene, OR, USA) at 1:400 dilution for one hour at room temperature. The slides were mounted by Vectorshield Mounting Medium with DAPI (Vector Laboratories, Burlingame, CA, USA) and imaged under a fluorescence microscope.
Statistical analysis
Coil and stent specifications, technique, and endothelial cell counts were tabulated in an Excel spreadsheet (Microsoft Inc., Redman, WA, USA). Descriptive statistics were completed within Excel. Inferential statistical analyses were performed using Stata/SE 13.1.22 Prior to statistical analysis, cell counts were transformed using the formula, ln(x+1) to reduce the skewness of the data. We added one to the cell count prior to log transforming to accommodate observations with no cell count. We preformed univariate and multivariate linear regression with log-transformed cell counts as the outcome for both coil and stent datasets. For the coil data, we tested for the effect of secondary coil diameter (relative to aneurysm diameter), coil type (P400 vs. GDC), coil modification, and coil stiffness. For the stent data, we tested for the effect of stent diameter (relative to aorta diameter), stent length, polishing, and stent retrieval technique. We considered p-values less than 0.05 to be significant.
Results
Coils
Using conventional detachable-type and modified aneurysm coils, we were able to successfully harvest viable endothelial cells from carotid-ligation aneurysms in vivo (Table 1). Eleven of 14 (78.6%) coils harvested endothelial cells with a mean of 7.93 (±8.33) cells/coil. The operators noted no difference in coil delivery and there was no evidence of gross vascular injury as a result of placement or retrieval by angiography (Figure 1) or histopathology (Figure 2A,B,E,F). Coil stiffness was significantly associated with endothelial cell count in univariate analysis (Table 2, p = 0.044). Other tabulated variables, including coil type, coil size (secondary diameter or length), aneurysm size (diameter or length), and coil-to-aneurysm dimension ratios did not, however, demonstrate a significant association with endothelial cell numbers. There was a weak trend (p = 0.144) noted for the P400 coil type collecting more endothelial cells than the GDC coil type.
Table 1.
Coils.
| Type | Secondary diameter (mm) | Length (mm) | Modified | K | Aneurysm Ht | Aneurysm Dm | Cell number |
|---|---|---|---|---|---|---|---|
| GDC | 3 | 60 | No | Soft | 8.5 | 1.5 | 1 |
| GDC | 3 | 60 | No | Soft | 7.2 | 2.2 | 0 |
| GDC | 2 | 20 | No | Soft | 2.6 | 2.3 | 2 |
| P400 | 5 | 80 | No | STD | 8 | 3 | 19 |
| P400 | 4 | 80 | No | STD | 7.4 | 2.7 | 23 |
| P400 | 2 | 20 | No | Soft | 5.9 | 2.1 | 36 |
| P400 | 2 | 20 | No | Soft | 3 | 2.3 | 2 |
| P400 | 4 | 50 | Yes | Soft | 2.1 | 1.5 | 1 |
| P400 | 2 | 30 | Yes | Soft | 1.6 | 1.2 | 9 |
| P400 | 3 | 40 | Yes | Soft | 3.8 | 2.2 | 5 |
| P400 | 5 | 60 | Yes | Soft | 12.4 | 3.9 | 11 |
| P400 | 4 | 40 | Yes | Soft | 6.5 | 3.5 | 2 |
| P400 | 3 | 40 | Yes | Soft | 4.2 | 2.9 | 0 |
| P400 | 2 | 30 | Yes | Soft | 3.6 | 2.6 | 0 |
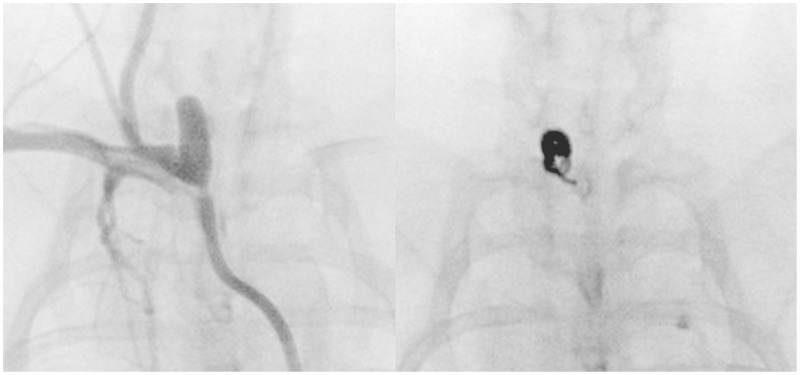
Figure 1.
Coil deployment. AP fluoroscopic images taken of a rabbit carotid-ligation aneurysm (A) and of a coil (5 mm × 80 mm, P400) within the aneurysm (B). The coil yielded 19 endothelial cells.
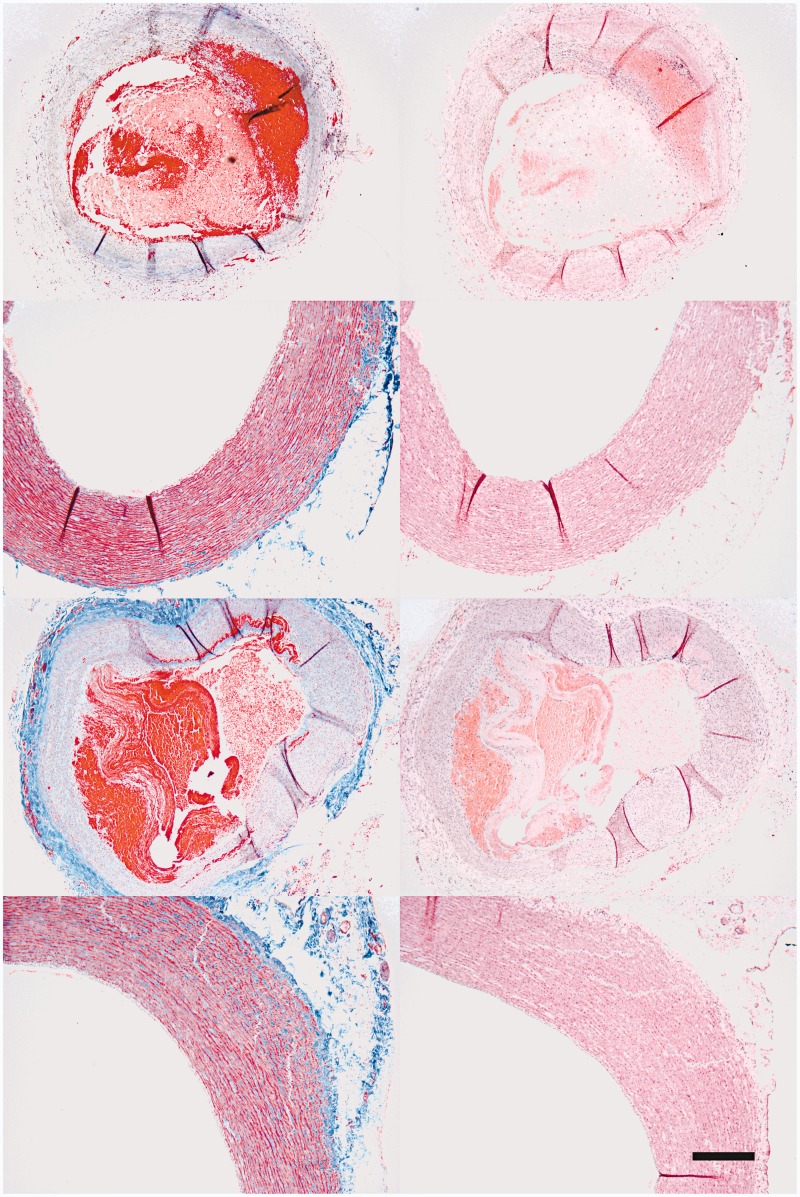
Figure 2.
Morphology of aneurysm and aortic arch were checked by H&E (B,D,F,H) and Masson Trichrome (A,C,E,G). No morphological evidence of injury was found in sampled aneurysm (A,B) and sampled aorta (C,D), compared with control aneurysm (E,F) and control aorta (G,H). Scale bar: 500 µm.
Table 2.
Statistical analysis: coils.
| Outcome: Cell Count (log transformed) | ||||
|---|---|---|---|---|
| Univariate | Predictor | PI† | 95% CI | p-value |
| Coil dm:10*Aneurysm dm | 1.01‡ | (0.87, 1.17) | 0.918 | |
| P400 | 3.17 | (0.60, 16.82) | 0.158 | |
| STD Coil | 6.34 | (1.06, 38.02) | 0.044 | |
| Modified | 0.54 | (0.13, 2.29) | 0.371 | |
PI is the proportional increase in cells for each unit increase in the predictor ‡Results interpreted as proportional change in cell count for each increase in coil diameter equal to 1/10th the aneurysm diameter.
Stents and stent-like devices
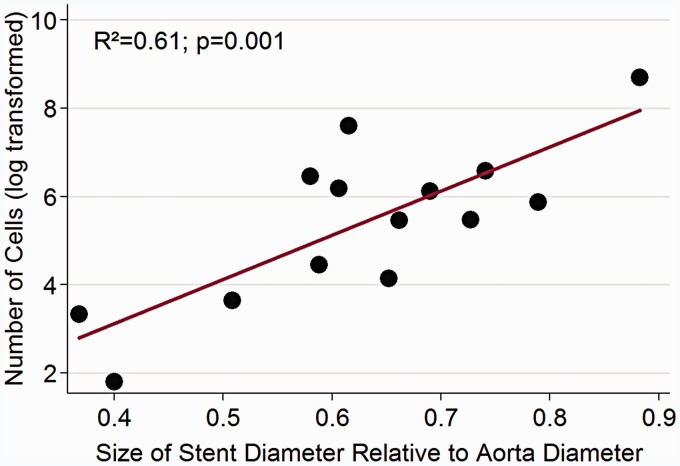
Using conventional stents, stent-like devices and modified stents, we were able to successfully harvest viable endothelial cells from rabbit aortas in vivo (Table 3). All devices harvested endothelial cells with a mean of 831.33 ( ± 887.73) cells/device. The operators noted no difference in stent delivery and there was no evidence of gross vascular injury as a result of placement or retrieval by angiography (Figure 3) or histopathology (Figure 2C,D,G,H). Univariate analysis demonstrated stent-to-aorta diameter ratios, stent length, and the use of a pulling retrieval technique significantly predictive of endothelial cell counts (Table 4). A multivariate model using these variables demonstrated only the stent-to-aorta diameter ratio predicative of endothelial cell count (Figure 4).
Table 3.
Stents.
| Type | Diameter (mm) | Length (mm) | Polished | Technique | Aorta Dm | Cell number |
|---|---|---|---|---|---|---|
| Merci V firm | 3 | 10 | No | No pull | 7.5 | 5 |
| Enterprise | 2.5 | 28 | Yes | No pull | 6.8 | 27 |
| Solitaire | 4 | 20 | Yes | No pull | 6.8 | 85 |
| Trevo PR | 4 | 20 | Yes | No pull | 6.5 | 2000 |
| Trevo PR | 4 | 20 | Yes | No pull | 5.5 | 236 |
| 3XS | 4.5 | 26 | No | No pull | 6.8 | 233 |
| 3XS | 4.5 | 26 | No | No pull | 6.9 | 62 |
| Liberty | 3 | 25 | Yes | No pull | 5.9 | 37 |
| Liberty | 4.5 | 45 | Yes | Pull | 2.91 | 1152 |
| Liberty | 4.5 | 45 | Yes | Pull | 5.1 | 6000 |
| Liberty | 4.5 | 45 | Yes | Pull | 5.7 | 351 |
| Liberty | 4 | 25 | No | Pull | 5.4 | 715 |
| Liberty | 4 | 25 | No | Pull | 5.8 | 453 |
| Liberty | 4 | 25 | No | Pull | 6.6 | 482 |
| Liberty | 4 | 25 | No | Pull | 6.9 | 632 |
1 – This animal was in clinical extremus prior to and during the cell harvesting procedure and as such there was generalized vasoconstriction. This data point was excluded from statistical analysis as detailed in Figure 5.
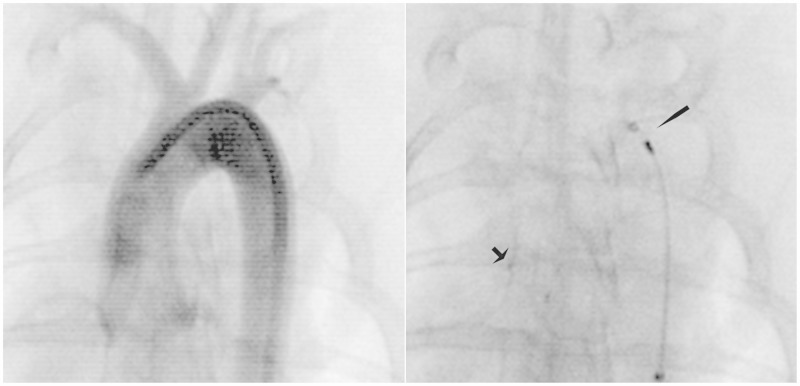
Figure 3.
Stent deployment. AP fluoroscopic images taken of a rabbit aorta (A) and of a stent (Trevo PR) within the aorta (B). The arrow (B) denotes the distal device markers and the arrowhead, the proximal markers and distal end of the microcatheter. This device was deployed and re-captured in position without use of a pulling technique. The device yielded 2000 endothelial cells.
Table 4.
Statistical analysis: stents.
| Outcome: Cell Count (log transformed) | ||||
|---|---|---|---|---|
| Univariate | Predictor | PI† | 95% CI | p-value |
| Stent dm:10*Aorta dm | 2.73‡ | (1.65, 4.51) | 0.001 | |
| Stent length (mm) | 1.11 | (1.00, 1.23) | 0.049 | |
| Technique | 2.16 | (0.42, 3.91) | 0.019 | |
| Polished | 1.40 | (0.16, 12.36) | 0.744 | |
| Multivariate | ||||
| Stent dm:10*Aorta dm | 2.32 | (1.11, 4.81) | 0.029 | |
| Stent length (mm) | 0.96 | (0.84, 1.10) | 0.519 | |
| Technique | 4.68 | (0.49, 44.42) | 0.155 | |
| Polished | 2.30 | (0.34, 15.61) | 0.351 | |
PI is the proportional increase in cells for each unit increase in the predictor ‡Result interpreted as proportional change in cell count for each increase in stent diameter equal to 1/10th the aorta diameter.
Figure 4.
Stent diameter : aorta diameter ratio vs. endothelial cell count. Scatter plot of the log(e) transformation of endothelial cell count (log) (y-axis) relative stent diameter : aorta diameter ratio (x-axis).
Macroscopic and microscopic morphology of carotid aneurysm and aorta
Fluoroscopic examinations at three weeks confirmed dilation and aneurysm in all treated right carotid vessels compared with the left untreated carotid arteries. The magnitude of aneurysms varied within the cohort (Table 1). Microscopic studies revealed intraluminal thrombus and intact aneurysmal carotid vessels (Figure 2A,B,E,F). There was no evidence of injury in the aortic arches.
Endothelial cell identification
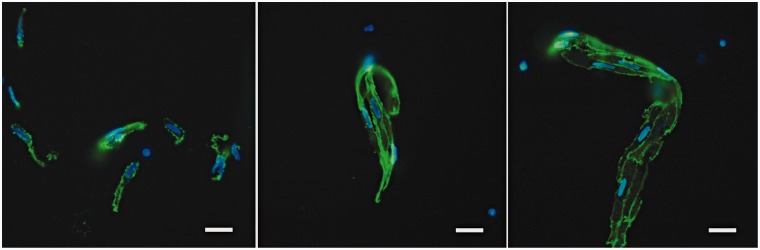
For quantification of endothelial cell yield, both morphology of endothelial-like nucleus shown by DAPI and positive cell membrane signal of CD31 were used to identify endothelial cells on the chamber slides (Figure 5). Basically, endothelial cells were found under fluorescence microscopy in three ways, single cell (Figure 5A), small aggregates that had two to four cells together (Figure 5B), and large aggregates of more than five cells (Figure 5C).
Figure 5.
Endothelial cells were identified by both morphological features (>20 µm, oval-shape with kidney-shaped nuclei) and cell surface CD31 staining. Endothelial cells presented in three ways: single cells (A); small aggregates of 2 to 4 cells (B); and larger aggregates more than 5 cells (C). (Scale bar: 20 µm).
Discussion
We have demonstrated the safety and efficacy of commercial and custom neurointerventional coils, stents, and stent-like devices in collecting viable vascular endothelial cells. This expands on our original work19 establishing the proof of principle that a conventional detachable aneurysm embolic coil could collect endothelial cells. This report confirms that finding, documenting the ability of coils to collect endothelial cells from experimental aneurysms proper. Compared to our prior work, we noted that the stiffness of the coil was associated with the magnitude of cell collection. However, given that the two standard coils were the two longest coils in the experiment and were both unmodified P400 coils, we cautiously note that the observed association with cell collection may be attributable to other coil characteristics. Otherwise we did not notice any statistically significant dimensional relationship of the aneurysm or coil to cell collection quantity. There was a weak trend for larger primary diameter (0.020″) P400 coils to collect more cells than the smaller (0.010″) GDC coils.19 This result complements that of our prior work, suggesting that in addition to the force exerted against the vascular intima by the coil as it deploys to its prescribed secondary diameter, the surface area of the coil itself may also facilitate cell collection.19 Our experimental wider-pitch, flat-wire coils did not demonstrate any greater or lesser performance relative to conventional coils. This result, though negative, is helpful as an early foray into modification techniques as it establishes the safety of such coil types.
The paucity of cells collected from coils is both helpful and harmful. That we are able to collect cells consistently and in small numbers supports the reproducibility of the method as well as the potential safety of the technique not to “denude” the aneurysm fundus. The scant cell number does, however, limit material for molecular analysis. To remedy this we have established a fluorescence-activated cell sorting method (FACS) to isolate the endothelial cell population, thus limiting cell loss.23 We have also employed PCR techniques to enable expression analysis at the single cell level.23
Compared to coils, the stent and stent-like devices were able to collect endothelial cells by greater than two orders of magnitude (7.93 (±8.33) cells/coil vs. 831.33 (±887.73) cells/device)). Furthermore, there were significant relations noted between stent-to-aorta diameter ratios, stent length, and the use of a pulling retrieval technique relative to cell counts. Controlling for the stent-to-aorta diameter ratio in multivariate analysis diminished the effects of stent length and a pulling technique on cell collection efficacy. As such, we noted a 173% increase in cell count for each increase in stent diameter equal to 1/10th the aorta diameter. This supports our original work, in which we noted a relative increase in cells with an increase in the secondary coil diameter.19 Though the devices are different, the concept that a greater force against the intima yields more cells is similar. Our non-polished stents demonstrated a weak trend toward a lower number of cells controlling for stent-to-aorta diameter, an unexpected effect and one that may be spurious given the small number (6 of 15) of devices it represents. This result, as with the modified coil outcomes, is, however, helpful as an early foray into modification techniques as it establishes the safety of such stent types.
That a pulling retrieval technique collected more cells is not surprising as this introduces friction beyond that generated with immediate device deployment. It is, however, noteworthy even without the use of the more dynamic pulling method, as is used in the practice of mechanical embolectomy, simple deployment and re-capture yielded 335.63 (±416.10) per device. It is encouraging that a stent or stent-like device might be opened in a vessel of interest and recaptured without the need of further manipulation inside that vessel for the express purpose of cell collection. Any manipulation of a stent, pulling or otherwise, in a vessel increases the potential for injury and should be avoided if not surgically indicated.
There are limitations to our study, in large part related to the small number of animals and, in turn, devices tested. We recognize the relatively narrow scope of the study and caution the generalized extrapolation of its results to endovascular devices writ large. Furthermore, the study is not an immediate endorsement of device deployment specifically for cell collection, especially within the cerebral vasculature. Any such study requires institutional oversight and approval as well as informed consent beyond the details of whatever clinically prescribed procedure is required.
That said, our ultimate goal is to collect endothelial cells from the human cerebral vasculature. The progression of surgical practice toward minimally invasive methods has been stepwise following the synchronous efforts of pioneering physicians and engineers. Endovascular surgery, in or outside the central nervous system, has permitted operators to treat diseases faster and safer than traditional open methods. This had been beneficial to patients, though endovascular surgery has limitations. One such constraint is the relative dearth of tissue collection for pathological analysis. Dedicated biopsy or tissue sampling during open surgery is common, though not performed routinely during endovascular procedures. As such, our molecular and histological understanding of many vascular diseases has been slowed in part because we lack a safe and reliable method to collect vascular tissue as part of modern endovascular practice. A non-traumatic endovascular biopsy method would address a wide range of questions in vascular biology relevant to clinical management. Its implementation would expand the availability of tissue for genetic analysis to further hone therapeutic targets and metrics to assess clinical risk.
Conclusion
Neurovascular disease represents a wide spectrum of pathophysiological processes, the elucidation of which has been limited to date by lack of a means to safely collect CNS vascular tissue in vivo. Conventional detachable-type aneurysm coils, stents, and stent-like devices can successfully collect viable endothelial cells during their routine usage in their respective vascular targets. The use of such a technique may prove to be a viable endovascular cell collection method for future clinical studies.
Acknowledgments
The authors wish to acknowledge Dr William Young and the group at the UCSF Center for Cerebrovascular Research and the UCSF Department of Radiology and Biomedical Imaging for their support of the project.
Funding
The project was supported in part by The Aneurysm and AVM Foundation, and the Clinical and Translational Science Institute Translational Technology grant UL1 TR000004.
Conflict of interest
The authors report no conflict of interest.
References
- 1.Peters D, Kassam A, Feingold E, et al. Molecular anatomy of an intracranial aneurysm: coordinated expression of genes involved in wound healing and tissue remodeling. Stroke 2001; 32: 1036–1042doi: 10.1161/01.STR.32.4.1036. [DOI] [PubMed] [Google Scholar]
- 2.Molyneux A, Kerr R, Stratton I, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 2002; 360: 1267–1274doi: 10.1016/S0140-6736(02)11314-6. [DOI] [PubMed] [Google Scholar]
- 3.Molyneux A, Kerr R, Birks J, et al. Risk of recurrent subarachnoid haemorrhage, death, or dependence and standardised mortality ratios after clipping or coiling of an intracranial aneurysm in the International Subarachnoid Aneurysm Trial (ISAT): long-term follow-up. Lancet Neurol 2009; 8: 427–433doi: 10.1016/S1474-4422(09)70080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mackey J, Brown RD, Moomaw CJ, et al. Unruptured intracranial aneurysms in the Familial Intracranial Aneurysm and International Study of Unruptured Intracranial Aneurysms cohorts: differences in multiplicity and location. J Neurosurg 2012. doi: 10.3171/2012.4.JNS111822. [DOI] [PMC free article] [PubMed]
- 5.Rahman M, Smietana J, Hauck E, et al. Size ratio correlates with intracranial aneurysm rupture status: a prospective study. Stroke 2010; 41: 916–920doi: 10.1161/STROKEAHA.109.574244. [DOI] [PubMed] [Google Scholar]
- 6.Wiebers D, Whisnant J, Huston 3rd, Jr, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 2003; 362: 103–110doi: 10.1016/S0140-6736(03)13860-3. [DOI] [PubMed] [Google Scholar]
- 7.Frösen J, Tulamo R, Paetau A, et al. Saccular intracranial aneurysm: pathology and mechanisms. Acta Neuropathol 2012; 123: 773–786doi: 10.1007/s00401-011-0939-3. [DOI] [PubMed] [Google Scholar]
- 8.Krings T, Mandell DM, Kiehl TR, et al. Intracranial aneurysms: from vessel wall pathology to therapeutic approach. Nat Rev Neurol 2011; 7: 547–559doi: 10.1038/nrneurol.2011.136. [DOI] [PubMed] [Google Scholar]
- 9.Aoki T, Nishimura M. The development and the use of experimental animal models to study the underlying mechanisms of CA formation. J Biomed Biotechnol 2011; 2011: 1–10Article ID: 535921. doi: 10.1155/2011/535921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aoki T, Kataoka H, Nishimura M, Ishibashi R, Morishita R, Miyamoto S. Regression of Intracranial Aneurysms by Simultaneous Inhibition of Nuclear Factor-κB and Ets With Chimeric Decoy Oligodeoxynucleotide Treatment. Neurosurgery 2012; 70: 1534–1543doi: 10.1227/NEU.0b013e318246a390. [DOI] [PubMed] [Google Scholar]
- 11.Hasan DM, Mahaney KB, Magnotta VA, et al. Macrophage imaging within human cerebral aneurysms wall using ferumoxytol-enhanced MRI: a pilot study. Arterioscler Thromb Vasc Biol 2012; 32: 1032–1038doi: 10.1161/ATVBAHA.111.239871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hasan D, Hashimoto T, Kung D, et al. Upregulation of Cyclooxygenase-2 (COX-2) and Microsomal Prostaglandin E2 Synthase-1 (mPGES-1) in Wall of Ruptured Human Cerebral Aneurysms: Preliminary Results. Stroke 2012; 43: 1964–1967. doi: 10.1161/STROKEAHA.112.655829. [DOI] [PMC free article] [PubMed]
- 13.Ishibashi R, Aoki T, Nishimura M, et al. Imidapril inhibits cerebral aneurysm formation in an angiotensin-converting enzyme-independent and matrix metalloproteinase-9-dependent manner. Neurosurgery. 2012; 70: 722–730doi: 10.1227/NEU.0b013e3182326188. [DOI] [PubMed] [Google Scholar]
- 14.Marbacher S, Schläppi JA, Fung C, et al. Do statins reduce the risk of aneurysm development? A case-control study. J Neurosurg 2012; 116: 638–642doi: 10.3171/2011.10.JNS11153. [DOI] [PubMed] [Google Scholar]
- 15.Habashi JP, Doyle JJ, Holm TM, et al. Angiotensin II type 2 receptor signaling attenuates aortic aneurysm in mice through ERK antagonism. Science 2011; 332: 361–365doi: 10.1126/science.1192152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanematsu Y, Kanematsu M, Kurihara C, et al. Critical roles of macrophages in the formation of intracranial aneurysm. Stroke 2011; 42: 173–178doi: 10.1161/STROKEAHA.110.590976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aoki T, Nishimura M, Kataoka H, et al. Complementary inhibition of cerebral aneurysm formation by eNOS and nNOS. Lab Invest 2011; 91: 619–626doi: 10.1038/labinvest.2010.204. [DOI] [PubMed] [Google Scholar]
- 18.Aoki T, Nishimura M, Matsuoka T, et al. PGE(2) -EP(2) signalling in endothelium is activated by haemodynamic stress and induces cerebral aneurysm through an amplifying loop via NF-κB. Br J Pharmacol 2011; 163: 1237–1249doi: 10.1111/j.1476-5381.2011.01358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cooke DLSH, Sun Z, Guo Y, et al. Evaluating the feasibility of harvesting endothelial cells using detachable coils. Interv Neuroradiol 2013; 19(4): 399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng L, Stern D, Pile-Spellman J. Human endothelium: endovascular biopsy and molecular analysis. Radiology 1999; 212: 655–664doi: 10.1148/radiology.212.3.r99au28655. [DOI] [PubMed] [Google Scholar]
- 21.Kallmes DF, Helm GA, Hudson SB, et al. Histologic evaluation of platinum coil embolization in an aneurysm model in rabbits. Radiology 1999; 213: 217–222doi: 10.1148/radiology.213.1.r99oc16217. [DOI] [PubMed] [Google Scholar]
- 22.StataCorp. Stata Statistical Software. Release 13 ed. College Station, TX: StataCorp LP; 2013.
- 23.Sun Z, Su H, Long B, et al. Endothelial cell high-enrichment from endovascular biopsy sample by laser capture microdissection and fluorescence activated cell sorting. J Biotechnol 2014; 192PA: 34–39doi: 10.1016/j.jbiotec.2014.07.434. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]