Abstract
Endovascular embolization has evolved to become the primary therapeutic option for dural arteriovenous fistulas (DAVFs). While guaranteeing complete occlusion of the fistula orifice, the goal of DAVF embolization is also to ensure the patency of normal cerebral venous drainage. This paper describes a case of successful embolization of a complex DAVF in the superior sagittal sinus with a multistaged approach using a combination of transvenous and transarterial tactics. The strategies and techniques are discussed.
Keywords: Dural arteriovenous fistula, endovascular embolization, balloon, coil, Onyx
Introduction
Dural arteriovenous fistulas (DAVFs) are relatively rare lesions consisting of anomalous connections between dural arteries and the venous system within the dura mater, approximately accounting for 10%–15% of all intracranial arteriovenous shunts. These lesions usually involve the wall of a major dural sinus such as the cavernous sinus, superior sagittal sinus (SSS) or transverse-sigmoid sinus.1 The venous drainage pattern of the shunting is crucial in the risk assessment of these lesions. DAVFs with direct or indirect cortical venous drainage are considered to carry a high risk for hemorrhage that mandates prompt diagnosis and active treatment.2
Endovascular embolization via transarterial or/and transvenous approach has evolved to become the primary therapeutic option for DAVF in a major dural sinus with the goal of obliterating the shunts and disconnecting cortical venous reflux. However, these techniques may cause the sacrifice of the involved sinus. Therapeutic sinus occlusion and subsequent sinus thrombosis may activate angiogenetic factors on the adjacent dural sinus. In addition, the relatively high intracranial pressure due to excessive sinus occlusion may cause appearance of new lesions.3,4 If there is dysplasia or occlusion in the contralateral venous sinus, the inadvertent embolization of a sinus with normal venous drainage would cause a catastrophic outcome. While treating these patients, one should not only guarantee complete occlusion of the fistula, but also ensure the patency of normal cerebral venous outflow. Several researchers have reported successful embolization of transverse-sigmoid sinus DAVFs with the maintenance of vein patency using venous balloon-assisted or stent-assisted techniques.5–7 However, reports on the application of venous balloon-assisted techniques in the SSS are limited. Here we describe a case of successful embolization of a complex DAVF of the SSS with transvenous balloon-assisted techniques.
Case Presentation
A 23-year-old male patient presented with a five-year history of tinnitus. Over the past two years, he had developed right eye orbital symptoms, including chemosis, proptosis and decreased visual acuity. Then, he was evaluated by magnetic resonance imaging (MRI) of the head at a local hospital (Figure 1). The MRI scan showed a cluster of subarachnoid flow voids in the bilateral cerebral hemispheres, cerebellar hemisphere and around the brainstem, as well as venous ectasia of the right superior ophthalmic vein. Subsequent cerebral digital subtraction angiography (DSA) demonstrated a complex DAVF located at the junction of the middle and posterior one-third of the SSS with superficial cortical venous reflux. The right transverse sinus was occluded and there was a severe stenosis at the junction of the left transverse and sigmoid sinus (Figure 1). This patient was treated palliatively with occlusion of the major feeding artery (occipital artery) and stent angioplasty of the left transverse-sigmoid sinus in the local hospital. Postoperatively, there was no significant improvement of symptoms. Subsequently, the patient suffered a sudden headache once five months ago and had an abrupt slurred speech with vomiting three months ago. Physical examination showed bruit heard over the parietal and occipital area, right eye proptosis, conjunctival injection and decreased visual acuity.
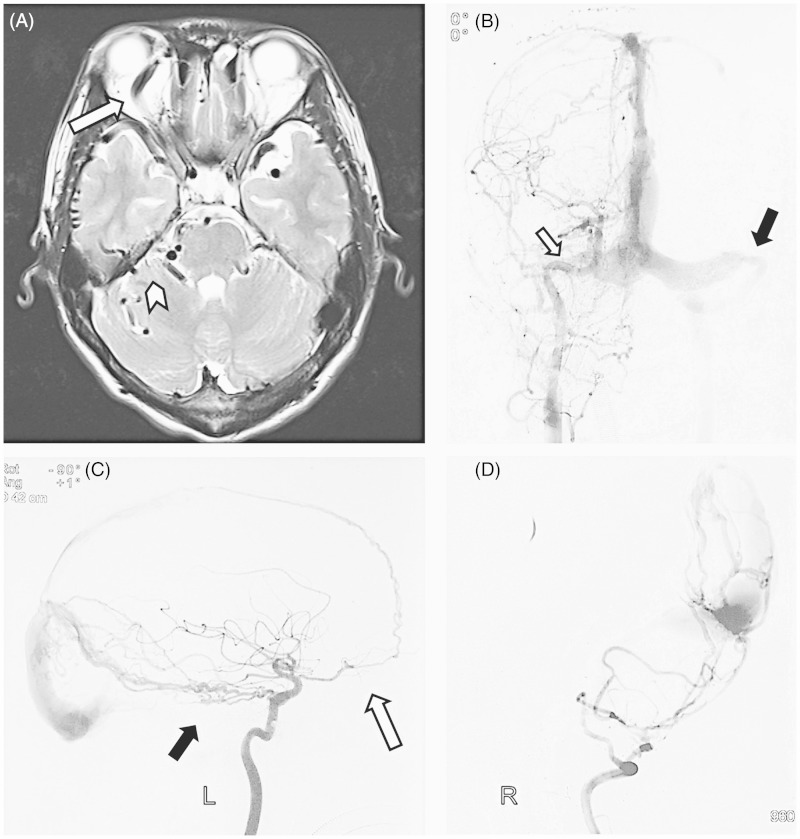
Figure 1.
A) The T2-weighted magnetic resonance images demonstrate subarachnoid flow voids in the bilateral temporal lobes and the brainstem (arrowhead), as well as ectasia of the right superior ophthalmic vein (arrow). B) Anteroposterior view of the left common carotid artery (CCA) injection showed a dural arteriovenous fistula (DAVF) with an occlusion of right transverse (white arrow) and a stenosis at the junction of the left transverse and sigmoid sinus (black arrow). C) Left internal carotid artery (ICA) injection, lateral view, documented that the anterior falcine artery arising from ophthalmic artery (white arrow) and marginal branches of the meningohypophyseal trunk (black arrow) fed the fistula. D) Lateral view of the right vertebral artery (VA) injection showed the DAVF was supplied by occipital artery. So, the patient was treated palliatively with occlusion of the right occipital artery and stent angioplasty of the left transverse-sigmoid sinus in a local hospital.
After written informed consent, an angiogram was obtained (Figure 2), which showed the SSS DAVF with superficial cortical venous reflux, partially through the cavernous sinus and right ophthalmic vein. The right transverse sinus was still occluded and the left transverse-sigmoid sinus was patent after stent angioplasty. The high-flow DAVF was mainly composed of two parts. One was located on the falx and supplied by the marginal branches of bilateral meningohypophyseal trunks arising from the internal carotid artery, bilateral middle meningeal arteries, bilateral maxillary arteries, meningeal branches of right ascending pharyngeal artery and bilateral posterior meningeal arteries, as well as the pial branches of bilateral posterior cerebral arteries. The other part was located on the left side convex dura beside the SSS and supplied by bilateral middle meningeal arteries, bilateral superficial temporal arteries and anterior falcine arteries arising from bilateral ophthalmic arteries. The venous pouch of each part joined the SSS at the junction of the middle and posterior one-third portion. There was a moderate stenosis in the confluence of the venous pouch and SSS. Antegrade flow was noted from the SSS to the left internal jugular vein through the left transverse-sigmoid sinus. Additionally, there was also retrograde flow in the anterior portion of the SSS. The superficial veins draining the fistula were significantly dilated compared with the former DSA and contrast stasis was noted in the drainage cortical veins.
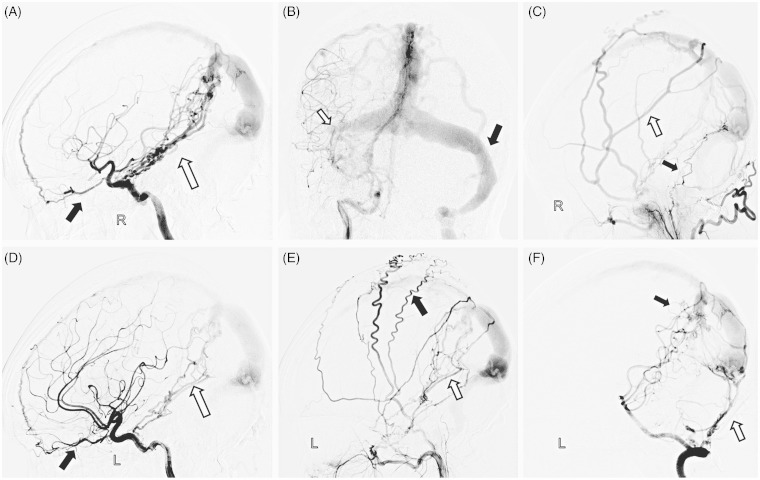
Figure 2.
A) Lateral view of right ICA injection showed the arterial feeders were from the anterior falcine artery arising from the ophthalmic artery (black arrow) and the meningohypophyseal trunk (white arrow). B) Right ICA anteroposterior angiography showed right transverse sinus was still occluded (white arrow) and the left transverse-sigmoid sinus was patent after stent angioplasty (black arrow). C) Selective right external carotid artery (ECA) angiogram showed that the DAVF was fed by the middle meningeal artery (MMA, white arrow) and meningeal branches of the right ascending pharyngeal artery (black arrow). D) Lateral view of the left ICA injection showed the DAVF was supplied by anterior falcine artery (black arrow) and the marginal branches of the meningohypophyseal trunk (white arrow). E) Left ECA lateral angiography demonstrated that the DAVF was fed by posterior meningeal artery (white arrow) and superficial temporal artery (black arrow). F) Lateral view of right vertebral arteriogram showed the feeders from occipital artery (white arrow) and pial branches of posterior cerebral artery (black arrow).
Endovascular Procedures
The patient was placed under general anesthesia and fully heparinized, and all procedures were performed via the bilateral femoral arteries and right femoral vein access. A 6 French guiding catheter was navigated into the distal left internal jugular vein. A 4/20 Hyperglide balloon (eV3, Neurovascular, CA, USA) was then delivered into the SSS through the left transverse-sigmoid sinus, with the distal one-third navigated into venous pouch and the proximal two-thirds located in the SSS (Figure 3). Meanwhile, an Echelon-10 microcatheter (EV3, Irvine, CA, USA) was positioned over a microwire transvenously into the venous pouch via the same guiding catheter. A Marathon microcatheter (EV3, Irvine, CA, USA) catheterized the distal middle meningeal artery (MMA) though the right external carotid artery and the tip was navigated as close to the fistula site as possible. The balloon was meticulously inflated and several coils were employed to obliterate the fistula through the Echelon-10 microcatheter. Then, Onyx-18 and Onyx-34 were injected through the Marathon microcatheter and the Echelon-10 microcatheter alternately. The embolization procedures were slow, with injection being stopped when Onyx reflux reached non-targeted areas or penetrated into the SSS past the balloon. After waiting 30 to 60 seconds, the injection was resumed. Several rounds of “waiting” and “injecting” took place through these two microcatheters. Caution was exerted to avoid inadvertent embolization of the SSS during the slow injection of Onyx. Patency of the SSS was checked frequently during the intervals between the intermittent injections of the embolic material. Only a small amount of Onyx flowed into the SSS. The injection was eventually stopped when the infiltration of Onyx into the venous pouch could not be continued despite several attempts, and excessive Onyx reflux in the MMA was present. Post-procedure angiograms demonstrated patency of the SSS and partial obliteration of the fistula. The residual component of the fistula was fed by several branches of the right MMA and meningohypophyseal trunk, but the blood flow had slowed down remarkably. The patient showed an immediate post-procedural improvement in tinnitus, chemosis and conjunctival injection, but he had transient cortical blindness. We performed an MRI scan as soon as the new neurological deficit was observed. The MRI showed no new abnormal sign (Figure 4). He received glucocorticoid and anticoagulant therapy and recovered to normality after eight hours.
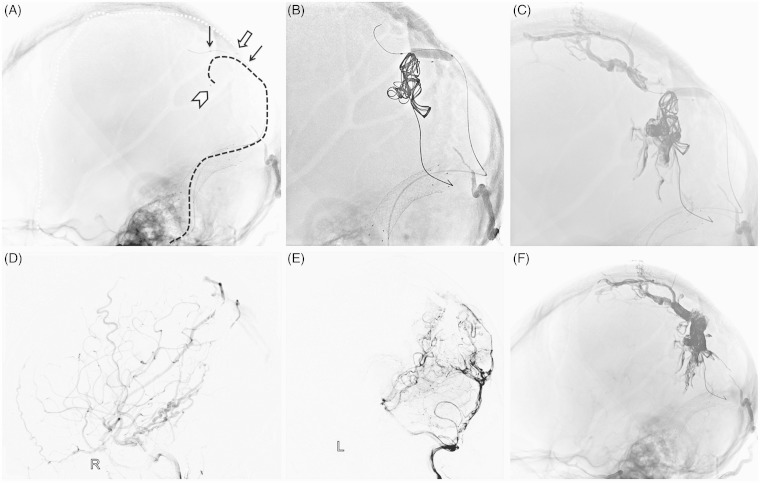
Figure 3.
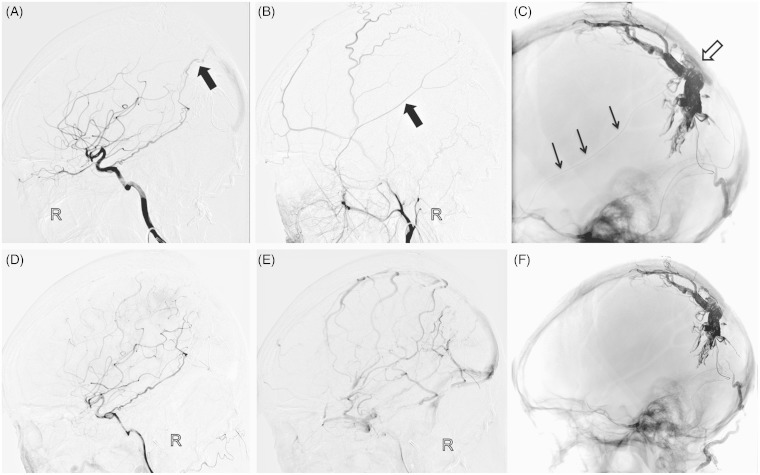
First stage operation. A) Unsubtracted lateral view showed the Hyperglide balloon position (black arrow), and the enchelon-10 microcatheter navigated into the venous pouch on the falx (catheter tip, arrowhead; catheter, black dotted line). A Marathon microcatheter catheterized the distal middle meningeal artery (catheter tip, white arrow; catheter, white dotted line). B) The balloon was inflated with the distal one-third in the venous pouch and the proximal two-thirds in the SSS, and several coils were employed through the Echelon-10 microcatheter. C) Onyx was injected through the Marathon microcatheter and Echelon-10 microcatheter alternately. Final right CCA lateral angiography (D) and left VA lateral angiography (E) showed partial obliteration of the fistula and blood flow slowed down. F) Post-embolization cast was seen on unsubtracted lateral view.
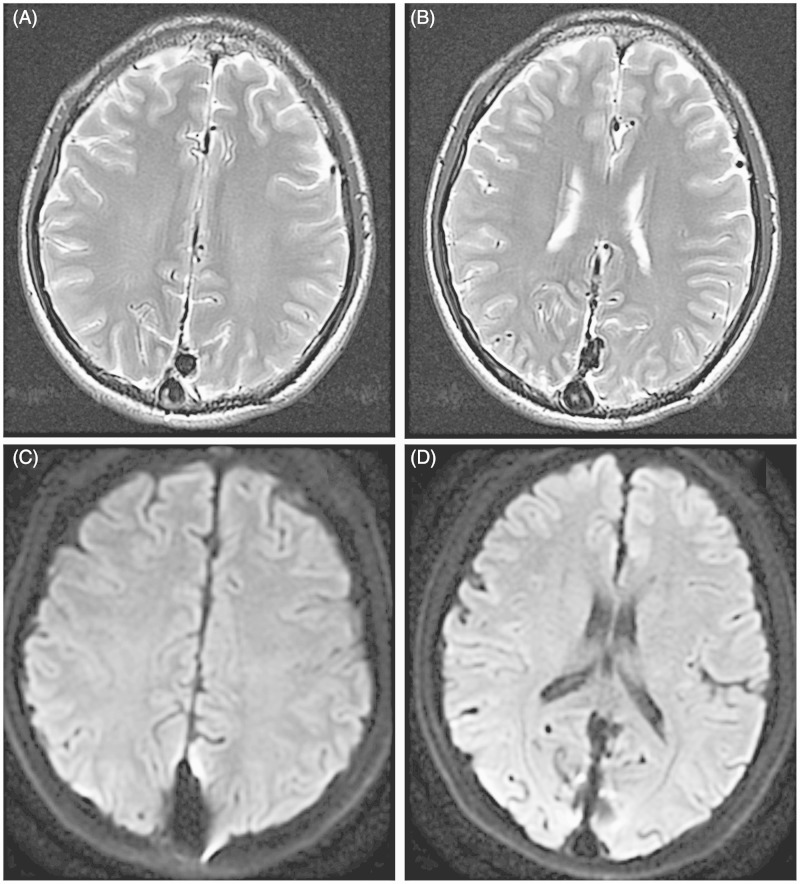
Figure 4.
A, B) T2-weighted MRI showed no new abnormal sign. C,D) There was no acute infarction in diffusion-weighted imaging.
After two uneventful months, the patient was admitted for a second stage operation (Figure 5). Preoperative DSA showed that the flow of the residual fistula had been greatly lowered. There was still a low-flow residual part, which was supplied by the right MMA, meningohypophyseal trunk and posterior meningeal artery. Therefore, the same therapeutic options were used again. Complete obliteration was achieved after repeat transarterial Onyx-18 injection through a Marathon microcatheter via the right MMA in combination with the venous balloon-assisted technique in the SSS. It was angiographically documented that the cortical vein and the SSS restored to antegrade flow. The DAVF was successfully occluded without complications. The patient’s visual acuity significantly improved and other symptoms were completely resolved at three-month follow-up.
Figure 5.
Second stage operation: the residual part was supplied by marginal branches of the right meningohypophyseal trunk (A, black arrow) and right MMA (B, black arrow). C) Unsubtracted lateral view showed the Hyperglide balloon (white arrow) and Onyx injection through a Marathon microcatheter via the right MMA (black arrow). Final angiograms documented complete occlusion of the fistula (D), and the cortical vein restored to antegrade flow (E). F) Post-embolization cast was seen on unsubtracted lateral view.
Discussion
Treatment options for DAVFs include endovascular embolization, microsurgery, stereotactic radiosurgery or a combination of these modalities.8 In some clinics, because of the relatively simple anatomical structure of these lesions, surgical resection of the diseased dura and sinus is still the main treatment of DAVFs which were classified as Cognard III-IV according to the Cognard classification.9 However, endovascular intervention has quickly evolved and become the first treatment option for most DAVFs, especially the lesions classified as Cognard I-II on the transverse-sigmoid sinus or SSS.10,11 The complete eradication of DAVF by transvenous embolization using different embolic material has been shown to be effective, but these endovascular techniques may cause occlusion of the dural sinus involved. Although complete occlusion of the venous sinus without normal function may not cause brain venous reflux disorder, the recurrence rate or rate of new lesion onset on the adjacent dural sinus is high.4
In some complex cases, the balloon-assisted technique served as an effective method to facilitate superselective catheterization, produce better distal penetration, or protect normal venous drainage from unwanted embolization. As we reported before,12 the key to interventional success is distal superselective microcatheterization to reach the fistulous zone. Superselective navigation of a microcatheter into the feeding artery can, however, be difficult, especially if there is an acute angulation between the parent vessel and feeding artery. In these instances, an inflated balloon was used to block the parent vessel temporarily to avoid microcatheter prolapse and to act as a buffer that also redirected the forward pressure applied on the microcatheter toward the feeding artery. In addition, use of a balloon via an arterial approach can increase proximal resistance, resulting in better control of reflux and enhanced distal penetration.7,13 Several papers.7,14,15 have reported that a deflated balloon via a venous approach can prevent untoward distal Onyx migration in the embolization of DAVF on the transverse-sigmoid sinus, but reports on venous balloon-assisted techniques in SSS are rare.
In this case, angiography revealed a complex type IIa + b DAVF of the SSS. We regard this DAVF as a “complex” fistula based on the fact that it had bilateral dural supply with cortical venous reflux and right transverse sinus occlusion. There was normal antegrade venous flow in the posterior one-third of the SSS. Once the posterior part of the SSS is occluded, normal cortical venous drainage may be obstructed, resulting in the potential sequelae of venous infarction or hemorrhage. Not only should we obliterat the fistulous site and disconnect cortical venous reflux, but we also need to restore the antegrade flow of the SSS and keep its patency. It is relatively easy to superselect the microcatheter into the fistula zone through the middle meningeal artery. However, it is difficult to occlude the fistula, and maintain the SSS patency in this high-flow DAVF as well. So it is of vital importance to use a venous balloon-assisted technique to avoid the SSS and cortical vein embolization, simultaneously achieving better penetration of the Onxy into the fistulous connections. Although the diameter of Hyperglide balloons available is smaller than the diameter of the SSS, the balloon is large enough to reduce the flow and prevent the SSS from obliteration. In addition, the complication caused by the complete block of the venous drainage with the balloon should be avoided. So the use of the balloon is to protect the SSS and reduce the blood flow. Adhesive between Onyx and balloon is also a major concern for this treatment. With the beneficial characteristics of Onyx, whether transarterial or transvenous balloon assisted, it has been proved to be safe in multiple centers.5,7,13,16
On the first stage operation, the patient had postoperative transient cortical blindness for eight hours. In the operation, we did not insert any microcatheter through the basilar artery or posterior cerebral artery. And no cerebral vasospasm was seen on the left VA lateral angiography (Figure 3D). Additionally, the MRI (Figure 4) performed postoperatively showed no infarction or hemorrhage. Contrast agents might have anticholinesterase activity on neurons, which could explain the temporary dysfunction of the cortex.17 Therefore, we think the cause of the temporary cortical blindness was contrast-induced encephalopathy.
It should be noted that this method is only suitable for selected patients due to its difficult technical points and potential associated complications. Firstly, high-compliance balloons, such as Hyperglide balloons designed to assist with coil embolization of intracranial wide-neck aneurysms, are preferable. Compared with the balloons used in angioplasty, the Hyperglide balloons are longer and able to be repeatedly inflated and uninflated. Secondly, caution must be taken when the balloon is inflated, in order not to overinflate and rupture the sinus or cortical vein. Inflation over a long period of time should be avoided to prevent embolic complications. Repeat inflation of the balloon is inevitable. Finally, the diameter of largest Hyperglide balloon available is 4 millimeters, but it is smaller than the diameter of the dilated vein. So the inflated balloon cannot closely fit the vessel wall. Although the balloon can prevent the coils prolapsing into the vein or sinus, it cannot completely avoid the flux of the Onyx into the sinus. However no other high-compliance balloons with enough length and diameter are currently available in China. This is also one of the reasons why the patient received a staged operation. Even so, we think this strategy, a multistaged approach with a combination of transvenous and transarterial tactics, is the optimum endovascular treatment for this patient.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Chaichana KL, Coon AL, Tamargo RJ, et al. Dural arteriovenous fistulas: Epidemiology and clinical presentation. Neurosurg Clin N Am 2012; 23: 7–13doi: 10.1016/j.nec.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 2.van Dijk JM, TerBrugge KG, Willinsky RA, et al. Clinical course of cranial dural arteriovenous fistulas with long-term persistent cortical venous reflux. Stroke. 2002; 33: 1233–1236doi: 10.1161/01.STR.0000014772.02908.44. [DOI] [PubMed] [Google Scholar]
- 3.Rahmanian A, Farrokhi MR, Alibai EA, et al. Multiple intracranial dural arteriovenous fistula. J Res Med Sci 2013; 18: 360–362. [PMC free article] [PubMed] [Google Scholar]
- 4.Kubo M, Kuwayama N, Hirashima Y, et al. Dural arteriovenous fistulae developing at different locations after resolution of previous fistulae: Report of three cases and review of the literature. Am J Neuroradiol 2002; 23: 787–789. [PMC free article] [PubMed] [Google Scholar]
- 5.Jittapiromsak P, Ikka L, Benachour N, et al. Transvenous balloon-assisted transarterial Onyx embolization of transverse-sigmoid dural arteriovenous malformation. Neuroradiology 2013; 55: 345–350doi: 10.1007/s00234-012-1107-8. [DOI] [PubMed] [Google Scholar]
- 6.Choi BJ, Lee TH, Kim CW, et al. Reconstructive treatment using a stent graft for a dural arteriovenous fistula of the transverse sinus in the case of hypoplasia of the contralateral venous sinuses: Technical case report. Neurosurgery 2009; 65: E994–E996. discussion: E996. [DOI] [PubMed] [Google Scholar]
- 7.Shi ZS, Loh Y, Duckwiler GR, et al. Balloon-assisted transarterial embolization of intracranial dural arteriovenous fistulas. J Neurosurg 2009; 110: 921–928doi: 10.3171/2008.10.JNS08119. [DOI] [PubMed] [Google Scholar]
- 8.Sarma D, ter Brugge K. Management of intracranial dural arteriovenous shunts in adults. Eur J Radiol 2003; 46: 206–220doi: 10.1016/S0720-048X(03)00092-5. [DOI] [PubMed] [Google Scholar]
- 9.Cognard C, Gobin YP, Pierot L, et al. Cerebral dural arteriovenous fistulas: Clinical and angiographic correlation with a revised classification of venous drainage. Radiology 1995; 194: 671–680. [DOI] [PubMed] [Google Scholar]
- 10.Cognard C, Januel AC, Silva NJ, et al. Endovascular treatment of intracranial dural arteriovenous fistulas with cortical venous drainage: New management using Onyx. Am J Neuroradiol 2008; 29: 235–241doi: 10.3174/ajnr.A0817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim DJ, Willinsky RA, Krings T, et al. Intracranial dural arteriovenous shunts: Transarterial glue embolization–experience in 115 consecutive patients. Radiology 2011; 258: 554–561doi: 10.1148/radiol.10100755. [DOI] [PubMed] [Google Scholar]
- 12.Zhao WY, Krings T, Yang PF, et al. Balloon-assisted superselective microcatheterization for transarterial treatment of cranial dural arteriovenous fistulas: Technique and results. Neurosurgery 2012; 71: s269–s273. discussion 273. [DOI] [PubMed] [Google Scholar]
- 13.Spiotta AM, Hughes G, Masaryk TJ, et al. Balloon-augmented Onyx embolization of a dural arteriovenous fistula arising from the neuromeningeal trunk of the ascending pharyngeal artery: Technical report. J Neurointerv Surg 2011; 3: 300–303doi: 10.1136/jnis.2010.003095. [DOI] [PubMed] [Google Scholar]
- 14.Andreou A, Ioannidis I, Nasis N. Transarterial balloon-assisted glue embolization of high-flow arteriovenous fistulas. Neuroradiology 2008; 50: 267–272doi: 10.1007/s00234-007-0322-1. [DOI] [PubMed] [Google Scholar]
- 15.Arat A, Cil BE, Vargel I, et al. Embolization of high-flow craniofacial vascular malformations with onyx. Am J Neuroradiol 2007; 28: 1409–1414doi: 10.3174/ajnr.A0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu Y, Li Q, Huang Q, et al. Embolization of direct carotid cavernous fistula with onyx and coils under transarterial balloon protection. Cardiovasc Intervent Radiol 2014; 37: 679–685doi: 10.1007/s00270-013-0732-x. [DOI] [PubMed] [Google Scholar]
- 17.Junck L, Marshall WH. Neurotoxicity of radiological contrast agents. Ann Neurol 1983; 13: 469–484doi: 10.1002/ana.410130502. [DOI] [PubMed] [Google Scholar]