Abstract
We experienced a rare complication after carotid artery stenting (CAS) characterized by transient neurological symptoms with no evidence of distal emboli or hyperperfusion. Using neuroimaging, we investigated the pathogenesis of the complication that occurred after CAS in three patients who developed neurological symptoms over a period of ten hours after CAS and improved within two days. None of the three patients showed signs of fresh infarctions on diffusion-weighted imaging or hyperperfusion on single-photon emission computed tomography. However, high signal intensity was observed in the leptomeningeal zone of the cerebral hemisphere on the stent side in all three patients and in the leptomeningeal zone of the contralateral anterior cerebral artery territory in one patient. These areas were assessed using fluid-attenuated inversion recovery (FLAIR) magnetic resonance imaging without gadolinium administration. The high signal intensity in the leptomeningeal zone disappeared as the symptoms improved. Based on the transient nature of the neurological disorders and the normalization of FLAIR imaging findings in these patients, the pathogenesis of this complication might have been vasogenic edema due to vasoparalysis of the local vessels caused by the hemodynamic changes occurring after CAS.
Keywords: Blood-brain barrier, carotid artery stenting, FLAIR MRI, reversible vasoparalysis
Introduction
Ischemic stroke due to distal emboli and hyperperfusion syndrome are well-known complications of carotid artery stenting (CAS). We describe a rare complication involving temporary late-onset symptoms unassociated with hyperperfusion or infarcts on neuroimaging in three patients after CAS. High signal intensity was detected in the leptomeningeal zone of the ipsilateral middle cerebral artery and anterior cerebral artery territories on the stent side in each patient and in the leptomeningeal zone of the contralateral anterior cerebral artery territory in one patient. This feature disappeared concomitantly with symptom improvement. We discuss the pathogenesis of this complication based on the published literature.
Materials and Methods
We enrolled 306 patients who received CAS for stenosis of the carotid artery and identified three patients with late-onset transient neurological symptoms without distal emboli or hyperperfusion after CAS between 2008 and 2012. Medical records were evaluated with magnetic resonance imaging (MRI), carotid ultrasound, single-photon emission computed tomography (SPECT), near-infrared spectroscopy, and cerebral angiography findings and reviewed for patient characteristics and clinical status at the time of hospital discharge. A modified Rankin score of <2 was considered a good outcome, and >3 was considered a poor outcome. The lack of a control group was a possible limitation of this study.
Results
In the 306 patients who received CAS for stenosis of the carotid artery, FLAIR hyperintensity in the subarachnoid space after CAS was detected in only three cases. Table 1 shows the time courses of the clinical manifestations in the three patients. The symptoms developed between 10 h 30 min and 11 h 20 min after CAS, and improved in each patient in less than two days. Fever was noticed in two patients, and one patient developed convulsions with elevated blood pressure (162/80 mm Hg) at the time of symptom onset, whereas blood pressure was normal in the other two patients. Table 2 shows a summary of the imaging findings. We detected high signal intensity in the leptomeningeal zone of the internal carotid artery (ICA) territory on the stent side in all three patients, which we assessed using fluid-attenuated inversion recovery (FLAIR) MRI. In Case 2, high signal intensity was observed in the leptomeningeal zone of the contralateral anterior cerebral artery territory. In this case, the contralateral proximal segment of the anterior cerebral artery (A1) was hypoplastic; therefore the contralateral anterior cerebral artery was assessed on the stent side using carotid angiography. The high signal intensity in the leptomeningeal zone on FLAIR MRI disappeared as the symptoms improved. In all three patients, no ischemic lesions or hyperperfusion was observed on diffusion-weighted imaging (DWI) or SPECT, respectively.
Table 1.
Time course of the clinical symptoms after carotid artery stenting.
| Patient No. | 1 | 2 | 3 | |
|---|---|---|---|---|
| Neurological symptoms | Onset time after CAS (hours) | 11.2 | 11 | 10.30 |
| Improvement time after CAS (hours) | 30 | 35 | 40 | |
| Fever (°C) | 39.1 | 38.9 | – | |
| Seizure | – | – | + | |
| Hypertension (mmHg) | 162/80 | – | – | |
Table 2.
Summary of the radiological findings.
| Patient No. | 1 | 2 | 3 | |
|---|---|---|---|---|
| High signal intensity in FLAIR MRI | Sites of the lesion (Leptomeningeal zone) | Ipsilateral MCA territory | Ipsilateral MCA and Bilateral ACA territory | Ipsilateral MCA territory |
| Onset time after CAS (hours) | 24 | 20 | 21 | |
| Disappearance time (hours) | 48 | 39 | 41 | |
Case Presentation
Case 1
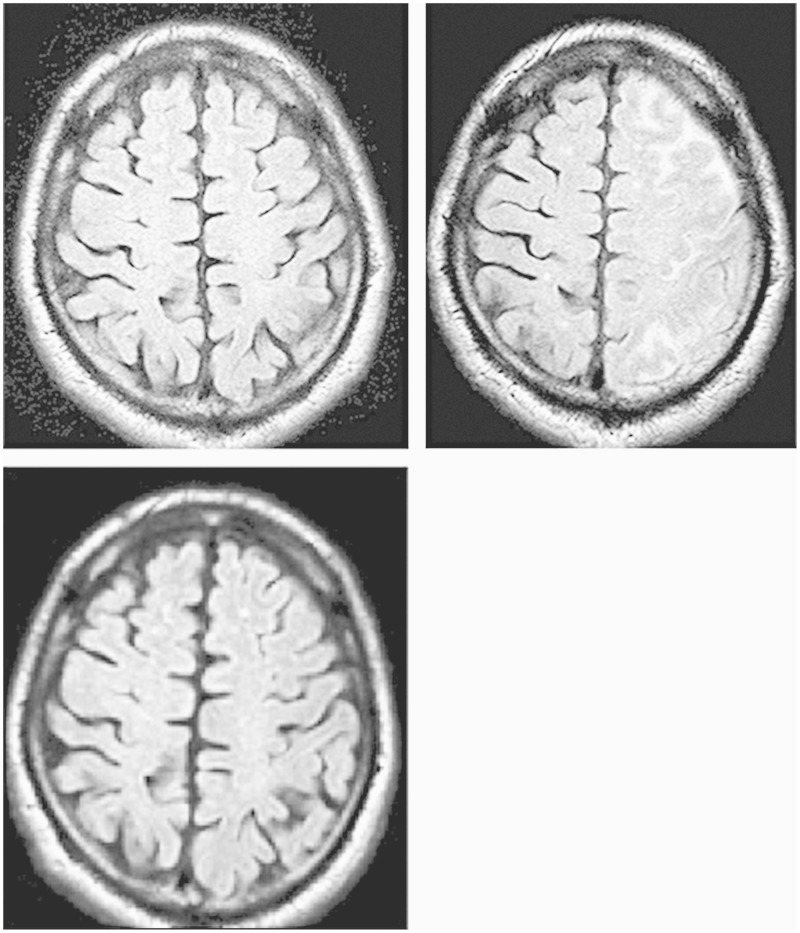
A 68-year-old man visited the emergency department of our hospital because of vertigo. He showed moderate segmental stenosis of the common carotid artery (CCA) on cervical ultrasonography (US) and was promptly hospitalized. The patient had a history of hypertension and hyperlipidemia, which were being treated medically. Neurological findings were normal on admission. The stenotic lesion showed high signal intensity on black blood MRI (BB-MRI) and decreased intensity on carotid US, suggesting a fragile plaque. Lipid-rich necrotic cores have high signal intensity on T1-weighted images during BB-MRI.1 Cerebral angiography detected moderate stenosis of the carotid artery and showed that the plaque was a short lesion localized in the CCA. Cerebral angiography revealed 65% stenosis according to NASCET criteria with an ICA/CCA diameter of 5.46/7.03 mm. The minimum lumen diameter (MLD) of the lesion was 1.05 mm. SPECT using n-isopropyl-123I-p-iodoamphetamine (IMP SPECT) revealed normal cerebral blood flow (CBF) at rest and normal vessel activity after an acetazolamide challenge test, suggesting a low risk of hyperperfusion after CAS. The patient strongly desired endovascular treatment, and given that the procedure appeared to have low risk, endovascular treatment was performed for CAS. Under local anesthesia, a 6-mm-diameter Angioguard XP system (Cordis; Johnson & Johnson, Warren, NJ, USA) was used for distal filter protection, and CAS was performed using the 10 mm × 4 cm PRECISE system (Cordis; Johnson & Johnson, Warren, NJ, USA) without pre-dilatation. After stenting, balloon angioplasty was performed only once post-dilatation using a Sterling balloon dilatation catheter (diameter, 6 mm; length, 30 mm; Boston Scientific, Natick, MA, USA) with 6 atm and an occlusion time of 30 s. Neurological examination during the procedure showed no abnormalities. Near-infrared spectroscopy (NIRS) showed regional cerebral oxygen saturation of <10% three minutes after reperfusion, indicating no evidence of hyperperfusion.2 No apparent debris was observed in the retrieved filter. After CAS, the patient’s condition was favorable with no neurological deficits in the intensive care unit. However, 11 h 20 min later, the patient’s condition deteriorated and he experienced aphasia. His vital signs included fever (up to 39°C) and blood pressure of 162/80 mm Hg. We suspected hyperperfusion syndrome, and diltiazem was immediately administered intravenously to keep the patient normotensive. However, there were no abnormal findings indicating distal embolism on DWI, such as a low apparent diffusion coefficient (ADC) of water (Figure 1A) or hyperperfusion on 99 mTc-ethyl cysteinate dimer (99 mTc-ECD) SPECT, both of which were performed promptly after symptom onset. The symptoms persisted so we performed cerebral angiography 15 h after stenting, which revealed no evidence of occlusion or thrombosis of the stent or ICA. However, FLAIR MRI performed 24 h after CAS detected significantly high signal intensity in the leptomeningeal zone of the ipsilateral cerebral hemisphere on the stent side (Figure 1B), with no bleeding on head CT. The patient’s symptoms gradually improved with blood pressure reduction and propofol sedation, and resolved completely 30 h later. The high signal intensity on FLAIR MRI also resolved completely 48 h after CAS (Figure 1C). IMP SPECT performed five days after CAS showed no indication of hyperperfusion.
Figure 1.
Case 1. A) Postprocedural fluid-attenuated inversion recovery (FLAIR) magnetic resonance (MR) image showing no abnormal findings after 11 h. B) A dense hyperintense region in the leptomeningeal zone after 24 h. C) Disappearance of the hyperintense lesions after 48 h.
Case 2
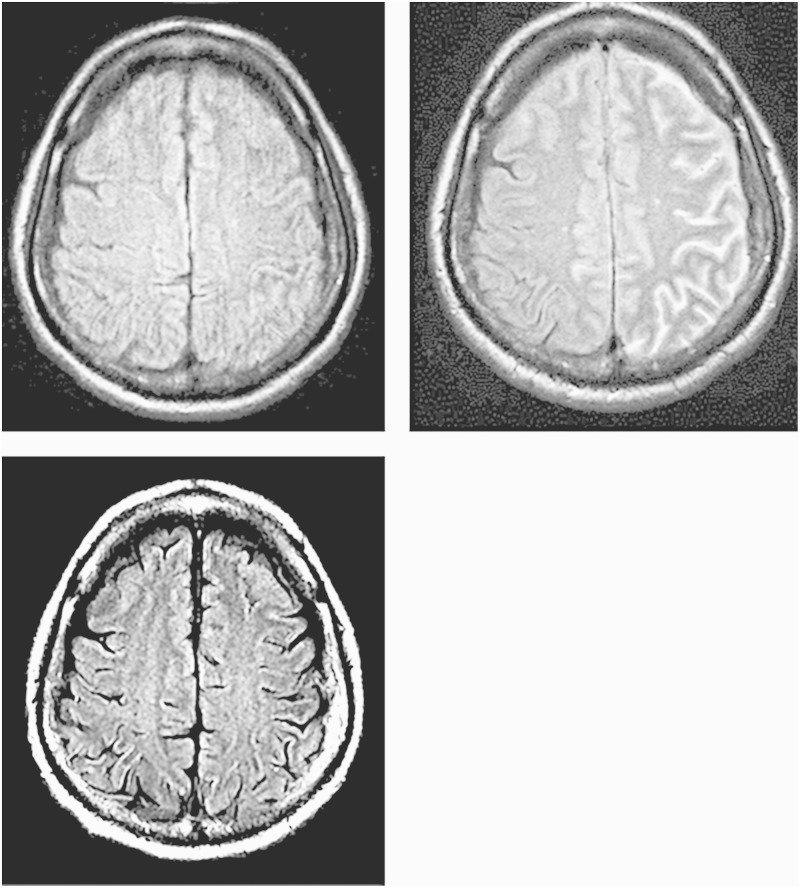
A 62-year-old man was assessed at the outpatient department after a diagnosis of left carotid artery stenosis. He was admitted to our department because of progression of the ICA stenosis, which was assessed by carotid US. The patient had a history of hypertension, diabetes mellitus and angina, and had received a coronary artery bypass graft. On admission, his neurological condition was normal. T1-weighted BB-MRI showed a slightly high signal intensity and calcification was observed on neck CT. Cerebral angiography revealed 77% stenosis according to NASCET criteria with an ICA/CCA diameter of 4.57/6.61 mm. The MLD of the lesion was 1.05 mm, and the contralateral anterior cerebral artery was assessed on the stent side via carotid angiography because of hypoplasia of the contralateral A1. IMP SPECT revealed normal CBF at rest and normal vessel activity after an acetazolamide challenge test, suggesting a low risk of hyperperfusion after CAS. Because of the indicated treatment criteria for asymptomatic carotid artery stenosis, CAS was performed to manage the progression of the stenosis using a 190-cm FilterWire EZ (Boston Scientific, Natick, MA, USA) and a 10 × 24 mm carotid WALLSTENT Monorail stent (Boston Scientific, Natick, MA, USA). Immediately after the procedure, the patient was neurologically normal, and no abnormal findings suggestive of hyperperfusion were observed on NIRS. Mental deterioration, aphasia, and right hemiparesis developed 11 h after CAS, with a fever of 38.9°C. His blood pressure was normal at 132/65 mmHg. MRI was performed 13 h after CAS and showed no abnormal findings, such as fresh infarcts on DWI or a low ADC of water (Figure 2A). Neither cerebral vasospasm nor occlusion of the stent was observed during immediate cerebral angiography. However, FLAIR MRI 20 h after CAS detected high signal intensity in the leptomeningeal zone of the ICA territory on the stent side and the contralateral anterior cerebral artery territory, which were perfused by the stent-side ICA (Figure 2B). No bleeding was observed on head CT at that time. ECD SPECT was performed 21 h after CAS and showed an absence of hyperperfusion. Although there was no suggestion of fresh infarcts on DWI, we administered intravenous heparin and edaravone, a free radical scavenger used to treat cerebral infarction that is available only in Japan. The patient’s symptoms gradually improved and resolved completely 35 h after CAS. The high signal intensity detected in the leptomeningeal zone by FLAIR MRI also resolved 39 h after CAS, with resolution of the symptoms (Figure 2C).
Figure 2.
Case 2. A) Postprocedural FLAIR MR image showing no abnormal findings after 11 h. B) A dense hyperintense region in the leptomeningeal zone after 20 h. C) Disappearance of the hyperintense lesions after 35 h.
Case 3
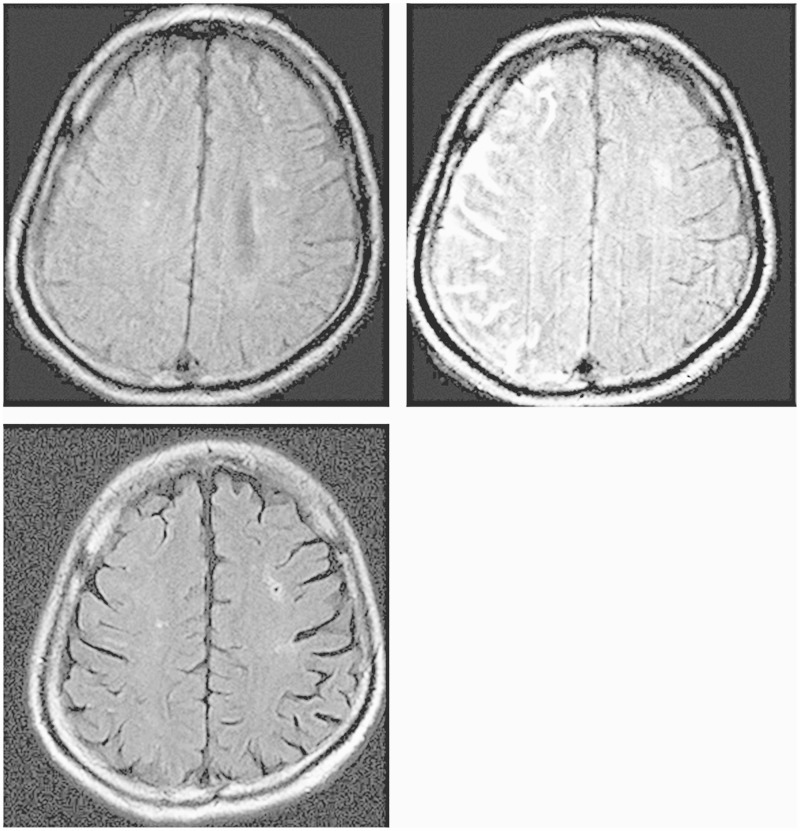
A 63-year-old man was admitted for treatment of asymptomatic right carotid artery stenosis. He had a history of CAS for symptomatic left carotid artery stenosis and iliac artery/femoral artery grafting for arteriosclerotic obliterans of the right leg. He was neurologically normal on admission. MRI revealed old cerebral infarcts in the right cerebellum and the left corona radiata. IMP SPECT showed normal CBF in each hemisphere at rest and normal vessel activity after an acetazolamide challenge test. Cerebral angiography showed that the stenosis was located mainly in the ICA and that its extent was 50% according to NASCET criteria. The MLD of the lesion was 2.39 mm with an ICA/CCA diameter of 5.40/10.50 mm. BB-MRI detected a stable plaque with no calcification. The patient had a history of cerebral infarction and strongly desired treatment. Therefore, he was a candidate for CAS. Bilateral arteriosclerotic obliterans was present with an aberrant right subclavian artery. Therefore, we made a direct puncture in the right CCA upon exposure, and CAS was performed using the 5-mm Angioguard XP system as a protective device and the 10 mm × 4 cm PRECISE system as a stent. After surgery, the patient was in favorable condition without neurological deficits; immediate post-operative NIRS showed no findings suggestive of hyperperfusion. However, left hemiparesis and left hemispatial neglect developed 10 h 30 min after CAS, followed by a focal seizure from the left side of the face to the left arm which was treated with intravenous diazepam. MRI, including DWI and determination of the ADC of water, and MR angiography were immediately performed and showed no abnormal findings including fresh infarcts or vessel occlusion (Figure 3A). Cerebral angiography showed no evidence of acute occlusion or stent thrombosis. Hexamethylpropyleneamineoxime (HMPAO) SPECT and transcranial Doppler revealed no evidence of hyperperfusion. However, the symptoms persisted; therefore, we started intravenous administration of edaravone 18 h after CAS. FLAIR MRI performed 21 h after CAS revealed high signal intensity in the leptomeningeal zone of the ICA territory on the stent side (Figure 3B). The symptoms improved, and the high signal intensity on FLAIR MRI disappeared 40 h after CAS (Figure 3C).
Figure 3.
Case 3. A) Postprocedural FLAIR MR image showing no abnormal findings after 12 h. B) Adense hyperintense region in the leptomeningeal zone after 18 h. C) Disappearance of the hyperintense lesions after 40 h.
Discussion
We observed three patients who developed neurological symptoms over a period of ten hours after CAS and who improved within two days. None of the three patients showed signs of fresh infarctions on DWI or hyperperfusion on SPECT. However, high signal intensity was observed in the leptomeningeal zone of the cerebral hemisphere on the stent side in all three patients and in the leptomeningeal zone of the contralateral anterior cerebral artery territory in one patient as assessed by FLAIR MRI. The high signal intensity in the leptomeningeal zone disappeared as the symptoms improved. Based on these findings, we consider that a hemodynamic change occurred in the hemisphere on the stent side after stenting in all three patients and in the contralateral anterior artery territory in one patient. We suggest that this hemodynamic change caused vasoparalysis of local vessels, leading to vasogenic edema.
Various complications after CAS have been reported, such as ischemic stroke due to distal embolism, stent occlusion caused by thrombosis or plaque prolapse, hyperperfusion syndrome, and effects related to carotid body reflexes.2–4 Among these complications, embolic events are related to the properties of the plaque in the carotid stenosis (i.e., the presence of soft or hard plaques) and the manner in which the catheter and the device are handled. A soft plaque was observed in one patient; however, there were neither catheter- nor device-related problems during stenting nor complications such as fresh infarctions on DWI and ADC of water, or cerebral vasospasms or stent occlusions on cerebral angiography. Therefore, we excluded the possibility of embolism or vessel occlusion during symptom development.
Hyperperfusion syndrome is a rare but well-known complication of carotid endarterectomy or CAS.2,5 Its symptoms include headache, vomiting, and neurological disorders, which are relieved in many cases by blood pressure management, although serious symptoms may persist because of bleeding in some cases.6 This presentation is similar to that in our patients with respect to the late onset of symptoms and their transient duration; however, our patients did not exhibit hyperperfusion on SPECT. Thus, hyperperfusion syndrome was excluded in our patients based on these findings.
To assess the clinical conditions associated with transient symptoms, it is necessary to differentiate the following clinical pathologies: adverse effects associated with the contrast media and reversible posterior leukoencephalopathy syndrome (RPLS)/posterior reversible encephalopathy syndrome (PRES).7 Shinoda et al. and Uchiyama et al. reported cases of adverse events after cerebral angiography associated with the use of a nonionic contrast medium8,.9 Previous studies have demonstrated abnormal enhancement of the cerebral cortex on CT, which was thought to have occurred via the disruption of the blood–brain barrier caused by nonionic contrast medium.8,9 In our patients, there were neither allergic reactions according to cerebral angiography findings nor areas of abnormal density in the cortex of the cerebral hemisphere according to CT findings. RPLS/PRES is a clinical entity in which the pathophysiology is related to hypertensive encephalopathy, eclampsia, collagen disease, severe infectious disease, or the administration of certain drugs such as immunosuppressive agents, anticancer drugs, or antiviral drugs.7,10 This pathology is associated with hypertension in 50–70% of cases.7 The most common clinical symptoms and signs are headache, seizures, mental confusion, and visual perception abnormalities, which are always reversible. Lesions appear mainly in the white matter of the occipital lobe and the parietal lobe. The detection of high-to-low signal intensity on DWI and high signal intensity on the ADC map are characteristic of subcortical edema without infarction.10 In this entity, the clinical and neuroimaging findings disappear within a few days to two weeks.4 In our three cases, the presentations were similar to RPLP/PRES with respect to the clinical features, but differed because the lesions were located in the leptomeningeal zone rather than in the white matter. These patients also had preferential sites and lesions were located in the unilateral ICA territory as assessed by neuroimaging.
We found FLAIR hyperintensity in the subarachnoid space without gadolinium administration in the three cases. Martin et al. studied the FLAIR sequences of MR imaging following CAS and enhancement of the subarachnoid space has been noted frequently in previous series.11 In one instance, FLAIR hyperintensity was found in the subarachnoid space prior to gadolinium administration. Similar findings have also been observed on post-contrast FLAIR following balloon occlusion.12 Köhrmann et al. analyzed cerebrospinal fluid samples of patients with the hyperintense acute reperfusion marker (HARM) on imaging with respect to the presence and concentration of gadolinium-based contrast agents and verified that the imaging phenomenon HARM was caused by leakage of gadolinium-based contrast agents into the cerebrospinal fluid.13 It is important to know whether gadolinium is administered prior to MR imaging; even when gadolinium is administered several days prior to MR imaging, delayed enhancement may be seen14 with HARM.15
The characteristics of our three cases were as follows: late-onset neurological symptoms developed after CAS and improved within a few days, while high signal intensity was observed in the leptomeningeal zone by FLAIR MRI after symptom onset and disappeared as the symptoms improved. The pathophysiology underlying this condition may be a hemodynamic change, but hyperperfusion did not occur after CAS; disruption of microcirculation autoregulation in the ipsilateral cerebral hemisphere followed endothelial damage and injury to the blood–brain barrier. A recent study of disruption of the blood–brain barrier owing to hemodynamic changes caused by reperfusion showed that matrix metalloproteinases were involved with the basal lamina, resulting in subsequent damage to the ultrastructure of the microvasculature.16,17 Additionally, injury to the microvasculature may occur by endothelial activation, excessive production of active oxygen species, inflammatory reaction, leukocytic upward titration, cytokine production, and edema formation.16,17 We used edaravone as a free radical scavenger in our cases, which might have contributed to the favorable results and may be a valid treatment for preventing the pathological processes in patients with similar symptoms.
Conclusion
We observed a rare complication after CAS in three patients. The pathophysiology might have been due to a hemodynamic change in the hemisphere on the stent side that caused vasoparalysis in the microvasculature of the leptomeningeal zone, leading to disruption of the blood–brain barrier. This may explain why the clinical symptoms and neuroimaging findings were transient and resolved completely within two days. To elucidate the pathological mechanism underlying this complication, it will be necessary to study additional cases and further investigate the details of this clinical condition.
Acknowledgement
The authors thank Dr Takeo Fukushima, Professor Emeritus at Fukuoka University, who served as a scientific advisor and critically reviewed this study proposal
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Yuan C, Mitsumori LM, Ferguson MS, et al. In vivo accuracy of multispectral magnetic resonance imaging for identifying lipid-ridge necrotic cores and intraplaque hemorrhage in advanced human carotid plaques. Circulation 2001; 104: 2051–2056doi: 10.1161/hc4201.097839. [DOI] [PubMed] [Google Scholar]
- 2.Matsumoto S, Nakahara I, Higashi T, et al. Near-infrared spectroscopy in carotid artery stenting predicts cerebral hypertension syndrome. Neurology 2009; 72: 1512–1518doi: 10.1212/WNL.0b013e3181a2e846. [DOI] [PubMed] [Google Scholar]
- 3.Bates ER, Babb JD, et al. American College of Cardiology Foundation, American Society of Interventional & Therapeutic Neuroradiology, Society for Cardiovascular Angiography, Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology. ACCF/SCAI/SVMB/SIR/ASTIN 2007 clinical expert consensus document on carotid stenting: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol 2007; 49: 126–170doi: 10.1016/j.jacc.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 4.Wilkinson ID, Griffiths PD, Hoggard N, et al. Unilateral leptomeningeal enhancement after carotid stent insertion detected by magnetic resonance imaging. Stroke 2000; 31: 848–851doi: 10.1161/01.STR.31.4.848. [DOI] [PubMed] [Google Scholar]
- 5.Karapanayiotides T, Meuli R, Devuyst G, et al. Postcarotid endoarterectomy hyperperfusion or reperfusion syndrome. Stroke 2009; 25: 21–26. [DOI] [PubMed] [Google Scholar]
- 6.Sundt TM, Sharbrough FW, Piepgras DG, et al. Correlation of cerebral blood flow and electroencephalographic changes during carotid endarterectomy: with results of surgery and hemodynamics of cerebral ischemia. Mayo Clin Proc 1981; 56: 533–543. [PubMed] [Google Scholar]
- 7.Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med 1996; 22: 494–500doi: 10.1056/NEJM199602223340803. [DOI] [PubMed] [Google Scholar]
- 8.Shinoda J, Ajimi Y, Yamada M, et al. Cortical blindness during coil embolization of an unruptured intracranial aneurysm – case report. Neurol Med Chir (Tokyo) 2004; 44: 416–419doi: 10.2176/nmc.44.416. [DOI] [PubMed] [Google Scholar]
- 9.Uchiyama Y, Abe T, Hirohata M, et al. Blood brain-barrier disruption of nonionic iodinated contrast medium following coil embolization of a ruptured intracranial aneurysm. Am J Neuroradiol 2003; 25: 1783–1786. [PMC free article] [PubMed] [Google Scholar]
- 10.Ay H, Buonanno FS, Schaefer PW, et al. Posterior leukoencephalopathy without severe hypertension: utility of diffusion-weighted MRI. Neurology 1998; 51: 1369–1376doi: 10.1212/WNL.51.5.1369. [DOI] [PubMed] [Google Scholar]
- 11.Martin AJ, Saloner DA, Roberts TPL, et al. Carotid Stent Delivery in an XMR Suite: Immediate Assessment of the Physiologic Impact of Extracranial Revascularization. Am J Neuroradiol 2005; 26: 531–537. [PMC free article] [PubMed] [Google Scholar]
- 12.Michel E, Liu H, Remley KB, et al. Perfusion MR neuroimaging in patients undergoing balloon test occlusion of the internal carotid artery. Am J Neuroradiol 2001; 22: 1590–1596. [PMC free article] [PubMed] [Google Scholar]
- 13.Köhrmann M, Struffert T, Frenzel T, et al. The hyperintense acute reperfusion marker on fluid-attenuated inversion recovery magnetic resonance imaging is caused by gadolinium in the cerebrospinal Fluid. Stroke 2012; 43: 259–261doi: 10.1161/STROKEAHA.111.632356. [DOI] [PubMed] [Google Scholar]
- 14.Ogami R, Nakahara T, Hamasaki O, et al. Cerebrospinal fluid enhancement on fluid attenuated inversion recovery images after carotid artery stenting with neuroprotective balloon occlusions: hemodynamic instability and blood-brain barrier disruption. Cardiovasc Intervent Radiol 2011; 34: 936–941doi: 10.1007/s00270-010-0035-4. [DOI] [PubMed] [Google Scholar]
- 15.Warach S, Latour LL. Evidence of reperfusion injury, exacerbated by thrombolytic therapy, in human focal brain ischemia using a novel imaging marker of early blood-brain barrier disruption. Stroke 2004; 35: 2659–2661doi: 10.1161/01.STR.0000144051.32131.09. [DOI] [PubMed] [Google Scholar]
- 16.Jean WC, Spellman SR, Nussbaum ES, et al. Reperfusion injury after focal cerebral ischemia: the role of inflammation and the therapeutic horizon. Neurosurgery 1998; 43: 905–911doi: 10.1097/00006123-199812000-00076. [DOI] [PubMed] [Google Scholar]
- 17.Latour LL, Kang DW, Ezzeddine MA, et al. Early blood-brain barrier disruption in human focal brain ischemia. Ann Neurol 2004; 56: 468–477doi: 10.1002/ana.20199. [DOI] [PubMed] [Google Scholar]