Abstract
This paper aimed to evaluate the effect of preoperative transarterial embolization (TAE) on estimated blood loss (EBL) during surgical excision of the vertebral tumors. Three hundred and forty-eight patients with spinal tumors were retrospectively analyzed. The preoperative TAE group consisted of 190 patients and the control group consisted of 158 patients. Gelatin sponge particles mixed withy contrast agent were used in the TAE group to embolize the tumor-feeding artery. The factors evaluated included: the time interval between embolism and surgery; the number of vertebrae involved by the tumor; pathological type of tumor; surgical approach; extent of excision and instrumental fixation. The time interval (P = 0.4669)between embolism and surgery had no significant correlation with EBL during surgery. The pathological diagnosis of vertebral tumor such as plasma cell myeloma, giant cell tumor, chondrosarcoma, hemangioma and metastasis had no significant correlation with EBL between the TAE group and control group during surgery, while the EBL of chordoma in the TAE group was significantly higher than that in the control group (p = 0.0254). The number of vertebrae involved (p = 0.4669, 0.6804, 0.6677), posterior approach (p = 0.3015), anterior approach (p = 0.2446), partial excision (p = 0.1911) and instrumental fixation (p = 0.1789) had no significant correlation with EBL during surgery between the TAE group and the control group. This study showed that preoperative TAE of the spinal tumor had no significant effect on intra-operative blood loss during surgical excision of the spinal tumor. In view of the risk of embolism, this method should be carefully considered.
Keywords: Spine, tumor, embolism, estimated blood loss
Introduction
Spinal tumors may often cause pain, mechanical instability and even neurological complications such as radiculopathy and compressed myelopathy, progressive paresthesia, etc. Tumor excision followed by reconstruction and stabilization of the spine is generally used to treat the spine tumor. However, excessive intra-operative blood loss may cause spinal cord injury or lead to incomplete tumor removal. Preoperative transarterial embolization (TAE) of the spinal tumor may reduce intra-operative blood loss and make the surgery easier and safer.1,2 This paper aimed to evaluate the effect of preoperative TAE on intra-operative blood loss.
Methods
Three hundred and forty-eight patients presenting with spinal tumors between March 2004 and September 2010 were retrospectively analyzed in this study. The preoperative TAE group consisted of 190 patients and the control group consisted of 158 patients.
TAE group
The preoperative TAE group consisted of 190 patients, 109 males and 81 females. Their ages ranged from 11 to 76 years, with a mean age of 45.6 years.
Lesion locations were: T1 1X, T4 5X, T5 7X, T6 2X, T7 5X, T8 5X, T9 3X, T10 12X, T11 5X, T12 5X, L1 5X, L2 7X, L3 7X, L4 5X, L5 7X, T3–41X, T4–51X, T3–51X, T5–63X, T5–71X, T6–71X, T7–82X, T7–92X, T8–91X, T9–102X, T10-L22X, T11–125X, T11-L11X, T3, T91X, T12-L11X, T12-L22X, L1–21X, L1–41X, L2–32X, L3–42X, L2–41X, L2–51X, L2-S41X, L3–51X, L4–S11X, L5–S11X, L5-S22X, L5-S43X, L5-S33X, S15X, S22X, S41X, S1–211X, S1–315X, S1-48X, S1-57X, S2-43X, S2-57X, S3-52X, S4-51X. X here means case(s).
The time interval was defined as days between TAE and surgery. Eight patients underwent surgery after less than 24 hours, 118 patients underwent surgery between 24 hours and 48 hours, 45 patients were between 48 hours and 72 hours 19 patients had surgery after more than 72 hours.
The pathological result of tumor was: aneurysmal bone cysts (5 cases), peripheral neuroectodermal tumor (15 cases), neurilemmoma (14 cases), malignant fibrous histiocytoma (3 cases), giant cell tumor of bone (19 cases), chordoma (22 cases), plasma-cell myeloma (11 cases), chondrosarcoma (8 cases), hemangioma (19 cases), metastatic tumor (55 cases), giant cell tumor of bone with aneurysmal bone cyst (6 cases), osteosarcoma (4 cases), lymphoma (5 cases), liposarcoma (1 case), bone cysts (2 cases) and spinal tuberculosis (1 case).
One hundred and twenty patients underwent the posterior operative approach, 30 patients underwent the posterior-lateral operative approach, 12 patients underwent the anterior operative approach, four patients underwent the anterior-lateral operative approach, 24 patients with the anterior combined with posterior operative approach. On hundred and eighty-one patients had partial excision and nine patients full excision; 184 patients had instrument internal fixation whereas six patients did not.
Control group
The control group consisted of 158 patients, 91 males and 67 females aged between 21 and 75 years, with a mean age of 50.1 years.
Lesion locations were: T1 8X, T2 7X, T3 9X, T4 3X, T5 4X, T6 3X, T7 5X, T81X, T91X, T10 2X, T11 4X, T12 4X, L1 5X, L2 3X, L3 5X, L4 4X, L5 4X, L1–31X, L2–33X, L3-41X, L4–51X, L5-S13X, L5–S31X, L3–51X, T1–31X, T1–22X, T4–61X, T5–63X, T4–52X, T7–82X, T8–91X, T9–103X, T10–112X, T11–122X, T11–L11X, C11X, C29X, C36X, C44X, C61X, C73X, C1–22X, C1–51X, C2–41X, C3–41X, C3–51X, C4–52X, C6–72X, C2–T11X, S12X, S32X, S1–25X, S1–35X, S2–42X, S3–41X, S3–52X, S4–52X.
The pathological result of tumor was: peripheral neuroectodermal tumor (2 cases), malignant fibrous histiocytoma (2 cases), giant cell tumor of bone (21 cases), osteosarcoma (5 cases), chordoma (18 cases), plasma cell myeloma (16 cases), chondrosarcoma (4 cases), hemangioma (14 cases), metastases (76 patients).
One hundred and eighteen patients underwent the posterior operative approach, four patients underwent the posterior-lateral operative approach, 16 patients underwent the anterior operative approach, 18 patients underwent the anterior-lateral operative approach, two patients had the anterior combined with posterior operative approach. One hundred and fifty-two 152 patients had a partial excision and six patients a full excision; 141 patients had instrumental fixation whereas 17 patients did not.
Inclusion criteria
Spinal tumor preoperative diagnosis by imaging examination and clinical diagnosis;
Patients had no serious complication, such as hypertension, coronary heart disease or psychiatric history, and no serious allergic history.
Patients had never undergone chemotherapy, radiotherapy or curative tumor resections;
Coagulation and routine blood tests were normal.
- Patients could tolerate the embolism treatment. Exclusion criteria
- After the operation, the pathologic diagnosis of the spinal tumor was unclear.
- The time interval between TAE and surgery was more than one week.
- Intra-operative blood loss could not be judged.
- Patients did not undergo o pre-operative embolism due to a lack of blood supply or other contraindication.
- Patients were unable to undergo surgery, or had an incomplete embolization.
Brief Embolize steps
DSA devices (Siemens Muhistar and TOSHIBA Infinx) were used for guidance. TAE patients were in a supine position. Under local anesthesia via a femoral approach in the groin, a 5F catheter sheath was inserted via femoral artery puncture using the Seldinger technique. Selective bilateral angiography with digital subtraction angiography (DSA) was performed in the affected segment as well as one or two segments above and below. The cobra catheter was inserted into the intercostal artery for thoracic tumors (below T3 segment), the lumbar artery for lumbar tumors, the internal iliac artery and the middle sacral artery for sacral tumors. The contrast agents were injected via a Mark V Plus high-pressure syringe. The total amount of the contrast agents was 6–10 ml, and the injection flow rate was 1–2 ml/s. Embolization was cancelled when it was found that the tumor-feeding artery was connected to the Adamkiewicz artery or anterior spinal artery. When embolization was decided, the gelatin sponge particles (diameter 710--1000um, China) were mixed with 20mml contrast agent (370 mg I/ml Ultravist, Germany) and 20mml 0.9 %NaCl saline, then manually inserted under fluoroscopy until no tumor staining was seen on DSA, which was regarded as a satisfactory embolization result (Figures 1–4).
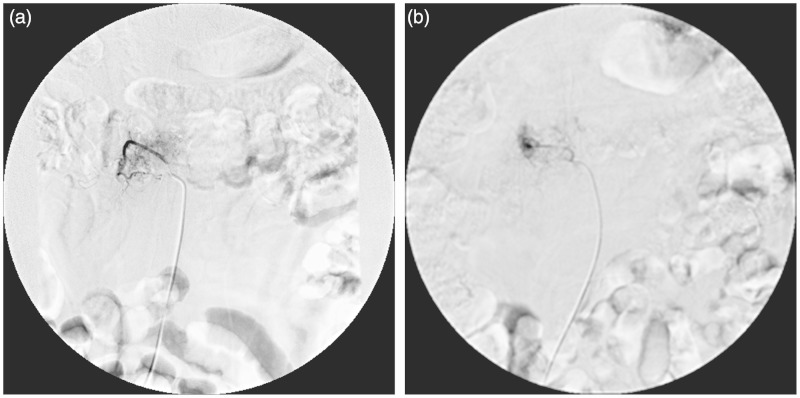
Figure 1.
One patient with an L1 metastatic tumor proved by pathology. Preoperative TAE showed L1 tumor staining supplied form right lumber artery.
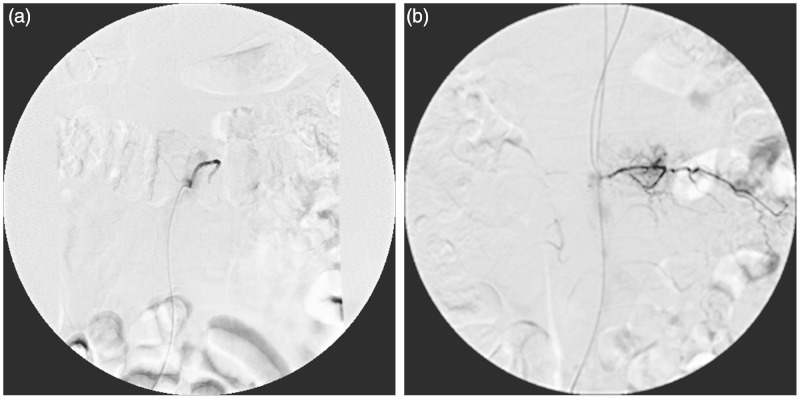
Figure 2.
Preoperative TAE showed L1 tumor staining supplied from left lumber artery.
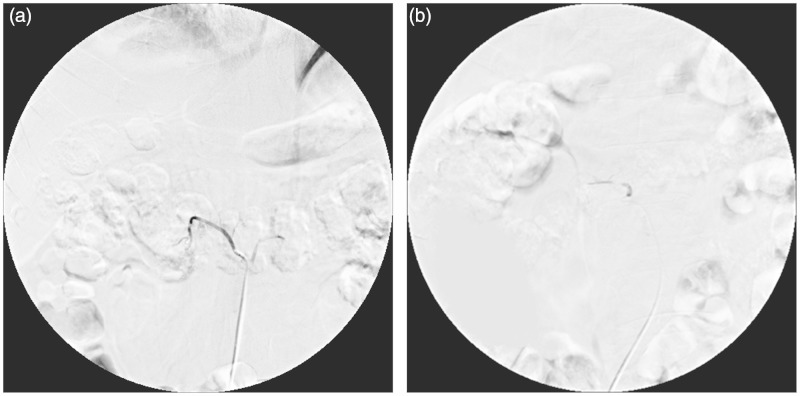
Figure 3.
After TAE the right lumber artery is filled with gelatin sponge particles, tumor staining disappeared on angiography.
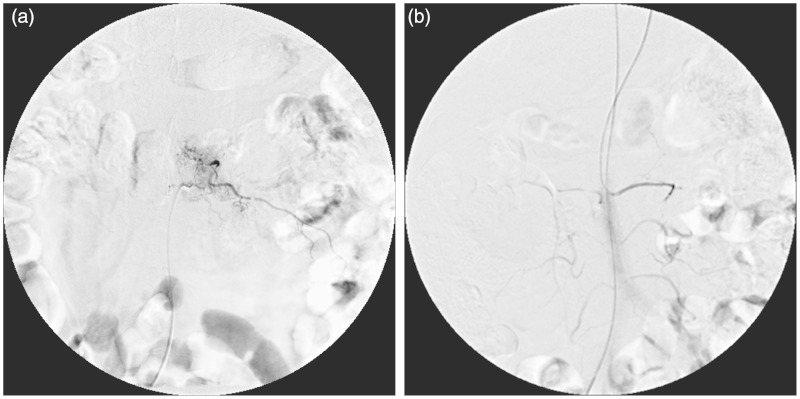
Figure 4.
After the TAE the left lumber artery is filled with gelatin sponge particles, tumor staining disappeared on angiography.
Statistical analysis
Numerical variables which followed normal distribution were analyzed by ANOVA or t-test, non-normal distribution variables were analyzed by Kruskal–Wallis tests or Wilcoxon rank sum test. Statistical analysis was performed using a commercially available software package (SPSS17.0). All tests were two-tailed, and P ≤ 0.05 was considered significant.
Results
The EBL range of the 190 patients in the TAE group was from 100 ml to 8000 ml (median blood loss: 2000.00 ml, Q1∼ Q3:1200.00 ∼ 3000.00) while the EBL range of the 158 patients in the control group was from 100 ml to 7000 ml (median blood loss: 2000.00 ml, Q1∼ Q3:1300.00 ∼ 2500.00 ml). No statistical difference in intra-operative blood loss was found between the two groups (P = 0.1183) (Supplemental Table A).
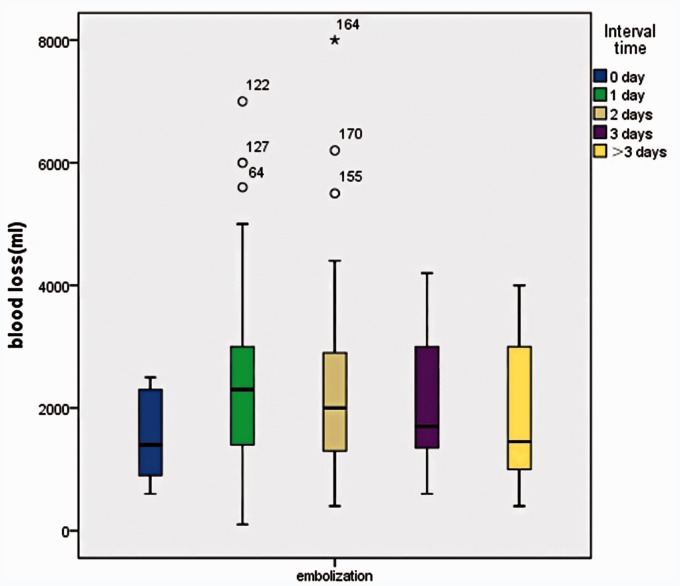
The time interval (no day, one day, two days, three days, more than three days) between embolization with gelatin sponge particles and surgery showed no significant difference (P = 0.4669) (Figure 5).
Figure 5.
Interval time between the TAE and surgery in correlation with EBL. The interval time (0 day, 1 day, 2 days, 3 days, more than 3 days) between embolization with gelatin sponge particles and surgery showed no significant difference.
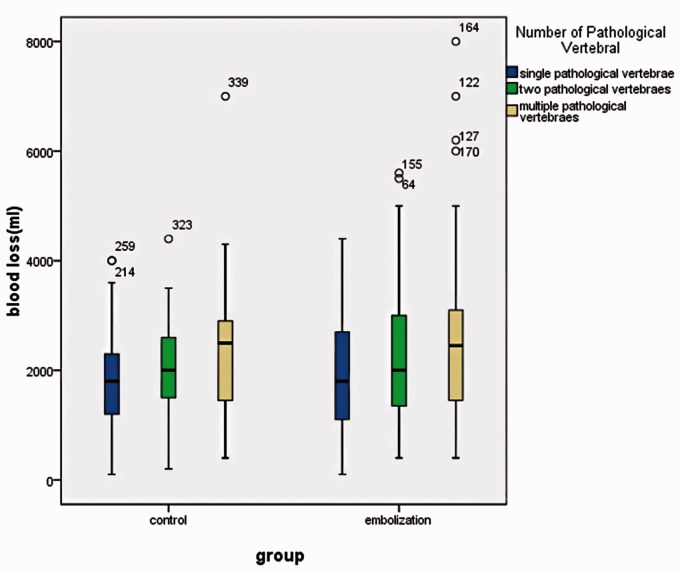
The two groups were analyzed according to the different number of pathological vertebral segments (single segment, two segments, and multiple segments). In the TAE group, the intra-operative EBL was 1800 ml (1100 ∼ 2700 ml) in the 86 patients with single pathological vertebrae, 2000 ml (1300 ∼ 3000 ml) in the 39 patients with two pathological vertebrae, and 2500 ml (1500 ∼ 3200 ml) in the 65 patients with multiple ( > =3) pathological vertebrae. In the Control group, the intra-operative EBL was 1800 ml (1200 ∼ 2300 ml) in the 102 patients with single pathological vertebrae, 2000 ml (1500 ∼ 2600 ml) in the 37 patients with two pathological vertebrae, and 2500 ml (1300 ∼ 3000 ml) in the 19 patients with multiple (>=3) pathological vertebrae. No significant difference was found between the two groups (p = 0.4669, p = 0.6804, p = 0.6677) (Figure 6).
Figure 6.
The different number of pathological vertebral segments (single segment, two segments, and multiple segments has no correlation with EBL in the TAE group and the control group.
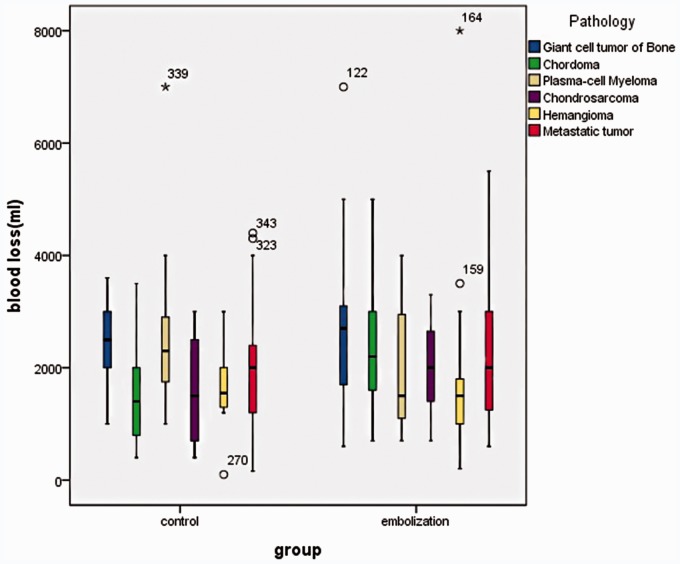
Of the 190 patients, the tumor types feasible for statistical analysis were plasma cell myeloma, giant cell tumor, chondrosarcoma, hemangioma, metastatic tumor and chordoma. For the plasma cell myeloma, giant cell tumor, chondrosarcoma, hemangioma and metastatic tumor, no significant difference was found in intra-operative blood loss in the two groups. The intra-operative blood loss (1400 ml) of the chordoma in the control group was obviously less (2200 ml) than that in the TAE group (p = 0.0254). This may result from the different number of pathological vertebral segments in the two groups. In the TAE group one patient had a single pathological vertebra, two patients had two pathological vertebrae and 19 patients had multiple pathological vertebrae; in the control group eight patients had a single pathological vertebra, five patients had two pathological vertebrae and five patients had multiple pathological vertebrae (Figure 7).
Figure 7.
Different pathological type of vertebral tumor in correlation with EBL during surgery between the TAE group and the control group. The intra-operative blood loss of the chordoma in the control group was obviously less than that in the TAE group.
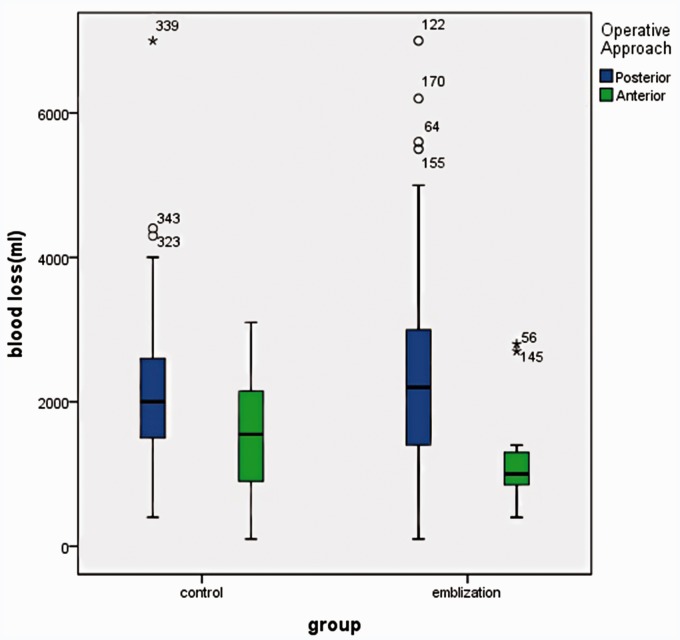
A statistical analysis of the posterior and anterior surgical approach showed was not possible due to the small number of patients. The intra-operative blood loss was 2200.00 ml (1400.00 to 3000.00) with the posterior approach in the TAE group of 120 patients, while it was 2000.00 ml (1500.00 ∼ 2600.00) in the control group of 118 patients. The intra-operative blood loss was 1000.00 ml (850.00 ∼ 1300.00) with the anterior approach in the TAE group of 12 patients, while it was 1550.00 ml (900.00 ∼ 2150.00) in the control group of 16 patients. There was no significant difference between the posterior approach (p = 0.3015) and the anterior approach (p = 0.2446) (Figure 8).
Figure 8.
There was no significant difference in posterior approach and the anterior approach surgery in correlation with EBL during surgery between the the TAE Group and Control Group.
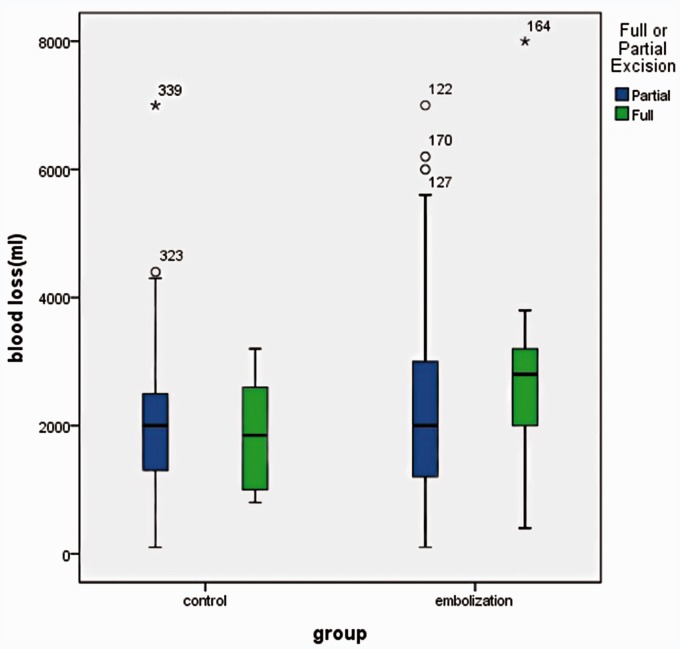
The intra-operative blood loss was 2000.00 ml (1200.00 ∼ 3000.00) for partial excision in the TAE group of 181 patients, while it was 2000.00 ml (1300.00 ∼ 2500.00) in the control group of 152 patients. Two sets of partial excision in correlation with EBL during surgery between the TAE group and the control group showed no significant difference (p = 0.1911) (Figure 9).
Figure 9.
There was no significant difference in partial excision in correlation with EBL during surgery between the TAE group and the control group.
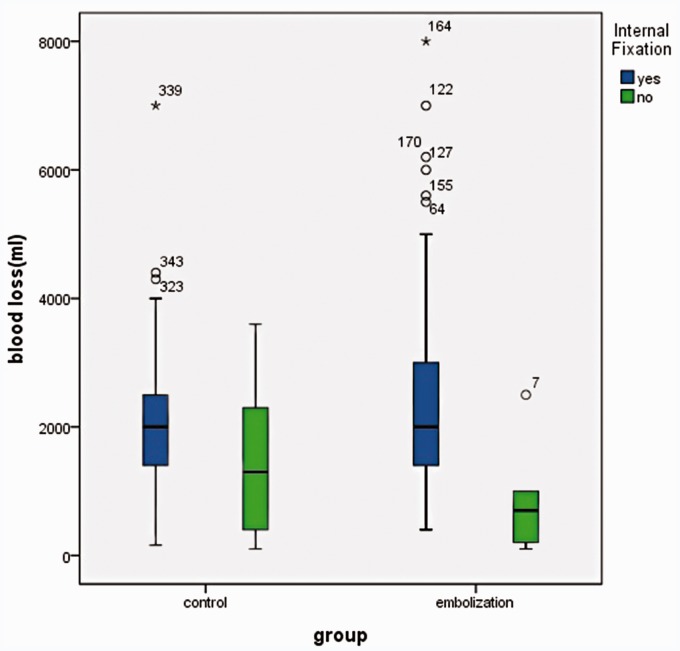
The intra-operative blood loss was 2000.00 ml (1400.00 ∼ 3000.00) for instrument fixation in the TAE group of 184 patients, while it was 2000.00 ml (1400.00 ∼ 2500.00) in the control group of 141 patients. Two sets of instrument fixation in correlation with EBL during surgery between the TAE group and the control group showed no significant difference (p = 0.1789) (Figure 10).
Figure 10.
There was no significant difference in instrument fixation in correlation with EBL during Surgery between the TAE group and the control group.
Discussion
Excessive intra-operative blood loss affects visualization of the operative field during spinal surgery and can lead to prolonged operative time and an increased risk of operative complications. Benati et al. first used TAE to treat spinal vascular malformations and tumors.3 Nowadays, TAE is widely used in the treatment of various spinal hyper-vascular benign tumors, primary tumors and metastatic tumors1,2,4–9. The intra-operative blood loss of the patients with embolization was obviously less than that of the patients without embolization (Supplemental Table B). Ozkan et al. even assumed the TAE could reduce intraoperative blood loss by 30%--50%1. However, some severe bleeding during surgery still occurred after preoperative TAE. Some factors may affect the result of intra-operative blood loss, such as the pathological type of tumor, the size of the tumor, the infiltrating range, and the complexity of surgery.10
TAE agents can be temporary and permanent. For permanent embolization, non-target embolization could cause neurological complications and skin or muscle necrosis.11 As a temporary embolization agent, the gelatin sponge particles have a protein sponge content and are thought to be a safe and effective embolic agent. The particles are degraded by proteolytic enzymes and absorbed seven days later. We first studied the correlation between EBL and the time interval between TAE and surgery. The time interval between transarterial embolization and surgical excision was grouped into: 0 day (within 24 hours), one day, two days, three days, and more than three days. The statistics showed that no significant difference in intra-operative blood loss was found (P = 0.4669). The result demonstrated that gelatin sponge particles had the same embolization effect within seven days.
Many papers reported that different pathological types of spinal tumors were involved in EBL, including renal cell carcinoma, spinal metastasis, metastatic melanoma, thyroid metastasis, breast metastasis and hepatocarcinoma metastasis, osteosarcoma and chondrosarcoma, plasmacytoma, pheochromacytoma, giant cell tumor, chordoma osteosarcoma, aneurysmal bone cyst, chordoma and hemangioma.1,2,5,9,12–15 In this study, there was no significant difference in EBL of the plasmacytoma, giant cell tumor, chondrosarcoma, hemangioma and metastatic tumor between the two groups. The EBL (1400 ml) of the chordoma in the control group was obviously less than that (2200 ml) in the TAE group (p = 0.0254), which may result from the different number of pathological vertebral segments in the two groups. In the TAE group there was one patient with a single pathological vertebra, two patients with two pathological vertebrae and 19 patients with multiple pathological vertebrae while in the control group there were eight patients with a single pathological vertebra, five patients with two pathological vertebrae and five patients with multiple pathological vertebrae.
Tumor size should be taken into consideration in the evaluation of EBL after embolization.16 The analysis was made according to the number of vertebral segments affected by the tumor (single vertebrae, two vertebrae, multiple vertebrae). The result showed no significant difference among the different vertebral segments in the two groups.
Other reasons affecting the amount of EBL include the surgical approach, laminectomy or en bloc, internal fixation or not.17–21 The surgical approach to excise the vertebral tumor is also in dispute.19–20 Most surgeons think that the posterior approach should be used for tumors located below the S3 level, while tumors located above the S3 level can undergo both an anterior and posterior approach. In our study, there was no significant difference in EBL according to the surgery approach, partial excision vertebral tumors and those that underwent instrument fixation between the TAE group and the control group. Based on our study, TAE has no significant effect on blood loss during spine tumor surgery. The reason maybe that intra-operative blood loss had a lot to do with the surgeon’s experience. The effect of TAE for spinal tumor on other aspects such as tumor size and operation time merit further study.
Acknowledgements
We thank Dr Qiao for data collection, Mrs He for data analysis and Dr Jia for general supervision of the research.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Ozkan E, Gupta S. Embolization of spinal tumors: vascular anatomy, indications, and technique. Tech Vasc Interv Radiol 2011; 14(3): 129–140doi: 10.1053/j.tvir.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Guzman R, Dubach-SS, Heini P, et al. Preoperative transarterial embolization of vertebral metastases. Eur Spine J 2005; 14(3): 263–268doi: 10.1007/s00586-004-0757-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benati A, Dalle Ore G, Da Pian R, et al. Transfemoral selective embolization in the treatment of some cranial and vertebrospinal vascular malformations and tumors. J Neurosurg Sci 1974; 18(4): 233–238. [PubMed] [Google Scholar]
- 4.Yang HL, Zhu LF, Liu JY, et al. Surgical treatment of sacral chordomas with pre-operative arterial embolization and prognostic indicators of outcome. Spine J 2007; 7(5): 34S doi: 10.1016/j.spinee.2007.07.084. [Google Scholar]
- 5.Manke C, Bretschneider T, Lenhart M, et al. Spinal metastases from renal cell carcinoma: effect of preoperative particle embolization on intraoperative blood loss. Am J Neuroradiol 2001; 22(5): 997–1003. [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson MA, Cooke DL, Ghodke B, et al. Retrospective analysis of preoperative embolization of the spinal tumor. Am J Neuroradiol 2010; 31(4): 656–660doi: 10.3174/ajnr.A1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roscoe MW, Mcbroom RJ, Louis E, et al. Preoperative embolization in the treatment of osseous metastases from renal cell carcinoma. Clin Orthop Relat Res 1989; 238: 302–307. [PubMed] [Google Scholar]
- 8.Gellad FE, Sadato N, Numaguchi Y, et al. Vascular metastatic lesions of the spine: preoperative embolization. Radiology 1990; 176(3): 683–686. [DOI] [PubMed] [Google Scholar]
- 9.Smith TP, Gray L, Weinstein JN, et al. Preoperative transarterial embolization of spinal column neoplasms. J Vasc Interv Radiol 1995; 6(6): 863–869doi: 10.1016/S1051-0443(95)71204-0. [DOI] [PubMed] [Google Scholar]
- 10.René S, Gerhard RH, Florian D, et al. Surgical therapy of vertebral metastases. Are there predictive parameters for intraoperative excessive blood loss despite preoperative embolization? Tumori 2011; 97(3): 66–73doi: 10.1700/611.7141. [DOI] [PubMed] [Google Scholar]
- 11.Bhatia KD, Wang L, Parkinson RJ, et al. Successful treatment of six cases of indirect carotid-cavernous fistula with ethylene vinyl alcohol copolymer (Onyx) transvenous embolization. J Neuroophthalmol 2009; 29(1): 3–8doi: 10.1097/WNO.0b013e318199c85c. [DOI] [PubMed] [Google Scholar]
- 12.Rossi G, Rimondi E, Bartalena T, et al. Selective arterial embolization of 36 aneurysmal bone cysts of the skeleton with N-2-butyl cyanoacrylate. Skeletal Radiol 2010; 39(2): 161–167doi: 10.1007/s00256-009-0757. [DOI] [PubMed] [Google Scholar]
- 13.Hosalkar HS, Jones KJ, King JJ, et al. Serial arterial embolization for large sacral giant-cell tumors: mid to long-term results. Spine 2007; 32(10): 1107–1115doi: 10.1097/01.brs.0000261558.94247.8d. [DOI] [PubMed] [Google Scholar]
- 14.Kwan RB, Erasmus AM, Hunn AW, et al. Pre-operative embolisation of metastatic paragon glioma of the thoracic spine. J Clin Neurosci 2010; 17(3): 394–396doi: 10.1016/j.jocn.2009.05.030. [DOI] [PubMed] [Google Scholar]
- 15.Yang H, Zhu L, Ebraheim NA, et al. Surgical treatment of sacral chordomas combined with transcatheter arterial embolization. J Spinal Disord Tech 2010; 23(1): 47–52doi: 10.1097/BSD.0b013e31819630ec. [DOI] [PubMed] [Google Scholar]
- 16.Řehák S, Krajina A, Ungermann L, et al. The role of embolization in radical surgery of renal cell carcinoma spinal metastases. Acta Neurochir 2008; 150(11): 1177–1181doi: 10.1007/s00701-008-0031-5. [DOI] [PubMed] [Google Scholar]
- 17.Sahakitrungruang C, Chantra K, Dusitanond N, et al. Sacrectomy for primary sacral tumors. Dis Colon Rectum 2009; 52(5): 913–918doi: 10.1007/DCR.0b013e3181a0d932. [DOI] [PubMed] [Google Scholar]
- 18.Puri A, Agarwal MG, Shah M, et al. Decision making in primary sacral tumors. Spine J 2009; 9(5): 396–403doi: 10.1016/j.spinee.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Fuchs B, Dickey ID, Yaszemski MJ, et al. Operative management of sacral chordoma. J Bone Joint Surg Am 2005; 87(10): 2211–2216doi: 10.2106/JBJS.D.02693. [DOI] [PubMed] [Google Scholar]
- 20.Hulen CA, Temple HT, Fox WP, et al. Oncologic and functional outcome following sacrectomy for sacral chordoma. J Bone Joint Surgery Am 2006; 88(7): 1532–1539doi: 10.2106/JBJS.D.02533. [DOI] [PubMed] [Google Scholar]
- 21.Prabhu VC, Bilsky MH, Jambhekar K, et al. Results of preoperative embolization for metastatic spinal neoplasms. J Neurosurg 2003; 98(2 Suppl): 156–164. [DOI] [PubMed] [Google Scholar]