Abstract
Endovascular embolization is an important modality in the treatment of brain AVMs. Nowadays staged embolization is the method of choice for the prevention of perioperative hemorrhagic complications.
Current theory suggests that simultaneous occlusion of more than 60% of AVM volume induces significant redistribution local blood flow. That, in turn, may lead to hemorrhage due to AVM rupture. Aside from angiographic findings, there is still no method that predicts the degree of safe partial embolization. Intraluminal measurement of flow velocity and pressure in the vicinity of the AVM nidus might allow detecting the changes in local hemodynamics. That can provide a valuable data and shed the light on the origin of vascular catastrophes.
Ten patients underwent 12 embolization sessions with intraluminal flow velocity and pressure monitoring. The measurements were performed by dual-sensor guidewire. The “Combomap” (Volcano) system with Combowire microguidewires was chosen for measurements, as there is a documented experience of safe use of said guidewires in the cerebral vasculature.
The findings observed during the study matched empirical data as well as the current physiological hypothesis of AVM hemorrhage.
In conjunction with DSA runs, intraluminal flow velocity and pressure monitoring has the potential to become a valuable tool in AVM treatment.
Keywords: Brain AVM, staged embolization, breakthrough hemorrhage
Introduction
Endovascular embolization is a valuable modality in the treatment of brain arteriovenous malformations (AVM). It sometimes serves a palliative or adjuvant role, but often achieves cure as a single method.1,2 The curative role of embolization became more prominent since the appearance of the Onyx (Covidien, Inc. Mansfield, MA, USA) on the market.3 The field grows and changes constantly with more sophisticated endovascular devices being introduced, increasing the reliability and safety of the procedures.
Though minimally invasive, embolization still carries a considerable risk of perioperative complications. Among them hemorrhage is the most common and potentially devastating complication, that plays a significant role in clinical course and long-term prognosis.4–6
The rate of hemorrhagic complications is reported to be approximately 1–5% depending on institution and patient selection.5,7,8 While in some cases the technical aspects of the embolization (e.g. vessel perforation with microcatheter, draining vein occlusion) might cause hemorrhage, rupture can often occur even after technically flawless embolization.
Currently, normal pressure breakthrough phenomenon and occlusive hyperemia are alternative, but not mutually exclusive hypotheses of perioperative hemorrhage biomechanics.9–12 The pathophysiological basis of those ruptures still does not have a reliable evidence-based background. The lack of an animal model of AVM hemorrhage adds to the difficulty of research in this field.
Nowadays, staged embolization is the main method decreasing the risk of hemorrhagic complications.13 Gradual flow occlusion over several months is considered to allow the cerebral vasculature to adapt to the hemodynamic changes, thereby minimizing the hemodynamic stress on the AVM-related vasculature.14–16 Decision-making on the degree of embolization per stage relies on the approximate percentage of the embolized nidus estimated by digital subtraction angiography (DSA). The fact that angiography reflects anatomical rather than hemodynamic changes is the main disadvantage of staging by DSA alone. Therefore, the decision on the permissible embolization volume depends primarily on operator experience, and is thus subjective.
Observation of the intraluminal flow parameters in the vicinity of the AVM should provide some objective criteria on the hemodynamic stress that embolization evokes.17,18 There is an experience of endovascular pressure evaluation in the feeding arteries with simultaneous flow velocity registration by transcranial Doppler (TCD).19 The main flaw of TCD-based assessment is that ultrasound can barely visualize the segments distal to the M1 and A1. Simultaneous pressure and flow pattern evaluation at the same point in place and time might precisely reflect the changes occurring in local flow.
This paper reports our first experience in evaluation of the periprocedural hemodynamic parameters by dual-sensor guidewire.
Patients and Methods
From January 2012 to January 2014, ten patients with brain AVMs underwent 12 embolization sessions with dual-sensor monitoring. We chose the “Combomap” system (Volcano Therapeutics, Inc., Rancho Cordova, CA, USA) with “Combowire” dual-sensor guidewire (Volcano Therapeutics, Inc., Rancho Cordova, CA, USA) for the measurements as there is existing experience on the safe use of “Combowire” guidewires in the cerebral vasculature.20
Patients with Spetzler-Martin I-III AVMs were selected for assessment as these AVMs are the most challenging in terms of decision-making for staged embolization. Informed consent was obtained from every patient. Institutional review board approved the study.
All procedures were performed under conditions of general anesthesia. After establishment of transfemoral arterial access with a 6 F introducer sheath, and placement of the 6 F guiding catheter, an “Echelon” microcatheter (Covidien, Inc. Mansfield, MA, USA) was navigated over the SilverSpeed (Covidien, Inc. Mansfield, MA, USA) guidewire into the arterial feeder chosen for embolization. The guidewire was then exchanged for the “Combowire” probe. Constant irrigation of the microcatheter lumen with heparinized saline was maintained throughout the procedure. The measurements were performed during the session of glue or “Onyx 18” (Micro Therapeutics, Inc. d/b/a ev3 Neurovascular, Irvine, CA, USA) embolization.
The main point of interest was the bifurcation of the feeding artery from the normal vessel (feeder branching point – FBP). The major flow changes should be observed at the said point according to our current understanding of AVM physiology.
In cases where the draining vein was solitary and accessible, the “Combowire” was introduced into the venous drainage site using the same technique as for afferent vessels, through transfemoral venous access with a 6 F introducer sheath and 6 F Envoy (Codman & Shurtleff, Inc, Raynham, MA, USA) or “Chaperon” (MicroVention, Inc. Tustin, CA, USA) guiding catheter. The “Echelon” microcatheter then was left in place to allow the “Combowire” to be introduced in the lumen on demand. To avoid additional risk, in every case the “Combomap” system was used only for data acquisition rather than for decision-making.
Use of dual-sensor monitoring had a variable impact on the length of each procedure. Depending on the difficulty of the afferent and venous site catheterization, the additional procedure time (which includes time for echelon navigation, venous catheterization and measurements themselves) varied from ten minutes to one hour. Duration was longer during the first cases and much less noticeable in the later ones. We associate this with the learning curve of dual-sensor guidewire use.
Results
The data obtained are presented in Table 1. In seven out of 12 cases a decrease in systemic-to distal pressure ratio was observed after embolization: the mean initial value of 1.4 shifted to 1.1.
Table 1.
Data obtained.
| S&M grade | Method and extension of embolization (in % of total volume) | Measurement point (feeder deriving artery and draining vein, if accessible) | MBP*/MDP** mmHg (ratio) |
MFV*** cm/s |
|||
|---|---|---|---|---|---|---|---|
| Pre-op | Post-op | Pre-op | Post-op | ||||
| Pt 1 | II | Onyx, total | RMCA, M4 | 69/41 (1.68) | 72/74 (0.97) | 69 | 25 |
| Pt 2 | III | Histoacryl, embolization of 2 intranidal fistulas | LMCA, M3 | 68/46 (1.47) | 75/56 (1.34) | 45 | 19 |
| Cortical vein draining in the left sigmoid sinus | 15 | 14 | 19 | 22 | |||
| Pt 3 | II | Onyx, total, second stage following partial embolization | LPCA, P2, inf. Temporal a. | 70/64 (1.09) | 78/75 (1.04) | 38 | 15 |
| Left transverse sinus | 32 | 18 | 14 | 5 | |||
| Pt 4 | II | Onyx, partial, 50% | LMCA, M4 | 65/34 (1.91) | 70/26 (2.69) | 66 | 22 |
| Pt 5 | II | Onyx, partial, 60% | RMCA, M4 | 58/69 (0.84) | 69/80 (0.86) | 78 | 53 |
| Pt 6 Stage I | III | Onyx, partial, 40% | RMCA, M2 | 62/34 (1.82) | 76/71 (1.07) | 67 | 22 |
| Pt 6 Stage II | III | Onyx, partial, 75% | RMCA, M2 | 77/58 (1.33) | 61/52 (1.17) | 62 | 10 |
| Pt 7 Stage I | III | Onyx, partial, 60% | LMCA M3 | 74/57 (1.30) | 81/50 (1.6) | 91 | 45 |
| Pt 7 Stage II | III | Onyx, partial 80% | LMCA M3 | 93/65 (1.43) | 83/57 (1.45) | 81 | 35 |
| Pt 8 | II | Onyx subtotal | LMCA, M4 | 72/46 (1,56) | 79/39 (2,02) | 54 | 22 |
| Pt 9 | II | Onyx, partial 60% | LACA A2 | 80/78 (1.02) | 82/86 (0.95) | 108 | 74 |
| Cortical vein draining into the superior sagittal sinus (anterior 1/3) | 26 | 12 | 19 | 9 | |||
| Pt 10 | III | Onyx, partial 80%, second stage following partial embolization | RMCA M3 | 67/48 (1.39) | 69/54 (1.27) | 111 | 79 |
| Cortical vein draining into the superior sagittal sinus (middle 1/3) | 32 | 22 | 13 | 7 | |||
mean blood pressure (arterial line).
mean distal pressure (guidewire sensor).
mean flow velocity (guidewire sensor).
In five cases an increase in systemic-to-distal ratio was observed. Among them in two cases the increase was within decimal range, in two cases (Pt7 and 8) the ratio increased from 1.3 to 1.6 and from 1.56 to 2.02 respectively. In one case we detected a significant (from 1.91 to 2.69) increase in pressure ratio with a simultaneous countercurrent prominent decrease (gradient of 44 cm/s) in flow velocity at the same point. The latter observation is probably related to the flow redistribution. In every single case, flow velocity in the feeder branching point decreased, with a mean difference of 37 cm/s.
When the draining vein was catheterized, a decrease in venous pressure and flow velocity was observed in every case with a mean transoperative difference of 10 mmHg for former pressure and 8.2 cm/s for velocity.
Throughout the series, we did not encounter complications related to the use of the dual-sensor guidewire.
Two patients developed treatment-related complications. In one case (patient 4), the patient developed intraoperative hemorrhage due to nidus perforation with the microcatheter. The hemorrhage was stopped with prompt superselective histoacryl injection. The patient developed transient motor deficiency that resolved over the following month. Though dual-sensor monitoring theoretically can increase the risk of technical complications, our Combowire method cannot evoke nidus perforation. In the other case (patient 6), the patient developed transient sensory deficit in the related dermatomes after each stage. MRI confirmed the ischemia in the region adjacent to the AVM each time. The dual-sensor detected a notable decrease in afferent flow velocity in both sessions as well as significant decrease in Mean/Distal pressure ratio in the first session. These changes might represent the flow redistribution that led to local ischemia.
Illustrative Cases
Case 1 (Patient 2)
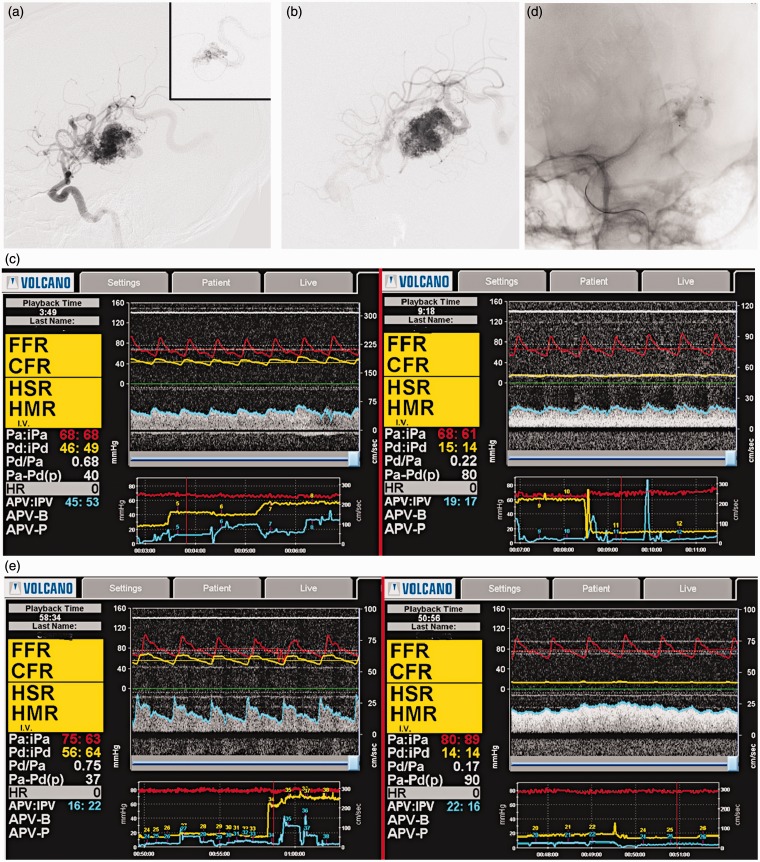
A 31-year-old woman with seizures was diagnosed with a Spetzler-Martin III AVM of the left temporal lobe. She chose endovascular intervention among all the modalities as a first step of treatment. Preprocedural DSA revealed that the feeders of the AVM were the left M3 branches, and the venous drainage went through the cortical veins into the left transverse sinus (Figure 1A). DSA also detected two intranidal fistulae, which were confirmed by superselective angiography. The “Echelon” microcatheter was navigated to the bifurcation of the pedicle from the maternal artery; a second “Echelon” catheter was introduced into the left sigmoid sinus (Figure 1B). The “Combowire” was consequently introduced into the lumens of the afferent artery and the left sigmoid sinus. Figure 1C shows the results obtained during the preoperative measurement. We occluded the fistula with “Histoacryl” (Figure 1D) and repeated the measurement. Figure 1E shows the patterns obtained.
Figure 1.
A) Left carotid injection and superselective DSA with fistula. B) The microcatheter in the draining vein. C) Pre-op measurements in the feeder (left) and drainage (right). D) Post-op DSA with occluded fistulas. E) Post-op measurements in the feeder (left) and drainage (right).
At the arteries, a significant rise in pressure was observed (Table 1). There was also a decrease in pressure and increase in flow velocity in the vein. Though the latter changes might not look considerable, even a small change in pressure and flow is significant in the venous system.
Case 2 (Patient 8)
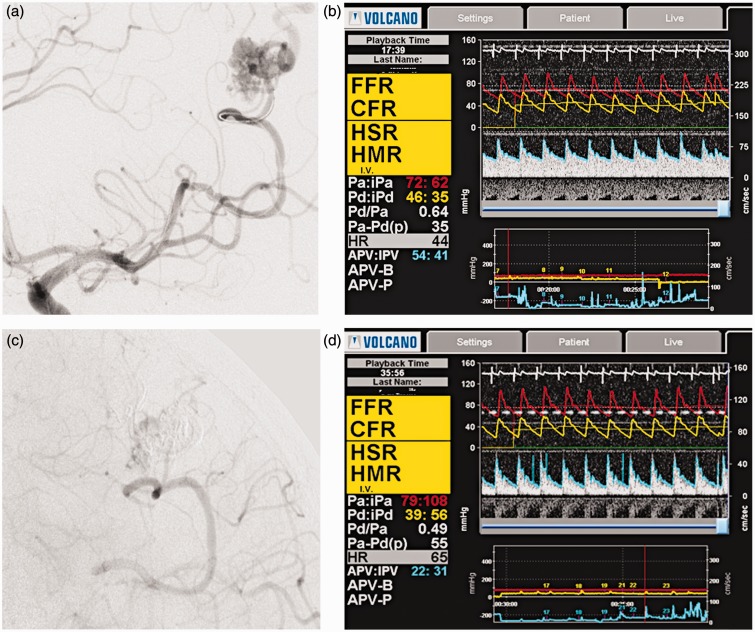
A 38-year-old man with headache and seizures was diagnosed with a Spetzler-Martin I AVM of the left parietal lobe. He chose endovascular embolization as a first step of treatment. Preprocedural DSA revealed that the feeders of the AVM were the left M4 branches, and the venous drainage went through the cortical veins into the superior sagittal sinus (Figure 2A). DSA did not reveal dangerous features and we attempted simultaneous total embolization. The “Echelon” microcatheter was navigated to the bifurcation of the feeder from the normal M4 with subsequent exchange of microguidewires for the Combowire. Subtotal embolization of the AVM nidus was achieved (Figure 2C) and microsurgical resection was planned the following day. Figures 2B and 2D show the results of perioperative monitoring: note the dramatic decrease in flow velocity. While systemic pressure remained approximately the same, there was a significant decrease in distal pressure. We accredit this finding to the flow redistribution after subtotal embolization.
Figure 2.
A) The Combowire in the feeder. B) Pre-operative measurements. C) Left carotid injection reveals residual late filling of the AVM. D) Post-procedural measurement: note the high systolic spikes of the flow velocity.
Discussion
Heidenreich et al.13 proposed the division of embolization into several sessions as a way to avoid hemorrhagic complications. While staged embolization allows AVM to be treated with a lower risk of intracranial bleeding, it increases general intervention and anesthesia-related risks. Thus, we presume that it is extremely important to find a method of hemodynamic assessment which will permit safe staging of the embolization.
As can be seen from Table 1, in most cases there was a significant rise in pressure with a countercurrent drop in flow velocity after embolization of the feeding compartment was performed. However, systemic pressure was stable throughout the sessions. This pattern was observed even after partial embolization of the minor compartments of small-vessel AVMs. The decrease in systemic to distal mean blood pressure ratio observed in most cases as well as the decrease in flow velocity in the feeder branching point denote a disappearance of the shunt-pattern even after partial embolization.
Hademenos et al.21 considered intranidal fistulae a source of significant flow redistribution following embolization. In illustrative case 1, we detected drastic changes in flow and pressure pattern after embolization of both fistulae. Measurements performed in the draining vein showed a disappearance of the arterial flow pattern in the efferent vessel (Figure 1E). Those findings lighten the hemodynamic impact of intranidal fistulae on the shunting function of AVM.
Illustrative case 2 shows dramatic changes. Flow velocity decreased from 54 to 22 cm/s, despite the fact that DSA did not disclose any angiographic “predictors”. The observation indicates the hemodynamic significance of the shunting function even of small low-flow AVMs.
The measurements performed in the draining vein in case 1 disclosed a decrease in pressure and disappearance of the arterial flow pattern after partial embolization, denoting shunt occlusion. This outlines the hemodynamic significance of every single compartment of the AVM.
Overall, the data gathered with the dual-sensor guidewire in each case match current theories on the AVM hemodynamic.
Conclusion
The risk of hemorrhagic complications is a serious limitation to endovascular treatment of AVMs. Staged embolization helps to decrease the rate of such complication. However, the permissible extension of each session is not always clear, especially with small AVMs. To achieve more precision in predicting such complications for reliable risk stratification, more data on the flow in AVM-related vessels is required. The possibility to introduce the “Combowire” into second to third order vessels will disclose flow changes in the proximity of the AVM nidus.
This method in conjunction conventional DSA has a potential to become a valuable asset in the assessment of flow changes in the AVM and clinical risk prediction.
Our initial experience confirms dual-sensor pressure and flow measurement as a useful tool in the exploration of AVM physiology. Nevertheless, large prospective studies are required to prove our observations.
Additional mathematical post-processing of the data might reveal a stable pattern of flow changes during embolization. Moreover, processed data might serve as a basis in experimental computational modeling of AVM hemodynamics.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interest
The authors declare no conflict of interest
References
- 1.Valavanis A, Yaşargil MG. The endovascular treatment of brain arteriovenous malformations. Adv Tech Stand Neurosurg 1998; 24: 131–214doi: 10.1007/978-3-7091-6504-1_4. [DOI] [PubMed] [Google Scholar]
- 2.Valavanis A, Pangalu A, Tanaka M. Endovascular treatment of cerebral arteriovenous malformations with emphasis on the curative role of embolisation. Interv Neuroradiol 2005; 11, Suppl 1 37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maimon S, Strauss I, Frolov V, et al. Brain arteriovenous malformation treatment using a combination of Onyx and a new detachable tip microcatheter, SONIC: short-term results. Am J Neuroradiol 2010; 31(5): 947–954doi: 10.3174/ajnr.A1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ledezma CJ, Hoh BL, Carter BS, et al. Complications of cerebral arteriovenous malformation embolization: multivariate analysis of predictive factors. Neurosurgery 2006; 58(4): 602–611; discussion 602-11doi: 10.1227/01.NEU.0000204103.91793.77. [DOI] [PubMed] [Google Scholar]
- 5.Liu L, Jiang C, He H, et al. Periprocedural bleeding complications of brain AVM embolization with Onyx. Interv Neuroradiol 2010; 16(1): 47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hademenos GJ, Massoud TF. Risk of intracranial arteriovenous malformation rupture due to venous drainage impairment. A theoretical analysis. Stroke 1996; 27(6): 1072–1083doi: 10.1161/01.STR.27.6.1072. [DOI] [PubMed] [Google Scholar]
- 7.Jayaraman MV, Marcellus ML, Hamilton S, et al. Neurologic complications of arteriovenous malformation embolization using liquid embolic agents. Am J Neuroradiol 2008; 29(2): 242–246doi: 10.3174/ajnr.A0793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lv X, Wu Z, Jiang C, et al. Complication risk of endovascular embolization for cerebral arteriovenous malformation. Eur J Radiol 2011; 80(3): 776–779doi: 10.1016/j.ejrad.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 9.Spetzler RF, Wilson CB, Weinstein P, et al. Normal perfusion pressure breakthrough theory. Clin Neurosurg 1978; 25: 651–72. [DOI] [PubMed] [Google Scholar]
- 10.al-Rodhan NR, Sundt TM, Jr, Piepgras DG, et al. Occlusive hyperemia: a theory for the hemodynamic complications following resection of intracerebral arteriovenous malformations. J Neurosurg 1993; 78(2): 167–175doi: 10.3171/jns.1993.78.2.0167. [DOI] [PubMed] [Google Scholar]
- 11.Batjer HH, Devous MD, Sr, Meyer YJ, et al. Cerebrovascular hemodynamics in arteriovenous malformation complicated by normal perfusion pressure breakthrough. Neurosurgery 1988; 22(3): 503–509doi: 10.1097/00006123-198803000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Zacharia BE, Bruce S, Appelboom G, et al. Occlusive hyperemia versus normal perfusion pressure breakthrough after treatment of cranial arteriovenous malformations. Neurosurg Clin N Am 2012; 23(1): 147–151doi: 10.1016/j.nec.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Heidenreich JO, Hartlieb S, Stendel R, et al. Bleeding complications after endovascular therapy of cerebral arteriovenous malformations. Am J Neuroradiol 2006; 27(2): 313–316. [PMC free article] [PubMed] [Google Scholar]
- 14.Tournade A, Riquelme C. Dialogues. Advances in the endovascular treatment of intracranial arteriovenous malformations. Clin Neurosci 2000; 2(3): 315–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosen RJ, Contractor S. The use of cyanoacrylate adhesives in the management of congenital vascular malformations. Semin Intervent Radiol 2004; 21(1): 59–66doi: 10.1055/s–2004-831406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strauss I, Frolov V, Buchbut D, et al. Critical appraisal of endovascular treatment of brain arteriovenous malformation using Onyx in a series of 92 consecutive patients. Acta Neurochir (Wien). 2013. [DOI] [PubMed]
- 17.Spetzler RF, Hargraves RW, McCormick PW, et al. Relationship of perfusion pressure and size to risk of hemorrhage from arteriovenous malformations. J Neurosurg 1992; 76(6): 918–923doi: 10.3171/jns.1992.76.6.0918. [DOI] [PubMed] [Google Scholar]
- 18.Duong DH, Young WL, Vang MC, et al. Feeding artery pressure and venous drainage pattern are primary determinants of hemorrhage from cerebral arteriovenous malformations. Stroke 1998; 29(6): 1167–1176doi: 10.1161/01.STR.29.6.1167. [DOI] [PubMed] [Google Scholar]
- 19.Fleischer LH, Young WL, Pile-Spellman J, et al. Relationship of transcranial Doppler flow velocities and arteriovenous malformation feeding artery pressures. Stroke 1993; 24(12): 1897–1902doi: 10.1161/01.STR.24.12.1897. [DOI] [PubMed] [Google Scholar]
- 20.Ferns SP, Schneiders JJ, Siebes M, et al. Intracranial blood-flow velocity and pressure measurements using an intra-arterial dual-sensor guidewire. Am J Neuroradiol 2010; 31(2): 324–326doi: 10.3174/ajnr.A1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hademenos GJ, Massoud TF. Risk of intracranial arteriovenous malformation rupture due to venous drainage impairment. A theoretical analysis. Stroke 1996; 27(6): 1072–1083doi: 10.1161/01.STR.27.6.1072. [DOI] [PubMed] [Google Scholar]