Abstract
Background
In most cases, glioblastomas are associated with seizures, headaches, neurological deficits, aphasia, or bleeding. But these tumors are rarely associated with cerebral infarction and never so deadly.
Case report
A 40-year-old man presented with sudden morning isolated aphasia. One hour later, he developed a motor deficit at right upper member, quickly completed with a total right hemiplegia. Imaging studies revealed a left frontotemporal enhancing glioblastoma with a perilesional edema which produced an important mass effect on the posterior arm of the external capsule, on the primary motor cortex posteriorly and the entire sylvian valley anteriorly. Due to major surgical risks associated with left middle cerebral artery (MCA) inclusion and large edema, we decided to postpone the tumor removal and introduce quickly high concentrations of steroids. Twenty-four hours after his admittance, the patient presented a sudden impaired consciousness, coma, and a left mydriasis. A brain magnetic resonance image (MRI) revealed a left malignant MCA infarction, deadly for the patient.
Conclusion
To our knowledge, glioblastomas complicated by fatal ischemic stroke have not been reported. We discuss the pathology of such an event and try to figure out if it was predictable based on MRI finding, and inevitable with precocious surgery.
Keywords: MRI diagnostic, glioblastoma, acute ischemic stroke
Introduction
Glioblastoma remains one of the most aggressive and lethal forms of malignant primary brain tumor in adults as it corresponds to the highest grade (IV) of the World Health Organization classification. Glioblastomas are often present with seizures or confusion and occasionally with intracranial hemorrhage. But these tumors are rarely associated with cerebral infarctions, which are even more deadly.1 Unexpected death from an undiagnosed primary intracranial neoplasm is a rare event, with reported variable frequencies in the range of 0.02%, 2.1%, up to 12% in different medico-legal autopsy series, and almost never because of glioblastomas.2,3
This is a report on a patient presenting an acute onset of aphasia and right hemiplegia caused by perilesional glial edema. Initially mimicking a stroke, clinical presentation was quickly complicated by a real fatal malignant middle cerebral artery (MCA) infarction. A radiological diagnostic approach allowed us to better understand the series of events without unfortunately changing the dramatic prognosis.
Case report
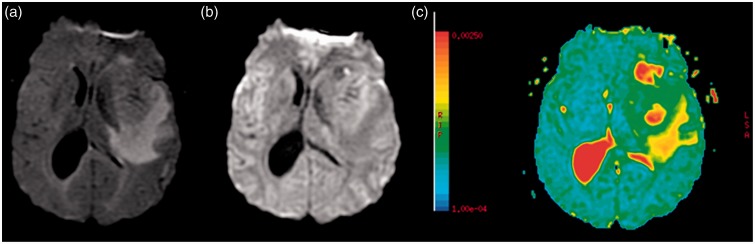
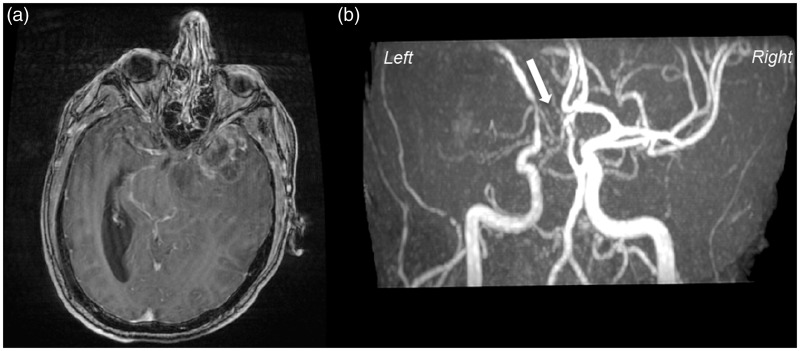
In October 2013, a 40-year-old man with no past medical history, no medical treatment, and no previous hospitalization presented with sudden morning isolated aphasia in a local emergency department. One hour later, he developed a motor deficit in his right upper member, quickly followed by a total right hemiplegia. Thanks to cranial magnetic resonance imaging (MRI), within 5 hours the diagnosis of acute ischemic stroke was ruled out. Fluid attenuated inversion recovery (Flair), diffusion weighted imaging (DWI) MRI, and apparent diffusion coefficient (ADC) showed no hypersignal in the left frontotemporal area (Figure 1). Brain MRI allowed diagnosis of a left heterogeneous necrotic frontotemporal glioblastoma, with peripheral contrast enhancement after gadolinium injection, temporal cystic component and a necrotic center (Figure 2(a)). A significant perilesional edema produced an important mass effect on the primary motor cortex posteriorly and the entire sylvian valley anteriorly (Figure 1(b)). A time of flight (TOF) angiography sequence determined that the left MCA was completely discharged upward and partially compressed by glial mass (Figure 2(b)). On the same, the left A1 segment of anterior cerebral artery was completely stenosis by the glial mass; therefore the arterial vasculature downstream could not be seen (Figure 2(b), white arrow).
Figure 1.
Flair T2-weighted MRI (a) demonstrates a hyperintense subcortical suggestive vasogenic edema. DWI MRI (b) showed no hyperintense region in the left frontotemporal area spreading the diagnostic of ischemic stroke, confirmed by ADC in this territory which is virtually not reduced (c).
Figure 2.
T1-weighted MRI, post-gadolinium (a) demonstrates a large necrotic lesion in the left frontotemporal region, typically ring-like, enhanced with perilesional edema, suggesting of malignant brain tumor. (b) MR angiography by TOF reveals the uprising and partial stenosis of the left middle cerebral artery (MCA) by a tumor and the major stenosis of A1 segment of anterior cerebral artery (ACA) (white arrow).
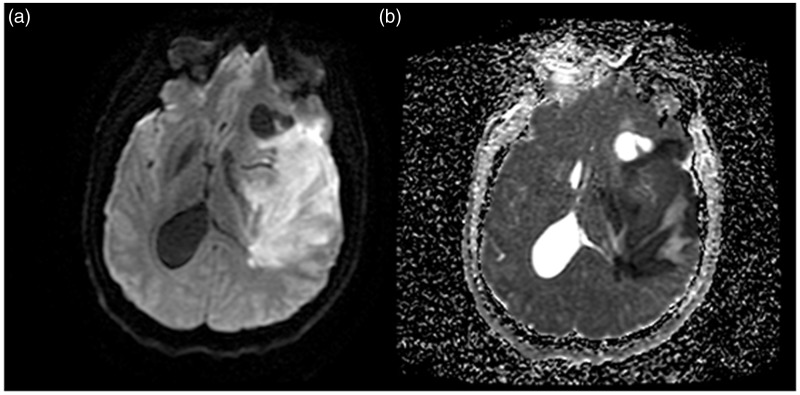
On his arrival in our neurosurgery department, the patient had no eye deviations and no Babinski sign, but remained hemiplegic, aphasiac, and presented right facial central palsy, and perfect consciousness with Glasgow coma scale (GCS) 15 (taking into account his aphasia and hemiplegia). He received intra-venous high concentrations of steroids (240 mg × 2) and due to the severity of the edema, was hospitalized to perform a surgical resection of the tumor in the following days. Cardiovascular assessment was unremarkable: the electrocardiogram detected no arrhythmias or conduction disorders, the transthoracic ultrasound was ordinary, and hemodynamic constants remained in regular standards (systolic blood pressure <150 mmHg). Twenty-four hours after his admittance, the patient presented a sudden impairment of consciousness with coma GCS 3 and appearance of a left mydriasis. A brain diffusion-weighted MRI revealed a hypersignal of the entire left MCA vascular area with midline deviation over 15 mm, which corresponded to a left malignant MCA infarction confirmed by the corresponding ADC mapping (Figure 3).
Figure 3.
Diffusion-weighted MRI (a) and ADC mapping (b) following the sudden patient deterioration at 24 hours demonstrates apparition of hyperintense left hemispheric areas corresponding to a middle cerebral artery (MCA) stroke territory, revealing the fatal acute ischemic stroke.
Because of the seriousness of the left malignant stroke with brainstem compression and the underlying aggressive high grade glioma, we did not propose any rescue surgery or treatment for stroke, despite the age of the patient. The intracranial pressure was quickly unmanageable and the patient died soon after.
Discussion
Glioblastoma multiforme (GBM) is known to sometimes present itself as a stroke.4–6 We present this observation in order to draw attention to the difficulties in radiologically and surgically managing such an unusual and delicate case.
With the use of MRI, the diagnosis of brain tumor associated with stroke is obvious in most cases. Gadolinium-enhanced MRI can usually identify brain tumor, while diffusion imaging and the ADC have shown to be highly accurate in detecting early stroke.7 In our observation, no brain computed tomography scan was realized. First, DWI MRI and ADC performed at admission eliminated the diagnosis of an acute ischemic stroke (Figure 1). Therefore, the patient’s initial neurological deficits (aphasia and hemiplegia) were likely due to the perilesional vasogenic edema of the glioblastoma (Figure 1(b)). Intra-tumor hemorrhage was ruled out by the T1-weighted MRI. Post-critical deficit was eliminated by the persistent of neurological signs under precocious antiepileptic treatment although no electroencephalogram had been realized.
We would like to discuss this case through several points.
The first issue concerns the indication for an emergency surgery to prevent this fatal complication. An a posteriori look on this report lets the reader think that the glioblastoma resection was mandatory at the admission in the neurosurgical department to prevent the fatal issue. Undeniably, on the initial MRI both subfalcine and uncal herniation could be detected, making a surgical indication pressing for the life-saving of the patient. But how could one know this ischemic complication would happen so quickly? Moreover, the first clinical examination was not so severe since the patient was conscious and the motor deficit was partially improving with intra-venous steroids. We must also remember that in this case, surgery presented many risks. Indeed, due to the proximity and the compression of the MCA (and the possible invasiveness of it by the tumor), the massive edema and the cerebral compression, this surgery could be considered risky.8 In case of acute hydrocephalus, the resection of the glial lesion may be delayed by the implementation of external ventricular derivation or ventriculocisternostomy.9
Indications for emergency resection of glioblastoma remain fairly limited. Surgery is recommended mainly to risk imminent engagement. In this case, debulking is the primary therapeutic measure. However, the introduction of high concentrations of steroids or intra-venous hyperosmotic therapy often reduces the mass effect by eliminating the perilesional vasogenic edema. We made that choice to ensure that in the following days, the exeresis was less risky. Retrospectively, with the first choice in this patient of a surgical GBM resection deemed too dangerous, a decompressive craniectomy with dural opening could have been made.
Additionally, once the stroke occurred, we wonder if the implementation of antiplatelet could have changed the outcome, despite the risk of bleeding.10 Off-label thrombolysis using recombinant human tissue-type plasminogen activator (rt-PA) can hardly be considered, because of the clinical and radiological severity and especially the type of tumor which remained a cons-indication to thrombolysis.11 Furthermore, it has been suggested that such rt-PA treatment of a malignant brain-tumor patient can cause tumor spread by degrading the extracellular matrix.4
The second issue to discuss is the stroke pathophysiology. Three mechanisms can be involved: (1) a mechanical compression of the artery by the tumor, (2) an invasion of the vessel by the glioblastoma fostered by tumor-induced hypercoagulable state, and (3) an embolic phenomenon. A hypercoagulable state (procoagulant factors secreted by gliomas) is common and partly accounts for the high incidence of deep vein thrombosis in these patients.12 Structural and/or functional vascular damages and changes in the hemostatic and inflammatory process in GBM may lead to a local or systemic prothrombotic state, which has yet to be fully elucidated. A systemic inflammatory state mediated by cytokines and acute phase proteins is involved in the increased expression of tissue factor (TF).13
GBM expresses the plasminogen activator inhibitor type 1 (PAI-1); it has been suggested that a dysregulation of fibrinolytic activity might contribute to the prothrombotic state associated with this tumor.14 Studies have demonstrated that in GBM, the loss of PTEN tumor suppressor genes and the enhanced expression of epidermal growth factor receptor induce TF expression and procoagulant activity in the tumor cells.15
Finally, gliomatous cells may compress and infiltrate the vessels at the tumor site,6,16,17 or in the meninges,7,18 leading to their occlusion or dissection.1 We can only regret that there was no post-stroke three-dimensional TOF MRI sequence that could have informed about the occlusion.
Fortunately, this kind of therapeutic dilemma is very rare. Most of the time, malignant brain tumors that present with intracranial hemorrhagic stroke are treated as such.19 On the contrary, ischemic strokes caused by malignant brain tumors are unusual.20,21 The majority of reported cases correspond to benign tumors, such as meningiomas, that cause progressive vascular encasement and compression of intracranial vessels which can generate ischemic strokes.11
The occurrence of postoperative stroke in glioblastomas is known and well described. These complications may result in interruptions of transcortical or deep arteries during the exeresis surgery. To our knowledge, we have not found other cases in the literature of such interaction between GBM natural history and fatal ischemic events.8,11
Conclusion
As a conclusion, we presume that with a glioblastoma involving the MCA, it is impossible to predict the occurrence of stroke, even with proper MRI interpretation. An a posteriori look at this report may show that the tumor removal was the solution, but one can not foresee the outcome after this surgery. Finally, this case encourages studies about glioblastomas and associated hypercoagulable states.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors have no conflicts of interest to declare.
References
- 1.Aoki N, Sakai T, Oikawa A, et al. Dissection of the middle cerebral artery caused by invasion of malignant glioma presenting as acute onset of hemiplegia. Acta Neurochir (Wien) 1999; 141: 1005–1008. [DOI] [PubMed] [Google Scholar]
- 2.Matschke J, Tsokos M. Sudden unexpected death due to undiagnosed glioblastoma: report of three cases and review of the literature. Int J Legal Med 2005; 119: 280–284. [DOI] [PubMed] [Google Scholar]
- 3.Riezzo I, Zamparese R, Neri M, et al. Sudden, unexpected death due to glioblastoma: report of three fatal cases and review of the literature. Diagn Pathol 2013; 8(1): 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klüenemann HH, Skljarevski V, Hamill RW. A stroke-like presentation of glioblastoma multiforme. J Stroke Cerebrovasc Dis 2003; 12(1): 47–48. [DOI] [PubMed] [Google Scholar]
- 5.Morgenstern LB, Frankowski RF. Brain tumor masquerading as stroke. J Neurooncol 1999; 44(1): 47–52. [DOI] [PubMed] [Google Scholar]
- 6.Hart MN, Byer JA. Rupture of middle cerebral artery branches by invasive astrocytoma. Neurology 1974; 24: 1171–1174. [DOI] [PubMed] [Google Scholar]
- 7.Herman C, Kupsky WJ, Rogers L, et al. Lepto meningeal dissemination of malignant glioma simulating cerebral vasculitis. Stroke 1995; 26: 2366–2370. [DOI] [PubMed] [Google Scholar]
- 8.Obeid M, Ulane C, Rosenfeld S. Pearls & Oysters: large vessel ischemic stroke secondary to glioblastoma multiforme. Neurology 2010; 30; 74: e50–e51. [DOI] [PubMed] [Google Scholar]
- 9.Diaz RJ, Girgis FM, Hamiltonn MG. Endoscopic third ventriculostomy for hydrocephalus due to tectal glioma. Can J Neurol Sci 2014; 41: 476–481. [DOI] [PubMed] [Google Scholar]
- 10.The National Institute of Neurological Disorders and Stroke rt- PA Stroke Study Group. Tissue plasminogen activator for acute chemic stroke. N Engl J Med 1995; 333: 1581–1587. [DOI] [PubMed] [Google Scholar]
- 11.Etgen T, Steinich I, Gsottschneider L. Thrombolysis for ischemic stroke in patients with brain tumors. J Stroke Cerebrovasc Dis 2014; 23: 361–366. [DOI] [PubMed] [Google Scholar]
- 12.Pan E, Tsai JS, Mitchell SB. Retrospective study of venous thromboembolic and intracerebral hemorrhagic events in glioblastoma patients. Anticancer Res 2009; 29: 4309–4313. [PubMed] [Google Scholar]
- 13.Milsom C, Rak J. Tissue factor and cancer. Pathophysiol Haemost Thromb 2007; 36: 160–176. [DOI] [PubMed] [Google Scholar]
- 14.Colin C, Voutsinos-Porche B, Nanni I, et al. High expression of cathepsin B and plasminogen activator inhibitor type-1 are strong predictors of survival in glioblastomas. Acta Neuropathol 2009; 118: 745–754. [DOI] [PubMed] [Google Scholar]
- 15.Rong Y, Belozerov VE, Tucker-Burden C, et al. Epidermal growth factor receptor and PTEN modulate tissue factor expression in glioblastoma through JunD/activator protein 1 transcriptional activity. Cancer Res 2009; 69: 2540–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dobkin BH. Stroke associated with glioblastoma. Bull Clin Neurosci 1985; 50: 111–118. [PubMed] [Google Scholar]
- 17.Züchner S, Kawohl W, Sellhaus B, et al. A case of gliosarcoma appearing as ischemic stroke. J Neurol Neurosurg Psychiatry 2003; 74: 354–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moonis M, Smith TW. Meningeal gliomatosis presenting as multiple cerebral infarcts: a case report. Neurology 1996; 46: 1760–1762. [DOI] [PubMed] [Google Scholar]
- 19.Kondziolka D, Bernstein M, Resch L, et al. Significance of hemorrhage into brain tumors: clinicopathological study. J Neurosurg 1987; 67: 852–857. [DOI] [PubMed] [Google Scholar]
- 20.Rojas-Marcos I, Martin-Duvemeuil N, Laigle-Donaday F, et al. Ischemic stroke in patients with glioblastoma multiforme. J Neurol 2005; 252: 488–489. [DOI] [PubMed] [Google Scholar]
- 21.Chen H, Cebula H, Schott R, et al. Glioblastoma multiforme presenting with ischemic stroke: case report and review of the literature. J Neuroradiol 2011; 38: 304–307. [DOI] [PubMed] [Google Scholar]