Abstract
Previous and more recent work of analyzing structural changes in the brain suggest that certain brain regions such as the frontal lobe are among the brain regions profoundly affected by the aging process across males and females. Also, a unified model of structural changes in a normally aging brain is still lacking. The present study investigated age-related structural brain changes in gray matter from young to early middle-age adulthood for males and females. Magnetic resonance images of 215 normal and healthy participants between the ages of 21–45 years were acquired. Changes in gray matter were assessed using voxel-based morphometry and gray matter volumetric analysis. The results showed significant decrease in gray matter volume between the youngest and oldest groups in the following brain regions: frontal, temporal, and parietal lobes. Grey matter loss in the frontal lobe was among the most widespread of all brain regions across the comparison groups that showed significant age-related changes in grey matter for both males and females. This work provides a unique pattern of age-related decline of normal and healthy adult males and females that can aid in the future development of a unified model of normal brain aging.
Keywords: Voxel-based morphometry, aging, magnetic resonance imaging, gray matter
Introduction
Gray matter (GM) volume of the adult human brain has been shown to diminish gradually with age. However, it is not clear if age-related changes occur uniformly across all cortical regions. Neurodegenerative diseases can reduce volume of GM regionally and globally long before they become symptomatic.1,2 Therefore, there exists an increased and crucial need for a full understanding of how the brain ages normally. Early detection of GM volume loss can help in distinguishing effects of certain neuropathology on the brain from normal aging. It appears that the pattern of GM volume reduction with age can be relatively easy to detect in elderly subjects when compared to young healthy subjects. However, for individuals who have already lost a substantial volume of GM, the implementation of any intervention, which can slow the accelerated volume loss of the brain, is obviously a late undertaking. Moreover, normal baseline values of GM for both males and females will certainly help better understand and distinguish the effects of certain neuropathology and neurodegenerative diseases on brain structure, and increased knowledge on brain shrinkage and associated patterns in normal aging can lead to more superior understanding of its causes, and may lead to interventions that can lessen the decline of brain functions associated with aging.3
There is plausible and convincing evidence from both post mortem and in vivo studies that the brain shrinks with age, but it remains the case that there is no clear quantification of age-related atrophy patterns. It still remains unclear whether there exist predictable patterns of age-related changes in the brain or whether such age-related changes are idiosyncratic.4 Moreover, specificity of brain tissue class loss is crucial towards understanding global brain shrinkage patterns. This issue can be addressed by segmenting magnetic resonance imaging (MRI) brain data into GM, white matter (WM), and cerebrospinal fluid (CSF): then using the voxel-based morphometry (VBM) technique to study and report regional changes in any tissue class, as well as using a volumetric analysis approach to see global volume changes with respect to tissue class.
Previous studies using VBM have reported global decrease in GM volume but with quantitative differences.5–8 More specifically, previous studies9,10 showed a significant decline in cortical GM volume, while Liu et al.11 found that global brain volume loss was around 0.385% per year in normal subjects over age 54 years in an MRI-based study. Another study12 provided similar results for whole brain in normal spouses of Alzheimer's Disease (AD) patients (∼0.33% per year). Furthermore, Coffey et al.13 found whole hemisphere loss of 2.79 cm3 per year, and Tisserand et al.14 also observed similar results.
Diffuse reductions of GM volume correlated with age in the frontal, parietal and temporal lobes, cerebellum and basal ganglia according to Smith et al.3 Also, accelerated brain atrophy was seen in the age range 35–54 years and cross-sectional analysis (i.e. VBM) revealed a significant association between age and reduction in brain volumes.11 Moreover, a voxel-based morphometry for ages between 18–79 years4 revealed a global age-related reduction in GM and reductions in the superior parietal gyri, insula, and cingulate sulci.
In a longitudinal study of brain volume changes in normal brain, Scahill et al. concluded that there existed a significant age-related decrease in global and regional brain volume, which may be related to nonlinear acceleration in atrophy rate in aging, and that better understanding of such a process may help differ structural changes in the brain due to normal aging from changes due to neurodegenerative diseases.12
Detection and quantification of subtle global and regional GM volume loss in earlier stages of adult life would allow identification of persons at high risk of more substantial accelerated volume loss with age and offer benefits of preventive interventions. Although we are able to measure regional brain volumes quite accurately using voxel-based morphometric MRI, it remains unclear whether there exist any pattern of age-related changes in the brain in adult subjects aged 21–45 years. Studies that suggest that there is no age-related volume loss in healthy subjects in that age span are supported by studies which have shown that blood flow parameters in basal cerebral arteries are fairly stable up to the age of 40–42 years, but the flow velocity starts to decrease and the flow impedance starts to increase definitely from this point in age.15,16
A lot, if not most, of previous work focused on associated volumetric-brain decline from early adulthood/middle adulthood to elderly age. In this study we examine the effect of normal aging on global brain volumes across an age range from early adulthood to early middle age (i.e. 21–45 years) in both males and females respectively. We performed a cross-sectional study using voxel-based morphometry on 215 normal T1-weighted MRI brain images, separately for males and females, in order to examine any reductions in GM with aging in this age range. We investigated normal brain images across ages 21–45 years and provide a pattern of regional global GM aging from age 21–45 years for males and females in order to help future work towards a unified model of normal brain aging. Furthermore we performed a volumetric analysis of GM volume to investigate global reductions in GM volume, if any, in females and males respectively. We hypothesized that greater regional and global effects of GM decline would be most evident between age groups 21–25 and 41–45 years for both males and females.
Methods
Subjects
All subjects were participants in a project lead by the Biomedical Image Analysis Group at Imperial College London.17 In that project more than 500 MRI images from normal and healthy subjects were acquired. The cohort included T1-weighted MRI images of normal and healthy subjects. Inclusion criteria were: minimum age of 21 and maximum age of 45 years, neurological and cognitive normality at time of MRI scan. A total of 215 T1-weighted MRI brain images of normal and healthy subjects were acquired from the brain development IXI dataset.17 The acquired brain images included randomly selected T1-weighted brain images of both males and females to create equal subgroups of data across age groups as follows: 21–25, 26–30, 31–35, 36–40, 41–45 years (see Table 1).
Table 1.
Mean and median age, number of females and males for each age group.
| Age group, years | Mean age (n = 43) | Median age (n = 43) | Number of males | Number of females |
|---|---|---|---|---|
| 21–25 | 24 | 24 | 20 | 23 |
| 26–30 | 28 | 28 | 21 | 22 |
| 31–35 | 33 | 34 | 22 | 21 |
| 36–40 | 38 | 38 | 24 | 19 |
| 41–45 | 42 | 42 | 22 | 21 |
MRI protocol
The T1-weighted images from brain-development.org17 were acquired at three different hospitals in London. Hammersmith Hospital used a Philips Medical Systems Intera 3T MRI system (TR = 9.6, TE = 4.6, number of phase encoding steps = 208, echo train length = 208, reconstruction diameter = 240, acquisition matrix = 208 × 208, flip angle = 8). The Institute of Psychiatry used a 1.5T GE MRI system, and Guy’s Hospital used a Philips Medical Systems Gyroscan Intera 1.5T MRI system (TR = 9.8, TE = 4.6, number of phase encoding steps = 192, echo train length = 0, reconstruction diameter = 240, flip angle = 8).
VBM and brain volume
VBM was performed using SPM8 (Wellcome Trust Centre for Neuroimaging, University College London, London, UK) and VBM8 toolbox (C. Gaser, Department of Psychiatry, University of Jena, Germany; http://dbm.neuro.uni-jena.de/vbm8/). All 215 T1-weighted MRI brain images were reoriented so that each respective image was centralized at the anterior commissure to optimize normalization. The images were segmented into GM, WM, and CSF, and normalized to standard space based on the Montreal Neurological Institute (MNI) template. A quality check was performed manually on all images to make sure that segmented images reflected segmentation category with proper and standard orientation. Then for the purposes of this study, the segmented GM data was smoothed using an 8 mm FWHM isotropic Gaussian kernel for later statistical data analysis.
For the VBM statistical analysis a second level analysis was performed in SPM8 (software from the Wellcome Department of Imaging Neuroscience, London, UK) for males and females respectively. The statistical analysis was performed on the smoothed GM data. Multiple regression analysis was performed with age and gender as covariates. Then age-group-specific statistical parametric maps of regional correlated changes in GM volume for age and gender were determined using a one-way analysis of variance (ANOVA) performed on the age groups provided in Table 1. The threshold for statistical significance in all VBM analysis was set to p < 0.05 with family wise error (FWE) correction, and voxel level and extent threshold of 10 voxels. Furthermore, the volume (ml) of segmented GM images was then calculated using Get_Totals (www0.cs.ucl.ac.uk/staff/g.ridgway/vbm/get_totals.m) SPM8 script. Then SPSS (IBM SPSS Statistics for Windows Version 22.0) was used to detect and exclude any outlier GM volumes for both genders (none found in this study), followed by a multiple linear regression where GM volume (ml) was the dependent variable and gender along with age as the independent variables.
Results
There was no significant reduction in GM between the following age groups in both males and females: 21–25 > 31–35, 21–25 > 26–30, 26–30 > 36–40, 26–30 > 31–35, 31–35 > 41–45, 31–35 > 36–40, and 36–40 > 41–45 years. The most significant reduction in GM was found in the following age-group contrasts for males and females respectively: 21–25 > 41–45, 21–25 > 36–40, and 26–30 > 41–45 years.
Comparison of the 21–25 and 41–45 age groups showed the most widespread age-related reduction in GM for both male and female groups. These reductions included the left frontal lobe, left temporal lobe, right frontal lobe, right temporal lobe, and right parietal lobe for males (Figure 1, Table 2) and for females (Figure 2, Table 3). Age-related reductions in GM between age groups 21–25 and 36–40 years were found in the right frontal lobe for both males (only region of GM loss: inferior frontal gyrus) and females while females showed more areas of regional GM loss (Table 4). Also, significant reductions in GM volume due to normal aging were exhibited between 26–30 and 41–45 years age groups for both males and females. In this comparison both males and females showed significant GM loss in the left frontal lobe while females showed more regional GM loss (Tables 5 and 6). Tables 7 and 8 provide the results of multiple linear regression with age and gender as covariates. More specifically Table 7 provides the brain regions that showed decrease in GM with increasing age (negative correlation), while Table 8 provides the brain regions that showed larger GM volume for females compared to males (females > males).
Figure 1.
Gray matter (GM) regional changes rendered onto a single subject brain for the comparison between 21–25 and 41–45 years age groups (21–25 > 41–45) for males. Four section configurations are shown.
Table 2.
Overall gray matter loss for males for the comparison between 21–25 and 41–45 years age groups (21–25 > 41–45).
| Region | Lobe | Hemisphere | MNI coordinates (mm) |
t-value | z score | p (FWE) | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Middle temporal gyrus | Temporal | Right | 63 | −28 | 0 | 5.93 | 5.72 | 0.001 |
| Cingulate gyrus | Limbic | Left | −3 | 21 | 34 | 5.79 | 5.6 | 0.001 |
| Inferior frontal gyrus | Frontal | Left | −56 | 18 | 4 | 5.74 | 5.55 | 0.002 |
| Superior frontal gyrus | Frontal | Left | −12 | 51 | 33 | 5.7 | 5.51 | 0.002 |
| Inferior frontal gyrus | Frontal | Right | 50 | 5 | 27 | 5.67 | 5.49 | 0.002 |
| Middle frontal gyrus | Frontal | Left | −48 | 11 | 31 | 5.67 | 5.48 | 0.002 |
| Medial frontal gyrus | Frontal | Left | −9 | 51 | 22 | 5.63 | 5.45 | 0.003 |
| Medial frontal gyrus | Frontal | Left | −3 | 21 | 52 | 5.63 | 5.45 | 0.003 |
| Cingulate gyrus (Brodmann area 32) | Frontal | Left | −3 | 11 | 40 | 5.6 | 5.43 | 0.003 |
| Supramarginal gyrus (Brodmann area 40) | Parietal | Right | 58 | −57 | 27 | 5.57 | 5.39 | 0.004 |
| Superior frontal gyrus (Brodmann area 9) | Frontal | Right | 4 | 53 | 27 | 5.54 | 5.37 | 0.004 |
| Anterior cingulate | Limbic | Right | 3 | 38 | 24 | 5.46 | 5.29 | 0.006 |
| Superior temporal gyrus | Temporal | Left | −58 | −55 | 12 | 5.42 | 5.26 | 0.007 |
| Medial frontal gyrus | Frontal | Right | 2 | 56 | 7 | 5.37 | 5.21 | 0.009 |
| Middle temporal gyrus | Temporal | Right | 54 | −67 | 1 | 5.33 | 5.18 | 0.01 |
| Superior frontal gyrus | Frontal | Right | 26 | 6 | 58 | 5.23 | 5.08 | 0.016 |
| Middle frontal gyrus | Frontal | Left | −27 | 15 | 58 | 5.2 | 5.06 | 0.018 |
| Sub-gyral (Brodmann area 6) | Frontal | Left | −26 | −4 | 58 | 5.1 | 4.97 | 0.027 |
FWE: family wise error; MNI: Montreal Neurological Institute; Region: name of specific brain region with significant gray matter loss.
Figure 2.
Gray matter (GM) regional changes rendered onto a single subject brain for the comparison between 21–25 and 36–40 years age groups (21–25 > 36–40) for females. Four section configurations are shown.
Table 3.
Overall gray matter loss for females for the comparison between 21–25 and 41–45 years age groups (21–25 > 41–45).
| Region | Lobe | Hemisphere | MNI coordinates (mm) |
t-value | z score | p (FWE) | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Precentral gyrus | Frontal | Left | −56 | 12 | 9 | 7.63 | 7.21 | 0 |
| Inferior frontal gyrus (Brodmann area 44) | Frontal | Right | 54 | 9 | 21 | 6.52 | 6.25 | 0 |
| Inferior parietal (Brodmann area 40) | Parietal | Right | 60 | −36 | 46 | 6.25 | 6.01 | 0 |
| Insula (Brodmann area 13) | Insula | Right | 38 | 18 | 6 | 5.8 | 5.6 | 0.001 |
| Caudate | Sub-lobar | Right | 14 | 8 | 16 | 5.75 | 5.56 | 0.002 |
| Insula | Insula | Right | 46 | −9 | 6 | 5.65 | 5.47 | 0.002 |
| Postcentral gyrus (Brodmann area 2) | Parietal | Right | 54 | −28 | 52 | 5.58 | 5.4 | 0.003 |
| Medial frontal gyrus (Brodmann area 9) | Frontal | Left | −10 | 39 | 18 | 5.54 | 5.37 | 0.004 |
| Inferior frontal gyrus (Brodmann area 47) | Frontal | Left | −15 | 18 | −21 | 5.46 | 5.29 | 0.006 |
| Precuneus (Brodmann area 7) | Parietal | Right | 3 | −63 | 52 | 5.41 | 5.25 | 0.007 |
| Superior temporal gyrus (Brodmann area 22) | Temporal | Right | 66 | −49 | 15 | 5.4 | 5.24 | 0.008 |
| Thalamus | Sub-lobar | Right | 16 | −28 | 1 | 5.28 | 5.13 | 0.013 |
| Fusiform gyrus (Brodmann area 37) | Temporal | Left | −48 | −45 | −21 | 5.25 | 5.1 | 0.015 |
| Culmen | Cerebellum | Right | 39 | −45 | −33 | 5.24 | 5.09 | 0.016 |
| Middle temporal gyrus (Brodmann area 22) | Temporal | Left | −64 | −39 | 1 | 5.18 | 5.04 | 0.02 |
| Superior temporal gyrus (Brodmann area 21) | Temporal | Right | 64 | −25 | −3 | 5.13 | 4.99 | 0.025 |
| Superior frontal gyrus (Brodmann area 9) | Frontal | Left | −26 | 45 | 37 | 5.12 | 4.98 | 0.026 |
| Middle temporal gyrus (Brodmann area 21) | Temporal | Right | 66 | −18 | −6 | 5.1 | 4.96 | 0.028 |
| Middle frontal gyrus (Brodmann area 6) | Frontal | Left | −44 | −1 | 45 | 5.09 | 4.95 | 0.03 |
FWE: family wise error; MNI: Montreal Neurological Institute; Region: name of specific brain region with significant gray matter loss.
Table 4.
Overall gray matter loss for females for the comparison between 21–25 and 36–40 years age groups (21–25 > 36–40).
| Region | Lobe | Hemisphere | MNI coordinates (mm) |
t-value | z score | p (FWE) | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Middle temporal gyrus (Brodmann area 21) | Temporal | Left | −63 | −19 | −8 | 6.17 | 5.94 | 0 |
| Superior frontal gyrus (Brodmann area 9) | Frontal | Right | 10 | 50 | 30 | 6.17 | 5.93 | 0 |
| Middle frontal gyrus (Brodmann area 6) | Frontal | Right | −32 | 5 | 54 | 5.76 | 5.57 | 0.002 |
| Middle frontal gyrus (Brodmann area 8) | Frontal | Left | −51 | 5 | 43 | 5.67 | 5.48 | 0.002 |
| Superior temporal gyrus (Brodmann area 22) | Temporal | Right | 60 | 0 | 4 | 5.66 | 5.48 | 0.002 |
| Inferior parietal lobe (Brodmann area 40) | Parietal | Right | 60 | −36 | 46 | 5.64 | 5.46 | 0.003 |
| Middle temporal gyrus | Temporal | Right | 62 | −33 | 0 | 5.58 | 5.41 | 0.003 |
| Superior temporal gyrus | Temporal | Right | 66 | −27 | −3 | 5.55 | 5.38 | 0.004 |
| Middle frontal gyrus | Frontal | Left | −48 | 9 | 33 | 5.54 | 5.37 | 0.004 |
| Medial frontal gyrus | Frontal | Left | −4 | 56 | −3 | 5.52 | 5.35 | 0.005 |
| Precentral gyrus | Frontal | Right | 57 | 14 | 7 | 5.44 | 5.28 | 0.006 |
| Insula (Brodmann area 13) | Insula | Right | 45 | −9 | −6 | 5.33 | 5.18 | 0.011 |
| Insula | Insula | Right | 46 | −6 | 4 | 5.28 | 5.13 | 0.013 |
| Anterior cingulate | Limbic | Right | 16 | 36 | 27 | 5.21 | 5.07 | 0.018 |
| Precentral | Frontal | Left | −57 | 12 | 7 | 5.14 | 5 | 0.024 |
| Middle frontal gyrus | Frontal | Left | −48 | 21 | 28 | 5.13 | 5 | 0.024 |
FWE: family wise error; MNI: Montreal Neurological Institute; Region: name of specific brain region with significant gray matter loss.
Table 5.
Overall gray matter loss for males for the comparison between 26–30 and 41–45 years age groups (26–30 > 41–45).
| Region | Lobe | Hemisphere | MNI coordinates (mm) |
t-value | z score | p (FWE) | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Inferior frontal gyrus | Frontal | Left | −57 | 18 | 4 | 5.56 | 5.38 | 0.004 |
| Inferior frontal gyrus | Frontal | Left | −56 | 14 | 12 | 5.31 | 5.15 | 0.012 |
| Superior temporal gyrus | Temporal | Left | −58 | −55 | 12 | 5.2 | 5.05 | 0.019 |
FWE: family wise error; MNI: Montreal Neurological Institute; Region: name of specific brain region with significant gray matter loss.
Table 6.
Overall gray matter loss for females for the comparison between 26–30 and 41–45 years age groups (26–30 > 41–45).
| Region | Lobe | Hemisphere | MNI coordinates (mm) |
t-value | z score | p (FWE) | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Superior frontal gyrus | Frontal | Left | 0 | 23 | 57 | 7.33 | 6.96 | 0 |
| Middle frontal gyrus (Brodmann area 11) | Frontal | Left | −40 | 36 | −15 | 6.57 | 6.29 | 0 |
| Subcallosal gyrus (Brodmann area 25) | Frontal | Left | −8 | 21 | −17 | 6.24 | 6 | 0 |
| Superior frontal gyrus (Brodmann area 8) | Frontal | Right | 28 | 26 | 54 | 6.22 | 5.98 | 0 |
| Superior temporal gyrus (Brodmann area 39) | Temporal | Right | 56 | −64 | 19 | 6.14 | 5.91 | 0 |
| Superior frontal gyrus (Brodmann area 9) | Frontal | Right | 22 | 39 | 40 | 6.11 | 5.88 | 0 |
| Middle temporal gyrus (Brodmann area 21) | Temporal | Right | 66 | −16 | −8 | 6.1 | 5.88 | 0.001 |
| Inferior frontal gyrus | Frontal | Left | −56 | 15 | 0 | 5.75 | 5.56 | 0.002 |
| Superior frontal gyrus (Brodmann area 9) | Frontal | Left | −24 | 42 | 37 | 5.67 | 5.49 | 0.002 |
| Middle frontal gyrus (Brodmann area 6) | Frontal | Right | 27 | 9 | 58 | 5.53 | 5.36 | 0.004 |
| Middle frontal gyrus (Brodmann area 9) | Frontal | Right | 54 | 15 | 31 | 5.53 | 5.36 | 0.004 |
| Postcentral gyrus (Brodmann area 2) | Parietal | Right | 54 | −30 | 51 | 5.51 | 5.34 | 0.005 |
| Thalamus | Sub-lobar | Right | 18 | −28 | 4 | 5.45 | 5.29 | 0.006 |
| Superior frontal gyrus | Frontal | Right | 33 | 48 | 25 | 5.41 | 5.25 | 0.007 |
| Medial frontal gyrus (Brodmann area 6) | Frontal | Left | −16 | 26 | 40 | 5.33 | 5.17 | 0.011 |
| Middle temporal gyrus (Brodmann area 21) | Temporal | Right | 62 | −27 | −6 | 5.25 | 5.1 | 0.015 |
| Orbital gyrus (Brodmann area 11) | Frontal | Right | 6 | 54 | −23 | 5.22 | 5.07 | 0.017 |
| Fusiform gyrus (Brodmann area 19) | Occipital | Right | 27 | −55 | −15 | 5.22 | 5.07 | 0.017 |
| Superior frontal gyrus (Brodmann area 10) | Frontal | Left | −26 | 66 | −6 | 5.16 | 5.02 | 0.022 |
| Insula (Brodmann area 13) | Insula | Right | 44 | −9 | 4 | 5.15 | 5.01 | 0.023 |
| Cingulate gyrus | Limbic | Left | 0 | 20 | 34 | 5.13 | 4.99 | 0.025 |
| Precuneus | Parietal | Left | 0 | −51 | 37 | 5.09 | 4.96 | 0.029 |
| Medial frontal gyrus (Brodmann area 10) | Frontal | Left | −10 | 56 | −6 | 5.07 | 4.94 | 0.032 |
FWE: family wise error; MNI: Montreal Neurological Institute; Region: name of specific brain region with significant gray matter loss.
Table 7.
Multiple linear regression results showing brain regions that have negative gray matter volume correlation with age.
| Region | Lobe | Hemisphere | MNI coordinates (mm) |
t-value | z score | p (FWE) | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Superior frontal gyrus | Frontal | Left | −8 | 47 | 33 | 9.89 | Inf | 0 |
| Superior frontal gyrus | Frontal | Left | −26 | 18 | 55 | 9.76 | Inf | 0 |
| Precentral gyrus | Frontal | Left | −56 | 14 | 7 | 9.32 | Inf | 0 |
| Thalamus | Sub-lobar | Right | 16 | −28 | 3 | 6.45 | 6.2 | 0 |
| Inferior temporal gyrus | Temporal | Left | −48 | −73 | −6 | 6.13 | 5.91 | 0 |
| Culmen | Cerebellum | Left | −34 | −52 | −33 | 6.09 | 5.87 | 0 |
| Cingulate gyrus | Limbic | Right | 12 | −57 | 25 | 6.07 | 5.85 | 0 |
| Cuneus | Occipital | Left | −27 | −84 | 31 | 5.93 | 5.73 | 0.001 |
| Precentral gyrus | Frontal | Left | −40 | −21 | 58 | 5.9 | 5.7 | 0.001 |
| Precuneus | Parietal | Right | 8 | −72 | 39 | 5.79 | 5.6 | 0.001 |
| Culmen | Cerebellum | Right | 38 | −48 | −32 | 5.77 | 5.59 | 0.001 |
| Culmen | Cerebellum | Right | 4 | −67 | −11 | 5.55 | 5.39 | 0.004 |
| Fusiform | Temporal | Right | 27 | −40 | −18 | 5.54 | 5.37 | 0.004 |
| Lingual gyrus | Occipital | Left | −21 | −93 | −11 | 5.46 | 5.3 | 0.006 |
| Superior temporal gyrus | Temporal | Left | −45 | −37 | 6 | 5.35 | 5.2 | 0.009 |
| Precentral gyrus | Frontal | Left | −54 | −7 | 37 | 5.19 | 5.05 | 0.019 |
| Thalamus | Sub-lobar | Right | 6 | −7 | 9 | 5.17 | 5.04 | 0.02 |
| Thalamus | Sub-lobar | Left | −15 | −30 | 3 | 5.16 | 5.02 | 0.021 |
| Inferior parietal | Parietal | Left | −40 | −61 | 42 | 5.16 | 5.02 | 0.021 |
| Precuneus | Parietal | Left | −2 | −66 | 52 | 5.14 | 5 | 0.023 |
FWE: family wise error; MNI: Montreal Neurological Institute; Region: name of specific brain region with significant gray matter loss.
Table 8.
Multiple linear regression results showing brain regions having gray matter volume correlated with gender (female > male).
| Region | Lobe | Hemisphere | MNI coordinates (mm) |
t-value | z score | p (FWE) | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Paracentral lobule | Frontal | Left | −3 | −27 | 45 | 8.56 | Inf | 0 |
| Cingulate gyrus | Limbic | Left | −2 | −6 | 42 | 7.28 | 6.92 | 0 |
| Caudate | Sub-lobar | Right | 8 | 4 | 7 | 7.06 | 6.73 | 0 |
| Cuneus | Occipital | Right | 9 | −87 | 4 | 6.87 | 6.57 | 0 |
| Cuneus | Occipital | Left | −6 | −87 | 6 | 6.82 | 6.52 | 0 |
| Declive of Vermis | Cerebellum | Left | −2 | −75 | −30 | 6.8 | 6.51 | 0 |
| Lingual gyrus | Occipital | Left | −6 | −84 | −2 | 6.72 | 6.44 | 0 |
| Postcentral gyrus | Parietal | Right | 38 | −25 | 42 | 6.65 | 6.37 | 0 |
| Precuneus | Occipital | Left | −24 | −82 | 24 | 6.53 | 6.26 | 0 |
| Superior temporal gyrus | Temporal | Left | −52 | −46 | 9 | 6.49 | 6.23 | 0 |
| Inferior frontal gyrus | Frontal | Left | −12 | 36 | −23 | 6.36 | 6.11 | 0 |
| Parahippocampa gyrus | Limbic | Left | −21 | −31 | −15 | 6.21 | 5.98 | 0 |
| Inferior frontal gyrus | Frontal | Right | 18 | 28 | −20 | 6.2 | 5.97 | 0 |
| Precuneus | Parietal | Left | −8 | −72 | 43 | 6.16 | 5.94 | 0 |
| Inferior frontal gyrus | Frontal | Left | −54 | 15 | 3 | 6.08 | 5.87 | 0 |
| Medial frontal gyrus | Frontal | Left | −2 | 48 | 13 | 6.05 | 5.84 | 0 |
| Medial frontal gyrus | Frontal | Right | 2 | 2 | 52 | 5.95 | 5.74 | 0.001 |
| Inferior parietal | Parietal | Right | 64 | −27 | 24 | 5.86 | 5.67 | 0.001 |
| Middle occipital gyrus | Occipital | Right | 33 | −82 | 12 | 5.86 | 5.66 | 0.001 |
| Superior temporal gyrus | Temporal | Right | 48 | −33 | 7 | 5.78 | 5.6 | 0.001 |
| Insula | Insula | Right | 46 | 9 | −2 | 5.72 | 5.54 | 0.002 |
| Precuneus | Parietal | Left | −8 | −64 | 58 | 5.67 | 5.49 | 0.002 |
| Precuneus | Parietal | Right | 8 | −67 | 31 | 5.47 | 5.31 | 0.005 |
| Postcentral gyrus | Parietal | Left | −40 | −25 | 40 | 5.28 | 5.13 | 0.013 |
| Middle temporal gyrus | Temporal | Right | 56 | −31 | −3 | 5.25 | 5.1 | 0.014 |
| Cerebellum posterior lobe | Cerebellum | Left | −39 | −64 | −42 | 5.19 | 5.05 | 0.018 |
| Cuneus | Occipital | Left | −26 | −84 | 10 | 5.18 | 5.04 | 0.019 |
| Precentral lobule | Frontal | Right | 10 | −49 | 55 | 5.17 | 5.03 | 0.02 |
| Superior frontal gyrus | Frontal | Left | −20 | 68 | 10 | 5.15 | 5.02 | 0.021 |
| Medial frontal gyrus | Frontal | Left | −8 | 63 | 12 | 5.15 | 5.01 | 0.022 |
FWE: family wise error; MNI: Montreal Neurological Institute; Region: name of specific brain region with significant gray matter loss.
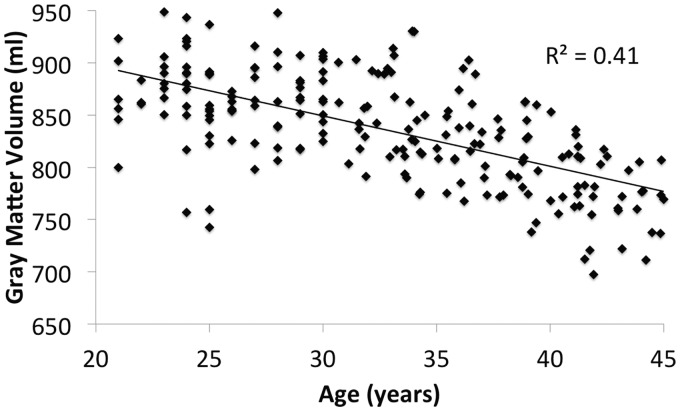
Results of the multiple linear regression, where GM volume (ml) was the dependent variable and gender along with age as the independent variables, showed standardized beta (B) for age as −0.629 (p < 0.001) at 95% confidence level, while B for gender was shown as 0.187 (p < 0.001) at 95% confidence level. Moreover scatter plots with respective coefficient of determination R2 values are shown in Figure 3 for males, Figure 4 for females, and Figure 5 for both males and females.
Figure 3.
Scatter plot of gray matter (GM) volume (ml) across age (21–45 years old) for males.
Figure 4.
Scatter plot of gray matter (GM) volume (ml) across age (21–45 years old) for females.
Figure 5.
Scatter plot of gray matter (GM) volume (ml) across age (21–45 years old) for both genders.
Discussion and conclusions
This study reports on a pattern of GM loss in a large series of normal adults using VBM analysis. GM volumes from normal adult males and adult females aged between 21–45 years were respectively compared across five different age subgroups (21–25, 26–30, 31–35, 36–40, 41–45 years). There was no significant reduction in GM volume between the following comparisons for both males and females: 21–25 > 31–35, 21–25 > 26–30, 26–30 > 36–40, 26–30 > 31–35, 31–35 > 41–45, 31–35 > 36–40, and 36–40 > 41–45 years. As hypothesized, distinct and widespread GM volume loss was seen in the 21–25 > 41–45 years comparison for both male and female subgroups. The reduction in GM for 21–25 > 41–45 years comparison was predominantly in the frontal lobe for both males and females, while such GM loss was also evident in the temporal and parietal lobes for both genders. The 21–25 > 36–40 years comparison showed significant GM loss in the frontal regions of the brain for both males and females, while 26–30 > 41–45 years comparison showed significant GM loss in both frontal and temporal regions for both genders.
In this study we also found that regional GM loss was more widespread in females than males (Figures 1 and 2, and Tables 2–6). We also found that the coefficient of determination (R2) value differed between males and females (females > males) with regards to age-related GM loss (Figures 3 and 4). Overall, according to the multiple linear regression of GM volume, we found that age as a much stronger predictor of GM loss (B = −0.629) than gender (B = 0.187) with 95% confidence level across early adulthood and early middle ages (i.e. 21–45 years of age).
In relating our findings to previous studies, Tisserand et al.18 found that advancing in age was strongly associated with volumetric decreases in the whole of frontal cortex with strongest age-related volume decrease found in the lateral and orbital frontal GM. In a later study Tisserand et al.19 found that the prefrontal cortex and temporal lobe were among the brain regions that showed the largest age effects, and concluded that such findings suggest that the prefrontal and temporal cortical brain regions are of particular relevance in aging as well as age-related cognitive decline in healthy elderly persons. Another study20 found that frontal and parietal, compared to temporal and occipital, brain regions showed greater tissue loss. These studies along this one and others21,22 seem to support the longstanding theory of frontal selectivity in cognitive aging.
The frontal lobe was among the brain regions that had the largest effect for GM volume loss across all the comparisons of this study that showed significant GM loss for both genders (21–25 > 41–45, 21–25 > 36–40, 26–30 > 41–45 years). In the 21–25 > 41–45 years comparison the temporal lobe along with the parietal lobe also showed significant loss in GM for males and females. For the 21–25 > 36–40 years comparison GM loss was only present in the left inferior frontal gyrus, while females showed more widespread regional GM loss that included the insula along with frontal, temporal, limbic, as well as parietal regions. As for the 26–30 > 41–45 years comparison males only showed GM loss in frontal and temporal regions while females showed GM loss in both temporal and frontal regions along with the thalamus, occipital lobe, cingulate gyrus, and insula.
With regards to the largest age gap comparison (21–25 > 41–45 years), along with the frontal lobe, the temporal lobe also showed significant decline in GM. It is well known and documented that the certain regions within the temporal lobe (i.e. entorhinal and perirhinal cortices) are devastated by neurofibrillary pathology and cell loss in very early course of Alzheimer’s disease.23,24 However it remains less clear whether and how such temporal cortical regions are affected by normal aging due to severely limited investigations.25–27
The multiple regression results for GM volume along with the scatter plots (Figures 3 and 4) and statistical parametric mapping results (Figures 1 and 2, and Tables 2–6) show that gender as a contributor to regional and global GM differences which is in line with previous work.28 Although gender may be a more minor contributor to GM loss (B = 0.187), age on the other hand is a major contributor to GM loss as indicated by Figures 3, 4, and 5 (R2 = 0.30/0.54/0.41 respectively), and B = −0.629.
In this work we provide a pattern of age-related GM loss in normal and healthy adults. Some of the brain regions that showed significant GM loss across different age groups are consistent with previous work. Also, this work is consistent with the frontal selectivity in cognitive aging theory. Moreover, this work provides a unique pattern for age-related GM decline associated with comparisons of young adults to early middle-aged males and females. Therefore, this work will aid in the development of a unified model of normal brain aging for both males and females which in turn may provide brain biomarkers of normal aging for both genders, and this may help ultimately in differentiating normal versus abnormal structural brain changes. On a final note; future work can include an investigation in inter-hemispheric differences, if any, in GM volume across age and gender.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The author(s) declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Kaye JA, Moore MM, Dame A, et al. Asynchronous regional brain volume losses in presymptomatic to moderate AD. J Alzheimer’s Dis 2005; 8: 51–56. [DOI] [PubMed] [Google Scholar]
- 2.Smith CD, Kryscio RJ, Schmitt FA, et al. Longitudinal functional alterations in asymptomatic women at risk for Alzheimer’s disease. J Neuroimaging 2005; 15: 271–277. [DOI] [PubMed] [Google Scholar]
- 3.Smith CD, Chebrolu H, Wekstein DR, et al. Age and gender effects on human brain anatomy: A voxel-based morphometric study in healthy elderly. Neurobiol Aging 2007; 28: 1075–1087. [DOI] [PubMed]
- 4.Good CD, Johnsrude IS, Ashburner J, et al. A voxel-based morphometric study of ageing in 465 normal adult human brains. In: Biomedical Imaging, 2002. 5th IEEE EMBS International Summer School on. IEEE, 2007, p.16.
- 5.Lemaître H, Crivello F, Grassiot B, et al. Age- and sex-related effects on the neuroanatomy of healthy elderly. Neuroimage 2005; 26: 900–911. [DOI] [PubMed] [Google Scholar]
- 6.Resnick SM. One-year age changes in MRI brain volumes in older adults. Cereb Cortex 2000; 10: 464–472. [DOI] [PubMed] [Google Scholar]
- 7.Raz N. Selective aging of the human cerebral cortex observed in vivo: Differential vulnerability of the prefrontal gray matter. Cereb Cortex 1997; 7: 268–282. [DOI] [PubMed] [Google Scholar]
- 8.Coffey CE, Wilkinson WE, et al. Quantitative cerebral anatomy of the aging human brain: A cross-sectional study using magnetic resonance imaging. Neurology 1992; 42: 527–527. [DOI] [PubMed] [Google Scholar]
- 9.Lim KO, Zipursky RB, Watts MC, et al. Decreased gray matter in normal aging: An in vivo magnetic resonance study. J Gerontol 1992; 47: B26–B30. [DOI] [PubMed] [Google Scholar]
- 10.Pfefferbaum A, Mathalon DH, Sullivan EV, et al. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol 1994; 51: 874–887. [DOI] [PubMed] [Google Scholar]
- 11.Liu RS, Lemieux L, Bell G, et al. A longitudinal study of brain morphometrics using quantitative magnetic resonance imaging and difference image analysis. Neuroimage 2003; 20: 22–33. [DOI] [PubMed] [Google Scholar]
- 12.Scahill RI, Frost C, Jenkins R, et al. A longitudinal study of brain volume changes in normal aging using serial registered magnetic resonance imaging. Arch Neurol 2003; 60: 989–994. [DOI] [PubMed] [Google Scholar]
- 13.Coffey CE, Saxton JA, Ratcliff G, et al. Relation of education to brain size in normal aging: Implications for the reserve hypothesis. Neurology 1999; 53: 189–196. [DOI] [PubMed] [Google Scholar]
- 14.Tisserand DJ, Visser PJ, van Boxtel MPJ, et al. The relation between global and limbic brain volumes on MRI and cognitive performance in healthy individuals across the age range. Neurobiol Aging 2000; 21: 569–576. [DOI] [PubMed] [Google Scholar]
- 15.Krejza J, Mariak Z, Walecki J, et al. Transcranial color Doppler sonography of basal cerebral arteries in 182 healthy subjects: Age and sex variability and normal reference values for blood flow parameters. AJR Am J Roentgenol 1999; 172: 213–218. [DOI] [PubMed] [Google Scholar]
- 16.Ringelstein EB, Kahlscheuer B, Niggemeyer E, et al. Transcranial doppler sonography: Anatomical landmarks and normal velocity values. Ultrasound Med Biol 1990; 16: 745–761. [DOI] [PubMed] [Google Scholar]
- 17.Brain Development Biomedical Image Analysis Group-Imperial College London, www.brain-development.org (accessed 14 April 2015).
- 18.Tisserand DJ, Pruessner JC, Sanz Arigita EJ, et al. Regional frontal cortical volumes decrease differentially in aging: An MRI study to compare volumetric approaches and voxel-based morphometry. Neuroimage 2002; 17: 657–669. [PubMed] [Google Scholar]
- 19.Tisserand DJ, van Boxtel MPJ, Pruessner JC, et al. A voxel-based morphometric study to determine individual differences in gray matter density associated with age and cognitive change over time. Cereb Cortex 2004; 14: 966–973. [DOI] [PubMed] [Google Scholar]
- 20.Resnick SM, Pham DL, Kraut MA, et al. Longitudinal magnetic resonance imaging studies of older adults: A shrinking brain. J Neurosci 2003; 23: 3295–3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Head D, Snyder AZ, Girton LE, et al. Frontal-hippocampal double dissociation between normal aging and Alzheimer’s disease. Cereb Cortex 2005; 15: 732–739. [DOI] [PubMed] [Google Scholar]
- 22.Raz N, Gunning-Dixon F, Head D, et al. Aging, sexual dimorphism, and hemispheric asymmetry of the cerebral cortex: Replicability of regional differences in volume. Neurobiol Aging 2004; 25: 377–396. [DOI] [PubMed] [Google Scholar]
- 23.Hyman B, Van Hoesen G, Damasio A, et al. Alzheimer’s disease: Cell-specific pathology isolates the hippocampal formation. Science (80-) 1984; 225: 1168–1170. [DOI] [PubMed] [Google Scholar]
- 24.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 1991; 82: 239–259. [DOI] [PubMed] [Google Scholar]
- 25.Sullivan E. Age-related decline in MRI volumes of temporal lobe gray matter but not hippocampus. Neurobiol Aging 1995; 16: 591–606. [DOI] [PubMed] [Google Scholar]
- 26.Jack CR, Petersen RC, Xu Y, et al. Rate of medial temporal lobe atrophy in typical aging and Alzheimer’s disease. Neurology 1998; 51: 993–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Du A-T, Schuff N, Chao LL, et al. Age effects on atrophy rates of entorhinal cortex and hippocampus. Neurobiol Aging 2006; 27: 733–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luders E, Narr KL, Thompson PM, et al. Mapping cortical gray matter in the young adult brain: Effects of gender. Neuroimage 2005; 26: 493–501. [DOI] [PubMed] [Google Scholar]