Abstract
The purpose of this study was to investigate the usefulness of diffusion tensor imaging (DTI) for early detection of pathological alterations in the myelon in patients with cervical spondylotic myelopathy (CSM) without T2-weighted imaging (T2W) signal abnormalities but with a narrowed spinal canal with corresponding clinical correlation. Axial DTI at 1.5T together with routine magnetic resonance imaging was performed on 18 patients fulfilling above mentioned criteria. Quantitative fractional anisotropy (FA) and apparent diffusion coefficient (ADC) maps were generated. Values at the narrowest cervical levels were compared to pre- and poststenotic levels and the interindividual means were tested for statistically significant differences by means of paired t-tests. The correlation between the grade and width of canal stenosis in the axial plane was measured. FA was significantly reduced at the stenotic level, compared to prestenotic level, whereas no significant differences were found when compared to poststenotic level. No significant differences between ADC values at stenotic level versus both adjacent non-stenotic levels were found, suggesting very early stage of degeneration. ADC values correlated significantly with the width of the spinal canal at the prestenotic level, but not at the poststenotic level. Findings indicate sufficient robustness of routine implementation of DTI at 1.5T to detect abnormalities in the spinal cord of CSM patients, before apparent T2W signal abnormalities and marked clinical deterioration. Therefore, larger and long-term studies should be conducted to establish the DTI scalar metrics that would indicate early intervention for a better clinical outcome in patients with clinical signs of CSM.
Keywords: Apparent diffusion coefficient, diffusion tensor imaging, fractional anisotropy, cervical spondylotic myelopathy, spinal canal stenosis
Introduction
Diffusion of water molecules in the human spinal cord is highly anisotropic due to its microstructural organization. Water protons diffuse in a directional dependent way primarily due to diffusion barriers represented by the cell membrane and the myelin sheath. However, this is governed by several other, yet not completely elucidated, parameters related to the diffusion physics and biophysical properties of the tissue, such as the cell membrane permeability and the free diffusion coefficients for the cellular compartments.1
CSM is the most common type of spinal cord dysfunction and most common cause of developed spastic paraparesis in patients older than 55 years.2 In CSM patients signal changes on T2-weighted magnetic resonance imaging (T2W-MRI) are often present. Such findings are suggestive of oedema, inflammation, ischaemia or gliotic changes of the myelon.3,4 Several groups assessed pre- and post-operative MRI in CSM patients and correlated the degree of T2W signal changes with clinical improvement.5,6 However, T2W signal hyperintensity is not seen in every patient presenting with classical signs of CSM.
Our aim in this study was to investigate whether scalar metrics, such as FA and ADC derived from DTI provide a sensitive means to assess early changes in the myelon not provided by T2W-MRI. Our hypothesis was that ADC and FA would be more sensitive to early pathological changes at microscopic level related to chronic trauma of the spinal cord from the spinal canal stenosis.
Materials and methods
Patient selection
To study the feasibility of DTI in CSM we prospectively included 18 patients (eight females, mean age was 66.4 and median age was 67 years) who fulfilled the following criteria: signs and symptoms of pure cervical myelopathy, imaging findings of canal stenosis without pathological signal changes in cervical myelon on T2W-MRI. We excluded patients with prior spine surgery, patients without radiological evidence of spinal canal stenosis or patients with other neurological conditions that might cause symptoms of cervical myelopathy.
Imaging protocols
MR examinations include routine imaging protocols, which were conducted on a 1.5-T clinical MR System (HDxt, GE Healthcare, Milwaukee, Wisconsin, USA). The protocol included routine axial and sagittal T2-, and axial and sagittal T1-weighted pre- and post-contrast sequences. DTI data were acquired as part of the routine protocol using the following parameters: 2D, spin-echo, single-shot echo-planar imaging (SS-EPI) sequence in oblique axial orientation; receive eight-channel head-neck-spine cervical-thoracic-lumbar top coil (HNS CTLTOP); anterior regional pre-saturation slab; repetition time (TR) 6300 ms; echo time (TE) 89.4 ms; slice thickness 5.0 mm; inter-slice gap 0.0 mm; field-of-view (FOV) 200 × 150 mm2; the actually acquired number of data points and matrix was 140 × 140. The matrix size of the images, however, was 256 × 256 after zero-interpolation during image reconstruction; receiver bandwidth 1953 Hz/pixel (±250 kHz); number of averages, six; b-values 0 and 700 s mm−2; number of diffusion-encoding directions, nine. The total DTI acquisition time was 6 min 24 s.
The MRI examinations were part of routine clinical imaging, which has already been approved by the Institutional Review Committee, and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All subjects gave informed consent.
Post-processing of measured data
To generate FA and ADC maps we used commercially available software (FuncTool DTI Processing, GE Healthcare, Milwaukee, Wisconsin, USA). Available options on the software were used to reduce artifactual factors that may in turn mislead the interpretation, such as EPI related geometric distortion artefacts, and Eddy current artifacts. To correct these artifacts distortion correction depending on the orientation of the diffusion-weighting gradient and separate Eddy-current correction were applied. We chose our regions of interest (ROIs) at pre- and post-stenotic axial slices on the same patients to compare the changes in FA and ADC values at the most stenotic level with non-stenotic levels, since FA and ADC values measured on healthy subjects and on radiologically unaffected areas of the patients did not differ statistically.7 ROIs were drawn manually on axial slices since dedicated software for automatic segmentation of cerebrospinal fluid (CSF) and myelon was not available at this time. The smallest ROI included approximately 70 pixels while the biggest ROI included approximately 204 pixels depending on the cross-sectional volume of the spinal cord. The sizes of ROIs in pixels have not been analysed for every ROI specifically. To investigate the possible correlation between scalar DTI metrics, we measured the width of the spinal canal in the anterior-posterior direction at the prestenotic level, the most stenotic and poststenotic levels. We paid utmost care not to include CSF and to avoid partial volume effects during measurements. ROIs included both white and grey matter of the spinal cord. Although relative isotropic diffusion in grey matter and the highly anisotropic diffusion characteristics of the spinal cord white matter both contribute to the measured FA and ADC values, we preferred to draw one ROI so as to keep the procedure as simple as possible for clinical routine application. There were no follow-up imaging of the patients; the data acquisition was carried out at one timepoint.
Statistical analysis
Altogether 54 ROIs, three ROIs for each level at prestenotic, the most stenotic and poststenotic segments were drawn on axial slices based on the width of the spinal cord to quantify FA and ADC. Statistical significance of differences was tested with the paired t-test. We also investigated the correlation between spinal canal width on axial plane and the ADC values derived from DTI (Wilcoxon test).
Results
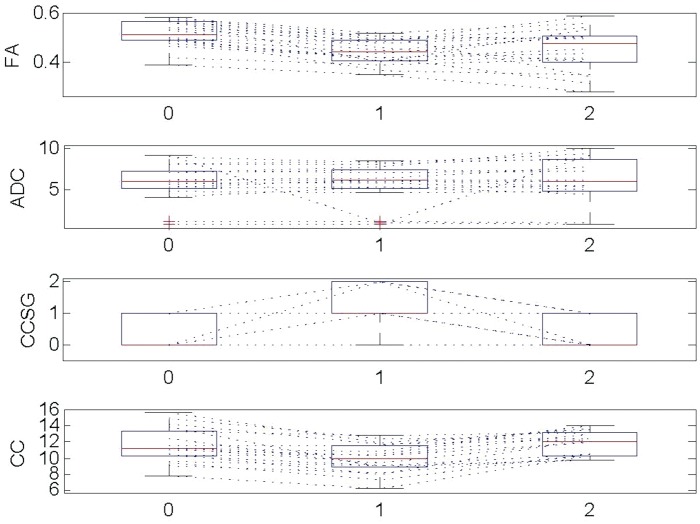
FA values were statistically reduced at the stenotic levels compared to prestenotic (p < 0.001, paired t-test and Wilcoxon test, Table 1, Figures 1 and 2). No statistically significant FA changes were observed at the stenotic levels in comparison to the poststenotic levels (p = 0.5). While mean FA at prestenotic levels were 0.51, which was statistically higher than at stenotic level (0.44), we did not observe statistically significant increment in ADC values at stenotic level in comparison to nonstenotic levels. Over the group of patients, ADC values correlated significantly with the width of the spinal canal at the prestenotic level (r = 0.5, p = 0.03), but not at the poststenotic levels (p = 0.4, Figure 3). We did not find significant correlations between FA values and the width of the spinal canal at pre-, post- and stenotic levels (Figure 3).
Table 1.
Showing quantificational FA and ADC values measured at Level 0, 1 and 2 with respective statistical paired t-Test and Wilcoxon Test results. Level 0 represents prestenotic axial slice level, level 1 and level 2 represent stenotic and poststenotic levels, respectively.
| Level 0 | Level 1 | Level 2 | |
|---|---|---|---|
| FA median values | 0.51 | 0.44 | 0.47 |
| ADC median values | 6.07 | 6.18 | 6.13 |
| FA mean values | 0.51 | 0.44 | 0.47 |
| ADC mean values | 5.99 | 6.18 | 6.13 |
ADC: apparent diffusion coefficient; FA: fractional anisotropy.
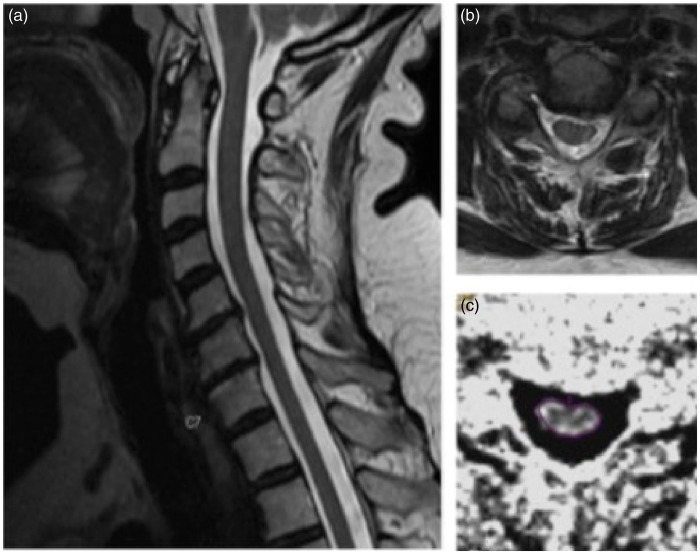
Figure 1.
Illustrative case 1 showing in a T2-weighted MRI sagittal image without signs of myelopathy at level C5-C6. In B axial T2-weighted MRI at most stenotic level between C5-C6. Exemplary measurement at the most stenotic level for FA and ADC values in C.
Figure 2.
(a) sagittal T2-weighted MRI imaging sequence of a patient showing degenerative changes and narrowing of cervical spinal canal, without T2w-MRI correlation. (b) axial T2-weighted MRI image slice of the same patient with measured FA and ADC maps in (c) and (d) respectively.
Figure 3.
FA was reduced at the stenotic level compared to prestenotic level (p < 0.001) which implies the loss of myelon integrity. 0: prestenotic level, 1: stenotic level, 2: poststenotic level, CCSG: cervical canal stenotic grade, CC: cervical canal.
Discussion
DTI has a proven clinical value in the investigation of various neurological pathologies affecting the brain. Its application in the human spinal cord however, has been associated with several challenges due to its fine structure and elasticity, its complex anatomical localization, and most importantly, the increased motion artefacts related to breathing and CSF pulsations, which generate increased susceptibility artefacts. Wilms et al. introduced a new technique, using reduced FOV in combination with an oblique spin echo acquisition and outer volume suppression (OVS) succeeding to reduce these limitations and optimize imaging. They achieved a reduction in SS-EPI that consecutively enabled high-resolution imaging not only the cervical spinal cord but also the entire cord.8 CSM benefits from timely radiological diagnosis by allowing better treatment planning and prompt intervention. Surgery performed at early stages was reported to have a better outcome, emphasizing the importance of readily diagnosis before pathological T2W signal alterations appear.1 Hyperintense lesions on T2W-MRI of the cervical myelon usually appear at later stages of CSM when pathological alterations may be irreversible. Mamata et al., studied age-related and spondylotic changes with DTI and found that ADC and FA show not only spondylosis-related changes, but also age-related increment in ADC and decrement in FA, suggesting age-related loss of nerve fibre density.9 They demonstrated pathological changes in FA and ADC values in 39 of 72 patients without T2W signal alterations. These findings showed consistency with the age-related changes reported in brain. Budzik et al. studied the feasibility of DTI and fibre tracking in CSM but they did not study the relationship of DTI scalars with cervical canal width.10 In our study over the group of patients, ADC correlated significantly with the width of the spinal canal at the prestenotic level (r = 0.5, p = 0.03), but not at the poststenotic level (p = 0.4) thus emphasizing the importance of a narrow spinal canal in the dynamic development of cervical myelopathy.11 Our results showed statistically significant reduction in FA at the stenotic as compared to prestenotic level due to degenerative changes, such as osteophyte formation, disk herniation with compression of the dural sac, hypertrophied ligament flava, erosive osteochondrosis and dehydrated intervertebral disc, which have led to consecutive spinal canal stenosis. Reduction in FA at stenotic levels suggests either direct mechanical disruption of the white matter tracts and/or secondary changes in the spinal cord white matter, such as oedema or proliferation of macrophages in the area of the damaged neuronal tissue, leading to increased diffusivity of water protons in longitudinal axis along the coherently oriented spinal cord white matter. Biomolecular factors such as ischaemic injury due to chronic compression, glutamate-mediated excitotoxicity and oligodendrocyte/neuronal apoptosis play conclusive roles in the pathophysiology of cervical myelopathy among other well-known static and dynamic factors.12 Studies suggest that apoptosis of the oligodendrocyte cells due to chronic trauma of the spinal cord is an early biomolecular characteristic of the CSM.3 Among other supporting function for neurons, satellite oligodendrocytes play a crucial role in formation of myelin sheaths. They remain opposed to neurons and regulate the extracellular fluid compartment. It is well known in the literature that experimental studies conducted on animal models support apoptosis in chronic compression related cervical myelopathy.3 Through histological examination of twy mice spinal cord, descending degeneration affecting the anterior and lateral columns and ascending degeneration along the posterior funiculus could be observed. Immunohistochemical studies could establish, analogically, the role of oligodendrocyte apoptosis in cervical myelopathy in a human spinal cord with myelopathy due to chronic trauma, caused by an ossified posterior longitudinal ligament that revealed a pattern of neuronal loss, demyelination and apoptosis closely resembling those observed in the twy mice. These changes interfere with the anisotropic movement of water protons and lead to restricted diffusion anisotropy. In our patients no statistical increment in ADC and no statistically significant reduction in FA values at the stenotic levels were observed when compared to post-stenotic levels. Since the nature of the injury at the immunohistochemical level is/was beyond the scope of this study, we cannot make any scientific/evidence based explanation about our findings regarding no statistically significant increment in ADC values despite of reduced FA values at the same level. These findings are in agreement with other studies done either with 1.5T or 3T MR,9,10,13–16 suggesting very early stage of degeneration. Reduced FA values at stenotic levels are interpreted as either extracellular oedema or decreased neuronal fibres due to microstructural damage with consecutive increment in extracellular space. Damage to oligodendrocytes causes dysregulation in the extracellular fluid compartment. Absence of statistically significant ADC changes at the stenotic level and of FA changes at the poststenotic, as compared to stenotic segments in patients with spondylotic myelopathy symptoms without pathological signal T2-signal abnormalities, probably reflect very early stage of myelopathy at the stenotic levels without cranial or caudal extension yet.
DTI studies in healthy subjects to determine normal FA and ADC values gave almost similar results emphasizing that these measures are relatively invariable to the MR manufacturer, type of sequence used and strength of the magnet, which makes this technique attractive for clinical studies. Mean FA values at the cervical levels range between 0.70 ± 0.08, 0.72 ± 0.03 and 0.75 ± 0.1 whereas ADC values between 0.97 ± 0.32, 0.87 ± 0.04 and 0.90 ± 0.10.8,17,18
We also studied the correlation of the FA and ADC values with the cervical canal width. The positive predictive value of reduced canal width suggested to us that the pathological changes could be potentially related to spondylotic changes only. Decrease in the width of the spinal canal due to degenerative changes of the vertebral column leads to chronic trauma to the spinal cord with consecutive damage to myelin, axonal membrane, neurofilaments and microtubules. Loss of integrity of these structures will let water protons move freely in a radial direction rather than only in a longitudinal direction, thus causing ADC values to increase at a given axial, most stenotic slice. Over the group of patients, ADC values correlated significantly with the width of the spinal canal at the prestenotic levels (r = 0.5, p = 0.03), but not at the poststenotic levels. We did not find significant correlations between FA values and the width of the spinal canal at pre- and post-stenotic levels. Based on these observations, we believe that ADC may be more sensitive than FA in depicting early pathological changes of CSM, prior to the appearance of T2W signal alterations, and could be used as a potential biomarker for early disease manifestation for better correlation with clinical symptoms and treatment planning.
The correlation of DTI scalar metrics with the width of the spinal canal proves the necessity of DTI in clinical setting in patients with signs and symptoms of CSM but without pathological T2 signal alterations. We would like to mention that we conducted this prospective study using a 1.5T MR scanner, which is still the most available scanner type used for clinical examinations, in patients with a relatively common disease process, to emphasize the potential of this method to provide additional useful information when implemented to routine clinical protocols of the spine. All patients underwent the 5–6 min additional scanning time of the cervical spine without any complaints.
The limitations of the study include the relatively small sample size and the lack of follow-up examinations. DTI measurements in patients who were treated conservatively could provide valuable information concerning the effectiveness of the non-surgical therapeutic modalities and eventually the prognostic value of the method. Further studies in a larger number of subjects correlating ‘signs and symptoms of pure cervical myelopathy' with reference to a scale of imaging characteristics would further enhance the clinical input of the method, establishing standardized diagnostic criteria relevant to the therapeutic strategy.
Conclusions
Our findings suggest that DTI at 1.5T seems to be sufficiently robust to detect pathological alterations at molecular level in the spinal cord of CSM patients, before a pathological T2 signal and marked clinical worsening are observed. Larger and long-term longitudinal studies should be conducted to further establish the potential of quantitative DTI scalar metrics as a routine biomarkers in patients with clinical signs of CSM to detect and enable intervention earlier for a better clinical outcome.
Acknowledgements
Guarantor of integrity of the entire study: S Kollias; study concepts: S Kollias; study design: S Kollias, U Ahmadli; definition of intellectual content: S Kollias, U Ahmadli; literature research: U Ahmadli, N Ulrich, Y Yuqiang; clinical studies: U Ahmadli, N Ulrich; data acquisition: U Ahmadli, N Ulrich, D Nanz; data analysis: U Ahmadli, N Ulrich, Y Yuqiang; statistical analyses: J Sarnthein; manuscript preparation: U Ahmadli, N Ulrich, Y Yuqiang; manuscript editing: U Ahmadli, N Ulrich, Y Yuqiang; manuscript review: S Kollias, D Nanz.
Ethical standards
The MRI examinations were part of a routine clinical imaging, which have already been approved by the Institutional Review Committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. We declare that all subjects gave informed consent.
Funding
This research received no support in the form of grants.
Conflict of interest
The author(s) declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Beaulieu C. The basis of anisotropic water diffusion in the nervous system–a technical review. NMR Biomed 2002; 15: 435–455. [DOI] [PubMed] [Google Scholar]
- 2.Montgomery DM, Brower RS. Cervical spondylotic myelopathy. Clinical syndrome and natural history. Orthop Clin North Am 1992; 23: 487–493. [PubMed] [Google Scholar]
- 3.Baron EM, Young WF. Cervical spondylotic myelopathy: A brief review of its pathophysiology, clinical course, and diagnosis. Neurosurgery 2007; 60: S35–S41. [DOI] [PubMed] [Google Scholar]
- 4.Kim DH, Vaccaro AR, Henderson FC, et al. Molecular biology of cervical myelopathy and spinal cord injury: Role of oligodendrocyte apoptosis. Spine J 2003; 3: 510–519. [DOI] [PubMed] [Google Scholar]
- 5.Matsuda Y, Miyazaki K, Tada K, et al. Increased MR signal intensity due to cervical myelopathy. Analysis of 29 surgical cases. J Neurosurg 1991; 74: 887–892. [DOI] [PubMed] [Google Scholar]
- 6.Mehalic TF, Pezzuti RT, Applebaum BI. Magnetic resonance imaging and cervical spondylotic myelopathy. Neurosurgery 1990; 26: 217–226. discussion 226–217. [DOI] [PubMed] [Google Scholar]
- 7.Facon D, Ozanne A, Fillard P, et al. MR diffusion tensor imaging and fiber tracking in spinal cord compression. AJNR Am J Neuroradiol 2005; 26: 1587–1594. [PMC free article] [PubMed] [Google Scholar]
- 8.Wilm BJ, Gamper U, Henning A, et al. Diffusion-weighted imaging of the entire spinal cord. NMR Biomed 2009; 22: 174–181. [DOI] [PubMed] [Google Scholar]
- 9.Mamata H, Jolesz FA, Maier SE. Apparent diffusion coefficient and fractional anisotropy in spinal cord: Age and cervical spondylosis-related changes. J Magn Reson Imaging 2005; 22: 38–43. [DOI] [PubMed] [Google Scholar]
- 10.Budzik JF, Balbi V, Le Thuc V, et al. Diffusion tensor imaging and fibre tracking in cervical spondylotic myelopathy. Eur Radiol 2011; 21: 426–433. [DOI] [PubMed] [Google Scholar]
- 11.Penning L. Some aspects of plain radiography of the cervical spine in chronic myelopathy. Neurology 1962; 12: 513–519. [DOI] [PubMed] [Google Scholar]
- 12.Baptiste DC, Fehlings MG. Pathophysiology of cervical myelopathy. Spine J 2006; 6: 190S–197S. [DOI] [PubMed] [Google Scholar]
- 13.Demir A, Ries M, Moonen CT, et al. Diffusion-weighted MR imaging with apparent diffusion coefficient and apparent diffusion tensor maps in cervical spondylotic myelopathy. Radiology 2003; 229: 37–43. [DOI] [PubMed] [Google Scholar]
- 14.Jones JG, Cen SY, Lebel RM, et al. Diffusion tensor imaging correlates with the clinical assessment of disease severity in cervical spondylotic myelopathy and predicts outcome following surgery. AJNR Am J Neuroradiol 2013; 34: 471–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kara B, Celik A, Karadereler S, et al. The role of DTI in early detection of cervical spondylotic myelopathy: A preliminary study with 3-T MRI. Neuroradiology 2011; 53: 609–616. [DOI] [PubMed] [Google Scholar]
- 16.Lee JW, Kim JH, Park JB, et al. Diffusion tensor imaging and fiber tractography in cervical compressive myelopathy: Preliminary results. Skeletal Radiol 2011; 40: 1543–1551. [DOI] [PubMed] [Google Scholar]
- 17.Bosma RL, Stroman PW. Characterization of DTI indices in the cervical, thoracic, and lumbar spinal cord in healthy humans. Radiol Res Pract 2012; 2012: 143705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maier SE, Mamata H. Diffusion tensor imaging of the spinal cord. Ann N Y Acad Sci 2005; 1064: 50–60. [DOI] [PubMed] [Google Scholar]