Abstract
The spine is a frequent localization of primary tumours or metastasis involving posterior arch, pedicles and vertebra body, and often causing unsustainable pain. The management of spinal metastasis remains complex, including medical therapy (corticosteroids, chemotherapy), radiotherapy and surgical treatment, or the recent percutaneous mini-invasive approach. The target of all these treatments is to improve the quality of life of patients affected by this type of lesion. Diagnosis of spinal metastasis and then its treatment should be based on the combination of different elements: clinical evaluation, CT, MRI and nuclear medicine patterns, considering the age of the patient, known primary tumour, location of the lesions, single/multiple lesions, pattern of morphology (border, matrix, expansile character, soft tissue extension), density or signal intensity, oncologic instability and expectancy of life. The percutaneous mini-invasive approach for patients affected by secondary lesions involving the spine has as treatment goal of: (1) pain relief improving the quality of life; (2) stability treatment re-establishing the spinal biomechanics, alterated by bone destruction or deformity, preventing pathological fracture; and (3) an anti-neoplastic effect. The aim of this paper is to provide a comprehensive diagnostic and percutaneous approach to the bone metastatic spine lesions, identifying which metamer should be treated to improve patient quality of life, showing the importance of a multi-disciplinary approach to this problem.
Keywords: Spinal metastasis, percutaneous mini-invasive procedure, vertebroplasty, radiofrequency, CT, MRI, nuclear medicine bone scan, Weinstein–Boriani–Biagini classification, polymethylmethacrylate, spinal instability
Introduction
The spine is a frequent localization of metastasis from breast, lung and prostate cancer, involving every part of the vertebra – posterior arch, pedicles and body – causing unsustainable pain. Less frequently it is the location of primary tumours. The majority of metastases have an osteolytic nature (70.9%), while 21.1% are mixed and only 8.0% are osteoblastic. Generally they involve more than one vertebra (86.3% of cases), with the vertebra body affected in 44.8% of patients and posterior elements affected in 40.5%.1
The management of spine metastasis remains complex, including medical therapy (corticosteroids, bisphosphonates, chemotherapy), radiotherapy and surgical treatment or, in selected patients, a percutaneous mini-invasive approach. It is recommended that a multi-disciplinary approach to spine metastasis is adopted to achieve the best result in each patient.
The target of all treatments is to improve the quality of life for the patient, which is decreased by the most important untreated symptom in cancer today, the ‘Fatigue’ concept, very often underlying in oncology.2 Fatigue incorporates a number of concepts including:
decreased mental and physical endurance;
decreased motivation;
depletion of reserves;
fatigability;
inability to rise to the occasion;
lassitude.
The causes of fatigue in the cancer patients include the following.
- Primary factors:
- pain;
- emotional distress;
- anaemia;
- sleep disturbance;
- hypothyroidism.
- Comorbidities:
- infection;
- cardiac dysfunction;
- pulmonary dysfunction;
- renal dysfunction;
- hepatic dysfunction;
- endocrine dysfunction.
- Nutritional/metabolic causes:
- changes in caloric intake/weight;
- fluid electrolyte imbalance.
- Conditioning causes:
- changes in exercise or activity patterns;
- deconditioning.
Pain is the most important aspect in the management of the metastasis treatment, and it can be evaluated using different scale or methods, for example, SF 36, VAS scale, Mc Gill Pain questionnaire, Mc Nab Method, Oswestry Low Back Pain disability questionnaire and the Quebec Back Pain Disability Scale.3–5
Pain is due to the stretching of periosteum nervous fibres by neoplastic tissue with/without pathological fractures or by the epidural, intra/extraforaminal extension of neoplastic tissue. It can be vertebrogenic pain, either nociceptive, caused by direct stimulation of nerve endings (receptors) in the structures affected by the disease (cortical bone, periosteum, subchondral) or neuropathic if it involves the nerve cord and/or nerve root.
Surgical treatment is indicated in the case of spinal cord compression (8% of tumours), spinal instability and acute or progressive neurological symptoms.6
The Weinstein–Boriani–Biagini (WBB) classification7,8 was developed to stage and facilitate surgical-treatment planning in patients with primary spine tumours, but this was later applied to single metastasis.9 The WBB surgical staging system describes the involvement of a specific vertebra in terms of sectors involved, arranged clockwise in 12 sectors on an axial vertebral face. The lesion is further confined to five layers of tissue penetration. Longitudinal extent is described in numbers of vertebrae involved. Recommendations can be made regarding the most appropriate approach and procedure to allow for en bloc removal of a neoplastic lesion, based on the sectors and layers involved in the specific case, including surrounding structures (as denominated by the tissue layers) as required for en bloc excision of the lesion with an appropriate clear margin. Three procedures are advocated: vertebrectomy, sagittal resection and posterior arch resection. A vertebrectomy is indicated for lesions involving only the soma with at least one pedicle free of tumour to remove the vertebral body containing the tumour in its entirety (en bloc). Sagittal resection is indicated for unilateral lesions involving an anterior–posterior half-soma with the lesion possibly involving the pedicle or transverse process. Posterior arch resection is indicated for lesions restricted to posterior elements.7
Tomita et al.10 defined a new surgical strategy for the treatment of patients with spinal metastases who had undergone a wide range of surgical interventions, ranging from limited decompression to radical tumour excision. They devised a 10-point scale that took into account tumour histology and the extent of visceral and bone metastases to determine the goal of intervention and thereby the aggressiveness of surgery. According to the authors, the treatment goal of patients with rapidly growing tumours, such as those of the lungs or stomach, and systemic metastases, is largely short-term palliation or terminal care; this makes these patients candidates for limited palliative decompression surgery or only supportive care. However, patients with slow-growing tumours, such as those of the breasts or thyroid, and solitary spinal metastasis, are candidates for wide or marginal excision of the tumour, with the goal of long-term control.10,11
Radiation therapy is generally the only option for patients who have radiosensitive tumour (lymphoma, myeloma, breast or prostate cancer) cannot tolerate surgery or have a poor survival prognosis.12
Pain relief may be delayed for up to two weeks following the procedure, and this treatment does not correct existing biomechanical abnormalities or stabilize the spine. Maximal benefit from radiation therapy for solitary or multiple metastases usually occurs in 12–20 weeks, but this treatment is not effective for preventing imminent vertebral body collapse. In fact almost half of patients undergoing radiotherapy subsequently experience vertebral body fractures.12,13
A mini-invasive percutaneous procedure has the advantage of combining the antalgic effect with spinal stabilization treatment, with a low rate of complications.
Vertebral compression fracture caused by multiple myeloma is another condition frequently causing spine pain in which a mini-invasive approach can help stabilize the involved metamer, reducing clinical symptomatology.
Diagnostic approach
Diagnosis of spinal metastasis and then its treatment should be based on the combination of different elements: clinical evaluation, CT, MRI and nuclear medicine patterns, considering the age of the patient, known primary tumour, location of the lesions, single/multiple lesions, pattern of morphology (border, matrix, expansile character, soft tissue extension), density or signal intensity alteration, oncologic instability and life expectancy (Table 1).
Table 1.
diagnostic algorithm for VP indication in patients affected by spine pain.
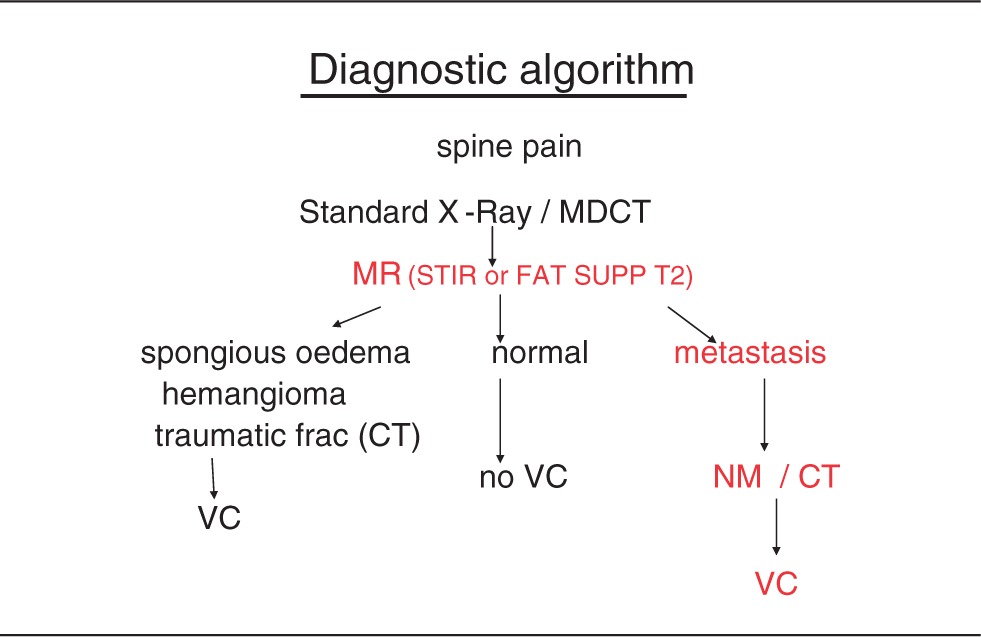
MRI (with the T2-STIR images to check the bone marrow oedema and fat-suppressed SE T1 with contrast-enhanced scans) is the gold standard among non-invasive imaging, with a sensitivity and specificity of 98.5% and 98.9%.14 However, CT can be frequently added for the planning of percutaneous treatment (i.e. vertebroplasty, radioablation, cryoablation). A combination of MRI pattern and CT imaging helps to recognize at which level causing symptoms should be treated, especially in patients affected by multi-level tumour infiltration. The pain may be caused not necessarily by fractured vertebra but by spinal axial-load alteration, and this will be the goal of percutaneous treatment.
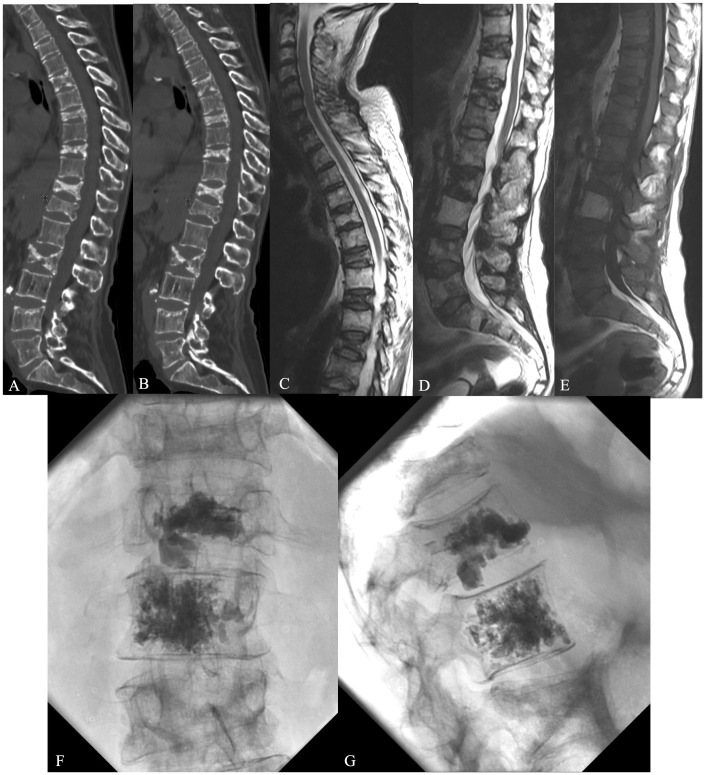
For example, in a patient affected by multiple myeloma involving different metamers, CT imaging with evidence of sclerotic reaction correlated with MRI intensity alteration was useful to distinguish the level causing pain; not tumour infiltration, but biomechanical modification, especially at the T12-L1 segment (Figure 1(a–g)).
Figure 1.
A 65-year-old male affected by multiple myeloma involving different metamers at thoraco-lumbar level; CT and MRI (a–e) showed a sclerotic reaction of multiple vertebral bodies infiltrated by tumour. VP was performed at T12-L1 segment with good pain relief (f–g). Symptoms were not caused by tumour infiltrations but by biomechanical alteration at T12-L1.
Strategy of the percutaneous mini-invasive treatment and indications
Multiple strategies can be considered with different outcomes and results according to the histology, tumour extension, type of lesion (lytic, mixed or osteoblastic metastasis) up to obtaining a ‘tailored’ best treatment, using a combination between:
vertebral cementoplasty;
radiotherapy;
chemotherapy;
ormonotherapy;
radiometabolic therapy;
bisphosphonates;
embolization (renal cell CA-thyroid);
surgery;
radiofrequency (RF).
The mini-invasive percutaneous approach for patients affected by neoplastic lesions involving the spine responds to two major concepts:
Pain treatment to improve the quality of life.
Stability treatment, especially for the spinal metastasis, in order to re-establish the spinal biomechanic alterated by bone destruction or deformity resulting in a decrease in spinal load-bearing capacity preventing pathological fracture, elements.15
The location of the tumour (and hence bone destruction) within the vertebral body plays a role in the patient’s risk of fracture and spinal instability.
The Spine Oncologic Study Group (SOSG)16 has defined spinal instability due to tumour extension as the loss of spinal integrity associated with pain related to movement, symptomatic deformity and progressive neurological deficit under physiological load.
Patients affected by malignant primary or metastasis tumours involving the spine have a decrease in spinal load-bearing capacity due to bone destruction or spine deformity resulting in modified biomechanics of the spine. This alteration and the risk factor of fracture of spine instability depends on tumour size and bone mineral density. Krishaney17 has divided the vertebral body in 27 similar cubes. When the destruction of all the cubes within 1/3 of the axial soma occurs, it creates instability due to a deficit of the anterior and middle column. In the case of sagittal destruction only, spinal stability is maintained and not alterated. A discrepancy between the thoracic and thoracolumbar or lumbar spine and spinal oncological instability is well demonstrated. In fact, according to Taneichi, the costo-vertebral joint with all thoracic muscular structures increases the stiffness and the resistance of the thoracic spine, maintaining spinal biomechanics. In fact, at the thoracic level, it is demonstrated that it is necessary to have about 50%–60% vertebral disruption to have pathological vertebral fracture and instability, against 35%–40% of thoraco-lumbar and lumbar level.18
Vertebroplasty indication
Vertebroplasty (VP) can be performed in patients affected by osteolytic or mixed lesions, symptomatics and resistance to medical therapy. The rationale of VP procedure responds to a double goal: mechanical vertebral stabilization and pain resolution.
The MRI examination with a sagittal T2WI-STIR sequence (or any fat suppression if using a 3 T MRI equipment) is mandatory to determine the treatment and the number of vertebral bodies to treat, checking the bone marrow oedema.19 Absolute VP contra-indications are the presence of local or systemic infections, painless vertebral fracture and allergy to polymethylmethacrylate (PMMA).20 A metastatic lesion with erosion of the posterior wall does not represent a contraindication to perform a VP. Using high viscosity cement it is possible to fill the lesion without cement leakage in the spinal canal.
The presence of a myelopathy and radicular compression in case of spine tumours represent an obvious and urgent indication to surgical decompression.21
The presence of an epidural soft tissue component with thecal sac compression does not represent an absolute contraindication for VP, but it requires accurate clinical evaluation in order to decide between mini-invasive therapy or surgical treatment.16,21
Multiple myeloma cannot be considered as typical spinal metastasis. It can associate with pre, para-vertebral or epidural soft-tissue or it can appear quite often as similar to porotic vertebral body fracture. In these cases, the therapeutic option depends on the clinical status associated or not with myelo-radicular deficits. VP indication responds to antalgic and spinal stability effects
VP technique
The technique is an imaging-guided technique and generally fluoroscopy control is most used.
The patient is in the prone position and under neuroleptoanalgesia. The procedure consists of a simple cement injection into the neoplastic vertebral-body lesion with an antalgic effect using the mono or bi-peduncular approach. An oblique approach with the typical ‘scottie dog’ imaging appearance is also useful to reach the third anterior of the vertebral body. If the neoplastic tissue involves the dens of C2 or the anterior arch of C1 a trans-oral approach is required.
Once local anaesthesia has been done, the needle is positioned in the vertebral body through a trans-peduncular approach, reaching its posterior wall; in AP view the medial margin of the peduncle is an absolute anatomical landmark to check before passing over the posterior wall of the vertebral body in LL view. It is enough to position the needle within the third anterior of the vertebral body on LL view.
The cement injection is performed under continuous fluoroscopy control. The amount of cement injected in the vertebral body is extremely variable: 2 to 4 ml by each peduncle depending on the spine metamer to treat (thoracic or lumbar) and the grading of the collapsed vertebra; however, there is no absolute rule regarding the amount of cement to be injected.20
High quality fluoroscopy is very important to achieve a complete anatomical control of the spine, in order to obtain a correct peduncular approach avoiding complications, even in case of patients affected by age scoliosis.22–31
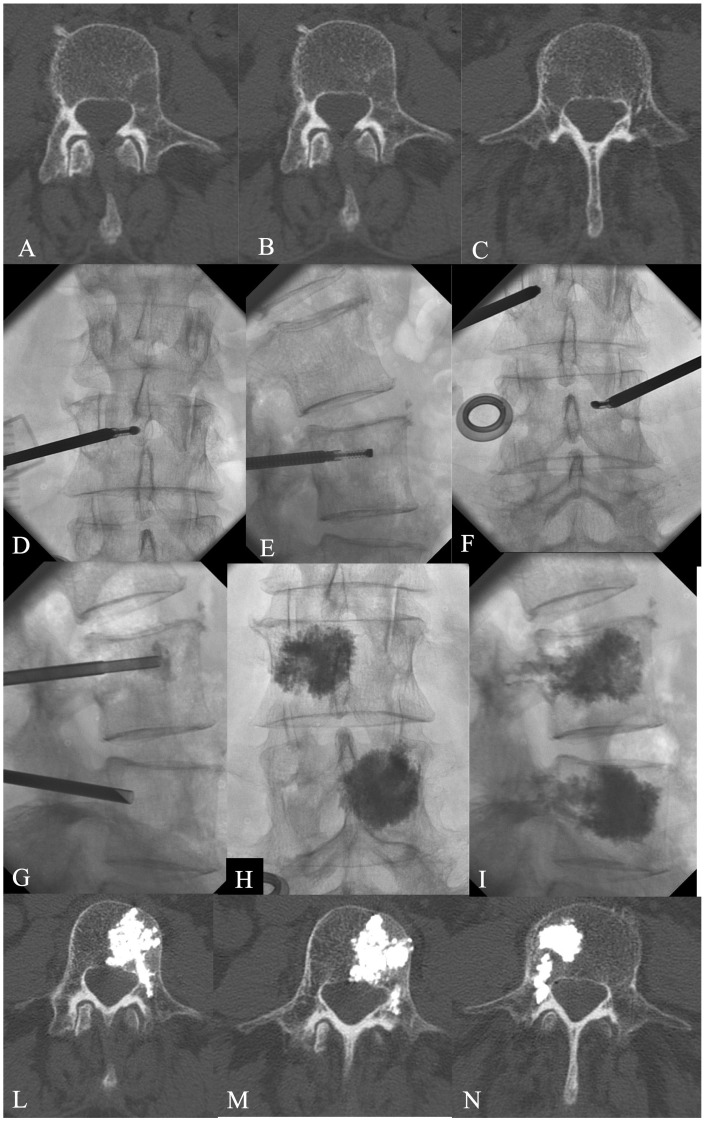
Instead of fluoroscopy control, the procedure can be performed under Fluoro-CT or CT guidance, especially for the treatment of extra-vertebral lesion location, such as sacrum bone or iliac bone, or the ‘bone-remodelling’ effect when the lesion involves the posterior element (lamina, peduncle, spinous process) of the spine. In any case the cement injection has to be performed always under fluoroscopy control (Figure 2(a–y)).
Figure 2.
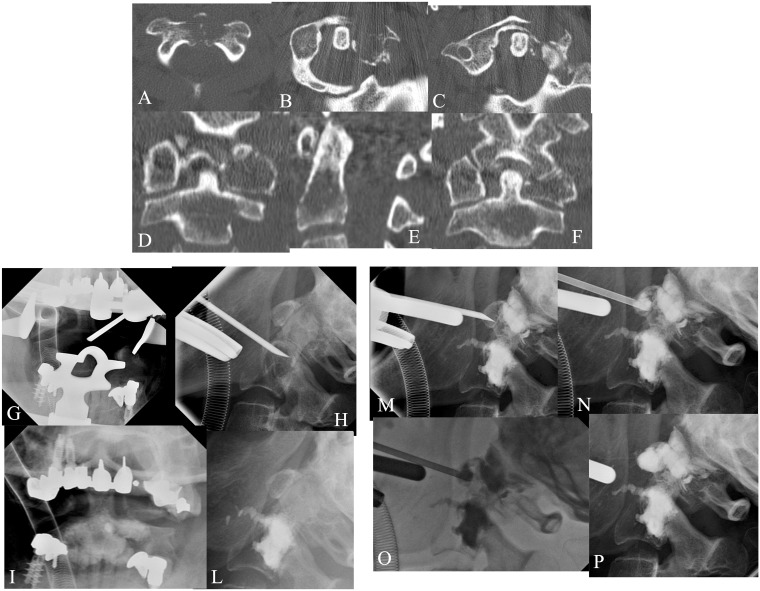
A 60-year-old female affected by renal carcinoma. MPR-MDCT (a–f) showed lytic metastases involving C1 and C2. VP was performed at C1–C2 levels under general anaesthesia and fluoroscopy guidance by the trans-oral approach (g–p).The lytic lesion involving the left lateral mass of C1 was performed by VP with a direct approach under fluoro-CT control. The post-treatment C1–C2 MPR-MDCT showed complete filling of both lesions without leakage, with vertebral stabilization and pain relief effects.
Sacroplasty can be performed precisely using a CT-guided technique in the case of small or massive sacral disease. The use of a CT-guided procedure avoids the frequent risk of incorrect needle position related to the difficult evaluation of the sacral bone on a conventional C-arm fluoroscopy, reducing the risk of intraforaminal leakage.32,33
VP + radiofrequency spinal tumour ablation
RF spinal tumour ablation can be performed alone or associated with VP in order to combine a carcinolytic effect with an antalgic effect.
The aim of performing RF heat ablation before VP is to destroy tumour tissue and to thrombose the paravertebral and intravertebral venous plexus, minimizing procedure-related complications. The purpose of the associated VP is then to stabilize the vertebra.35,36
RF is indicated in patients with unresectable osteolytic metastasis and who have not responded to radio-chemotherapy, or who have only moderately radiosensitive tumours (for example, prostate or breast cancer).37
The best indications are for patients with unremitting spine pain, in the absence of symptomatic spinal cord or roots compression, and refractory to conventional therapeutic options such as radiation therapy, chemotherapy, surgery and the use of analgesics. Absolute contraindications are patients with a pacemaker or other electronic device implant and C1–C2 lesions. This technique has the same approach as VP under fluoroscopic guidance with the patient in the prone position, under neuro-lepto-analgesia. The target is the joint lesion. Once the exact position of the needle has been verified, a 19-gauge needle electrode and a thermocouple are introduced coaxially through the inserted cannula into the central part of the lesion. After unsheathing the spiral electrode tine, which opens to a diameter of 9 mm and a length of 10 mm in the metastasis, the needle is connected with an RF generator. The RF heat ablation starts at an energy level of 15 W. The deployed energy is increased by 5 W every 2 minutes, up to 25 W. After the tumour necrosis by heat ablation, the lesion can be filled by cement (PMMA) injection in order to obtain pain relief and vertebral stabilization.35 The system has temperature sensors to monitor and display the temperature, minimizing patient risk during the RF energy release (Figure 3(a–n)).
Figure 3.
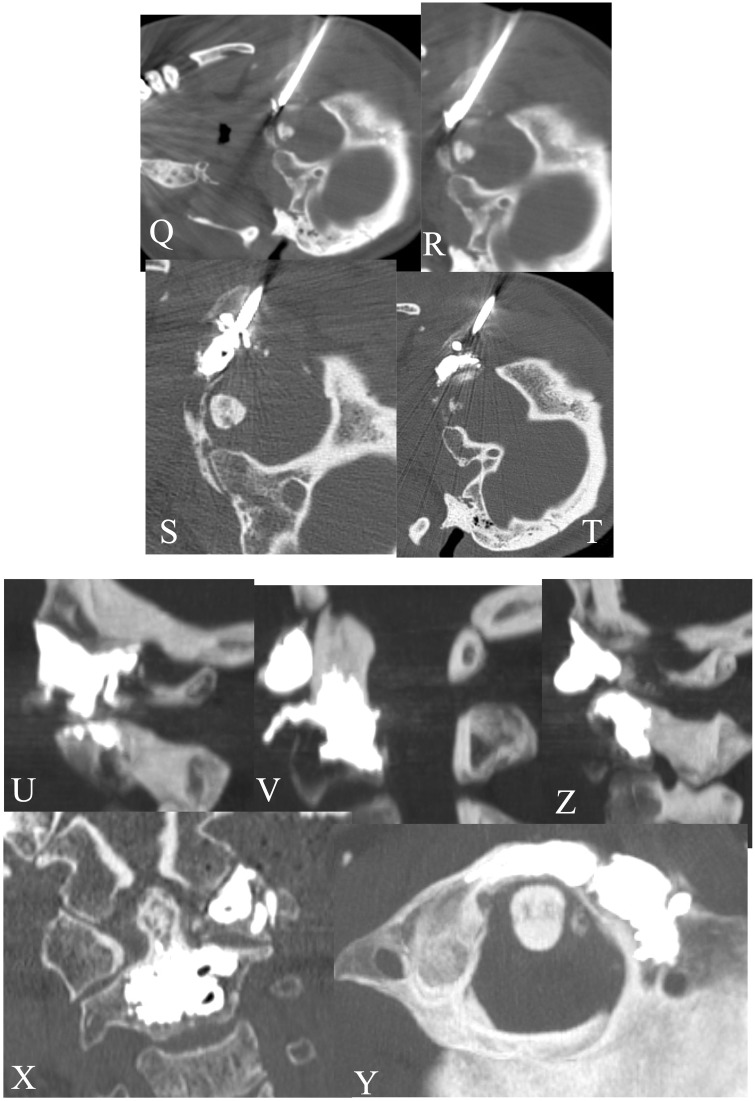
A 69-year-old male affected by lung cancer. CT (a–c) showed a lytic metastases involving the left peduncle/soma of L4 and right soma of L3. RF ablation + VP was performed at L3–L4 with a monopeduncular approach (d–i) with tumour ablation. The post-treatment CT showed a good filling of the lesions without leakage (l--n).
Contraindications for the combined procedure consist of osteoblastic tumours, fractures with retropulsion of the fragments within neural foramen, spread of tumour within the epidural space, local infection (osteomyelitis, discitis or epidural abscess), coagulative disorders, pain not related to vertebral collapse, steady asymptomatic fractures and tumour involvement or missing integrity of pedicles or joint facets.
Masala35 treated a group of patients with the association of RF and VP, registering a VAS reduction from an average of 8.6 points to 2.6 without any condition of extravasations of PMMA in the epidural or foraminal sites
VP-treatment efficacy and complications
There is a general scientific consensus supported by many international societies (Cardiovascular and Interventional Radiological Society of Europe [CIRSE], American Society of Neuroradiology [ASNR], American College of Radiology [ACR], Societe Francaise de Radiologie [SFR]) that percutaneous vertebral cementoplasty represents a successful, safe and effective minimally invasive procedure in selected patients for pain relief, performed with the correct medical indications.38
The pain resolution is demonstrated to occur in 84%–92% of patients within 24–72 hours. One of the main advantages of the procedure remains the possibility of avoiding drug abuse for patients in poor general condition. Because of the great psychological impact of pain relief and regained autonomic ambulation without a corset or crutches, the patient faces the disease in a more positive manner: for these reasons vertebroplasty is strongly advised before chemo/radiotherapy treatment.39,40
The Cancer Patient Fracture Evaluation (CAFE) study,41 the only multi-centre randomized controlled trial published, compared balloon kyphoplasty (70 patients) with non-surgical fracture management (64 patients) for treatment of painful VCFs in patients with cancer and one to three painful VCFs. The primary endpoint was a back-specific functional status measured by the Roland–Morris disability questionnaire (RDQ) score at one month .The mean RDQ score in the kyphoplasty group changed from 17.6 at baseline to 9.1 at one month (mean change −8.3 points, 95% CI − 6.4 to −10.2; p < 0.0001). The mean score in the control group changed from 18.2 to 18.0 (mean change 0.1 points; 95% CI − 0.8 to 1.0; p = 0.83). At one month, the kyphoplasty treatment effect for RDQ was −8.4 points (95% CI −7.6 to 9.2; p < 0.0001). The most common adverse events occurring within the first month were back pain (four of 70 in the BK group and five of 64 in the control group) and symptomatic vertebral fracture (two and three, respectively). This trial concluded that BK is an effective and safe treatment that rapidly reduces pain and improves function.
Concerning pain resolution related to neoplastic involvement of the vertebral bone, other therapy options seem to act slower than VP, as patients become painless generally in 2–4 months. Moreover, none of the treatments above can assure immediate stabilization of the affected vertebra, leading to the risk of vertebral collapse, despite delayed pain resolution.22–30
An antineoplastic effect can be obtained during VP associating the RF or crioablation systems, already used for the treatment of liver, lung, prostate or kidney lesions, with clinically significant pain relief in >90% of patients, and significant decrease in pain medication.29,30–35
There are two major drawbacks of this procedure; the extra-vertebral leakage of cement into the spinal canal or into pre/paravertebral venous plexus, with consequent compression of nervous structures or pulmonary embolism, and the risk of vertebral re-fractures at adjacent or distant metamers.
Extra-vertebral leakage is directly related to the pressure regulation capability of the injection, to the low viscosity of the cement and to the type of VCF. It occurs more frequently in metastatic vertebral fractures (30%–60% of cases), due to rich tumour vascularization, and less in osteoporotic fractures (10% of cases).42 The type of cement used and its density and viscosity are important elements to reduce the rate of leakage. New high-viscosity bone cement can reduce the venous leakage to 0.5%–1%.42 Anselmetti et al.43 performed VP in 60 patients dived into two groups comparing high viscosity cement versus PMMA, demonstrating a highly significant reduction of extravasation rate and leakage-related complications with high-viscosity cement versus standard one.
Using an RF cement injection system (StabiliT® Vertebral Augmentation system; DFINE Europe GmbH, Mannheim) under fluoroscopy control can reduce the rate of leakage, and this device seems to be a good option when a vertebra ‘at-risk’ for leakage into the spinal canal (i.e. posterior wall discontinuity) has to be treated. Trumm et al.44 performed CT fluoroscopy-guided, RF-induced VP (StabiliT®) in 25 neoplastic patients without reporting major extravasation. Osteoporotic fractures are now reduced about 0.5%–1%.
Performing the ‘remodelling technique’ using small fractionated injection of PMMA generally reduces significantly the risk of extra vertebral leakage, even in more risky areas. This explains why, despite originally being considered a contraindication, vertebral augmentation can be performed even in selected cases of epidural extension of the tumour.45–50
The cause of vertebral re-fractures at distant or adjacent metamer remains controversial and complex. Vertebral re-fracture is the natural evolution of osteoporosis or neoplastic disease. It has been demonstrated that a patient with a first vertebral compression fracture has a 19.2% risk of developing new fractures in the following first year, especially at distant metamer.51–54 It has also been well demonstrated that PMMA-injection into the vertebral body increases spinal stiffness and resistance, so modifying spinal biomechanics and leading to new vertebral fracture, especially at adjacent levels.55
Conclusions
Spine metastasis requires a multidisciplinary approach involving oncology, neurosurgery, orthopaedics, radiotherapy and interventional neuroradiology. Each type of lesion should be treated considering multiple factors, such as primary lesion, oncologic instability, radio-chemo-therapy resistance and psychological aspects. Percutaneous vertebroplasty and interventional tumour removal can be considered as safe, effective, and minimally invasive palliative therapies for reducing pain and improving function in patients with metastatic spinal tumours and malignant vertebral compression fractures. The factors influencing the results are the correct selection of the patients, the quality of the angio system, operator experience, the cement viscosity to reduce leakage and new material with RF.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
None declared
References
- 1.Constans JP, de Divitiis E, Donzelli R, et al. Spinal metastases with neurological manifestations. Review of 600 cases. J Neurosurg 1983; 59: 111–118. [DOI] [PubMed] [Google Scholar]
- 2.Curt GA, Breitbart W, Cella D, et al. Impact of cancer-related fatigue on the lives of patients: new findings from the Fatigue Coalition. Oncologist 2000; 5: 353–360. [DOI] [PubMed] [Google Scholar]
- 3.Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine 2000; 25: 2940–2952. [DOI] [PubMed] [Google Scholar]
- 4.Bodian CA, Freedman G, Hossain S, et al. The visual analog scale for pain: clinical significance in postoperative patients. Anesthesiology 2001; 95: 1356–1361. [DOI] [PubMed] [Google Scholar]
- 5.Bombardier C. Outcome assessments in the evaluation of treatment of spinal disorders: summary and general recommendations. Spine 2000; 25: 3100–3103. [DOI] [PubMed] [Google Scholar]
- 6.Weinstein JN. Surgical approach to spine tumor. Orthopedics 1989; 12: 897–904. [DOI] [PubMed] [Google Scholar]
- 7.Boriani S, Weinstein JN, Biagini R. Primary bone tumors of the spine. Terminology and surgical staging. Spine (Philadelphia Pa 1976) 1997; 22: 1036–1044. [DOI] [PubMed] [Google Scholar]
- 8.Hart RA, Boriani S, Biagini R, et al. A system for surgical staging and management of spine tumors. A clinical outcome study of giant cell tumors of the spine. Spine (Philadelphia Pa 1976) 1997; 22: 1773–1782. [DOI] [PubMed] [Google Scholar]
- 9.Chan P, Boriani S, Fourney DR, et al. An assessment of the reliability of the Enneking and Weinstein–Boriani–Biagini classifications for staging of primary spinal tumors by the Spine Oncology Study Group. Spine 2009; 34: 384–391. [DOI] [PubMed] [Google Scholar]
- 10.Tomita K, Kawahara N, Kobayashi T, et al. Surgical strategy for spinal metastases. Spine (Philadelphia Pa 1976) 2001; 26: 298–306. [DOI] [PubMed] [Google Scholar]
- 11.Laufer I, Sciubba DM, Madera M, et al. Surgical management of metastatic spinal tumors. Cancer Control 2012; 19: 122–128. [DOI] [PubMed] [Google Scholar]
- 12.Georgy BA. Metastatic spinal lesions: state-of-the-art treatment options and future trends. Am J Neuroradiol 2008; 29: 1605–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janjan NA. radiation for bone metastases : conventional technique and the role of systemic radiopharmaceuticals. Cancer 1997; 80: 1628–1645. [DOI] [PubMed] [Google Scholar]
- 14.Buhmann Kirchhoff S, Becker C, Duerr HR, et al. Detection of osseous metastases of the spine: Comparison of high resolution multi-detector-CT with MRI. Eur J Radiol 2009; 69: 567–573. [DOI] [PubMed] [Google Scholar]
- 15.Katagiri H, Takahashi M, Inagaki J, et al. Clinical results of nonsurgical treatment for spinal metastases. Int J Radiat Oncol Biol Phys 1998; 42: 1127–1132. [DOI] [PubMed] [Google Scholar]
- 16.Fisher CG, DiPaola CP, Ryken TC, et al. A novel classification system for spinal instability in neoplastic disease: an evidence-based approach and expert consensus from the Spine Oncology Study Group. Spine 2010; 35: E1221–1229. [DOI] [PubMed] [Google Scholar]
- 17.Krishnaney AA, Steinmetz MP, Benzel EC. Biomechanics of metastatic spine cancer. Neurosurg Clin N Am 2004; 15: 375–380. [DOI] [PubMed] [Google Scholar]
- 18.Weber MH, Burch S, Buckley J, et al. Instability and impending instability of the thoracolumbar spine in patients with spinal metastases: a systematic review. Int J Oncol 2011; 38: 5–12. [PubMed] [Google Scholar]
- 19.McConnell CT, Jr, Wippold FJ, 2nd, Ray CE, Jr, et al. ACR appropriateness criteria management of vertebral compression fractures. J Am Coll Radiol 2014; 14: S1546–1440. [DOI] [PubMed] [Google Scholar]
- 20.Anselmetti GC, Muto M, Guglielmi G, et al. Percutaneous vertebroplasty or kyphoplasty. Radiol Clin North Am 2010; 48: 641–649. [DOI] [PubMed] [Google Scholar]
- 21.Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: A randomised trial. Lancet 2005; 366: 643–648. [DOI] [PubMed] [Google Scholar]
- 22.Cheung G, Chow E, Holden L, et al. Percutaneous vertebroplasty in patients with intractable pain from osteoporotic or metastatic fractures: A prospective study using quality-of-life assessment. Can Assoc Radiol J 2006; 57: 13–21. [PubMed] [Google Scholar]
- 23.Fourney DR, Schomer DF, Nader R, et al. Percutaneous vertebroplasty and kyphoplasty for painful vertebral body fractures in cancer patients. J Neurosurg 2003; 98: 21–30. [DOI] [PubMed] [Google Scholar]
- 24.Ahn H, Mousavi P, Roth S, et al. Stability of the metastatic spine pre- and post vertebroplasty. J Spinal Disord Tech 2006; 19: 178–182. [DOI] [PubMed] [Google Scholar]
- 25.Aebli N, Goss BG, Thorpe P, et al. In vivo temperature profile of intervertebral discs and vertebral endplates during vertebroplasty: an experimental study in sheep. Spine (Philadelphia Pa 1976) 2006; 31: 1674–1678. Discussion 1679. [DOI] [PubMed] [Google Scholar]
- 26.Fenton DS, Czervionke LF. Image-guided Spine Interventions, New York: Springer, 2004, pp. 69–93. [Google Scholar]
- 27.Mathis JM. Vertebroplasty for vertebral fractures with intravertebral clefts. Am J Neuroradiol 2002; 23: 1619–1620. [PMC free article] [PubMed] [Google Scholar]
- 28.Shaefer O, Lohrmann C, Markmiller M, et al. Combined treatment of a spinal metastasis with radiofrequency heat ablation and vertebrioplasty. Am J Roentgenol 2003; 180: 1075–1077. [DOI] [PubMed] [Google Scholar]
- 29.Van der Linden E, Kroft LJ, Dijkstra PD. Treatment of vertebral tumor with posterior wall defect using image-guided radiofrequency ablation combined with vertebroplasty: preliminary results in 12 patients. J Vasc Interv Radiol 2007; 18: 741–747. [DOI] [PubMed] [Google Scholar]
- 30.Gangi A, Buy X. Percutaneous bone tumor management. Semin Intervent Radiol 2010; 27: 124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirsch JA, Hirsch AE, Jha R, et al. Practical management of malignant compression fractures. J Neurointervent Surg 2010; 2: 219–220. [DOI] [PubMed] [Google Scholar]
- 32.Andreson DE, Andreson DE, Cotton JR. Mechanical analysis of percutaneous sacroplasty using CT imaged based finite element model. Med Eng Phys 2007; 29: 316–325. [DOI] [PubMed] [Google Scholar]
- 33.Pommershei W, Huang-Hellinger F, Baker M, et al. Sacroplasty: a treatment for sacral insufficiency fractures. Am J Neuroradiol 2003; 24: 1003–1007. [PMC free article] [PubMed] [Google Scholar]
- 34.Richards AM, Mears SC, Knight TA, et al. Biomechanical analysis of sacroplasty: Does volume or location of cement matter? Am J Neuroradiol 2008; 30: 315–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Masala S, Roselli M, Massari F, et al. Radiofrequency heat ablation and vertebroplasty in the treatment of neoplastic vertebral body fractures. Anticancer Res 2004; 24: 3129–3133. [PubMed] [Google Scholar]
- 36.Georgy BA. Metastatic spinal lesions: state-of-the-art treatment options and future trends. Am J Neuroradiol 2008; 29: 1605–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grönemeyer DH, Schirp S, Gevargez A. Image-guided radiofrequency ablation of spinal tumors: preliminary experience with an expandable array electrode. Cancer J 2002; 8: 33–39. [DOI] [PubMed] [Google Scholar]
- 38.Barr JD, Jensen ME, Hirsch JA, et al. Position statement on percutaneous vertebral augmentation: a consensus statement developed by the Society of Interventional Radiology (SIR), American Association of Neurological Surgeons (AANS) and the Congress of Neurological Surgeons (CNS), American College of Radiology (ACR), American Society of Neuroradiology (ASNR), American Society of Spine Radiology (ASSR), Canadian Interventional Radiology Association (CIRA), and the Society of NeuroInterventional Surgery (SNIS). J Vasc Interv Radiol 2014; 25: 171–181. [DOI] [PubMed] [Google Scholar]
- 39.Cheung G, Chow E, Holden L, et al. Percutaneous vertebroplasty in patients with intractable pain from osteoporotic or metastatic fractures: A prospective study using quality-of-life assessment. Can Assoc Radiol J 2006; 57: 13–21. [PubMed] [Google Scholar]
- 40.Fourney DR, Schomer DF, Nader R, et al. Percutaneous vertebroplasty and kyphoplasty for painful vertebral body fractures in cancer patients. J Neurosurg 2003; 98: 21–30. [DOI] [PubMed] [Google Scholar]
- 41.Berenson J, Pflugmacher R, Jarzem P, et al. Balloon kyphoplasty versus non-surgical fracture management for treatment of painful vertebral body compression fractures in patients with cancer: a multicentre, randomised controlled trial. Lancet Oncol 2011; 12: 225–235. [DOI] [PubMed] [Google Scholar]
- 42.Muto M, Perrotta V, Guarnieri G, et al. Vertebroplasty and kyphoplasty: friends or foes? Radiol Med 2008; 113: 1171–1184. [DOI] [PubMed] [Google Scholar]
- 43.Anselmetti GC, Zoarski G, Manca A, et al. Percutaneous vertebroplasty and bone cement leakage: clinical experience with a new high-viscosity bone cement and delivery system for vertebral augmentation in benign and malignant compression fractures. Cardiovasc Intervent Radiol 2008; 31: 937–947. [DOI] [PubMed] [Google Scholar]
- 44.Trumm CG, Jakobs TF, Stahl R, et al. CT fluoroscopy-guided vertebral augmentation with a radiofrequency-induced, high-viscosity bone cement (StabiliT(®)): technical results and polymethylmethacrylate leakages in 25 patients. Skeletal Radiol 2013; 42: 113–120. [DOI] [PubMed] [Google Scholar]
- 45.Luo J, Adams MA, Dolan P. Vertebroplasty and kyphoplasty can restore normal spine mechanics following osteoporotic vertebral fracture. J Osteoporos 2010; 2010: 729257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gibson J, et al. Does cement distribution influence the mechanical outcome of vertebroplasty? In Proceedings of the Society of Back Pain Research Meeting, Keele University, 2008.
- 47.Baroud G, Crookshank M, Bohner M. High-viscosity cement significantly enhances uniformity of cement filling in vertebroplasty: an experimental model and study on cement leakage. Spine 2006; 31: 2562–2568. [DOI] [PubMed] [Google Scholar]
- 48.Loeffel M, Ferguson SJ, Nolte L, et al. Vertebroplasty: experimental characterization of polymethyl- methacrylate bone cement spreading as a function of viscosity, bone porosity, and flow rate. Spine 2008; 12: 1352–1359. [DOI] [PubMed] [Google Scholar]
- 49.Sun K, et al. Cement filling pattern has a significant effect on biomechanics of vertebroplasty. In Proceedings of the 52nd Annual Meeting of the Orthopaedic Research Society, Chicago, IL, 2006.
- 50.Cortet B, Cotten A, Boutry N, et al. Percutaneous vertebroplasty in patients with osteolytic metastases or multiple myelomas. Rev Rhum Engl Ed 1997; 64: 177–183. [PubMed] [Google Scholar]
- 51.Voormolen MH, Lohle PN, Juttmann JR, et al. The risk of new osteoporotic vertebral compression fractures in the year after percutaneous vertebroplasty. J Vasc Interv Radiol 2006; 17: 71–76. [DOI] [PubMed] [Google Scholar]
- 52.Lindsay R, Silvermann LS, Seeman E, et al. Risk of new vertebral fracture in the year following a fracture. J Am Med Assoc 2001; 285: 320–323. [DOI] [PubMed] [Google Scholar]
- 53.Lee WS, Sung KH, Choi YW, et al. Risk factors of developing new symptomatic vertebral compression fractures after percutaneous vertebroplasty in osteoporotic patients. Eur Spine J 2006; 15: 1777–1783. [DOI] [PubMed] [Google Scholar]
- 54.Syed M, Patel NA, Jan S, et al. New symptomatic VCF within a year following VP in osteoporotic women. 20% of the patients. Am J Neuroradiol 2005; 26: 1601–1604. [PMC free article] [PubMed] [Google Scholar]
- 55.Molloy S, Riley III LH, Belkoff SM. Effect of cement volume and placement on mechanical-property restoration resulting from vertebroplasty. Am J Neuroradiol 2005; 26: 401–404. [PMC free article] [PubMed] [Google Scholar]