Abstract
Branch vessel occlusion is a potential consequence following flow diverter placement for intracranial aneurysms, but the frequency and clinical impact has not been completely elucidated. In this case of a 45-year-old woman with a large left internal carotid artery aneurysm, the ophthalmic artery was covered by two flow diverters and was acutely occluded along with the aneurysm. Common carotid injections failed to demonstrate collateral flow to the ophthalmic artery via the external carotid artery. Nonetheless, the patient woke from anesthesia with objectively stable and subjectively improved vision. This case demonstrates that an acute occlusion of the ophthalmic artery without external carotid artery collaterals can be tolerated clinically.
Keywords: Intracranial aneurysm, flow diversion, ophthalmic artery
Introduction
Flow diversion (FD) is being used increasingly for treatment of giant, wide-necked, and atypical intracranial aneurysms and functions by inducing progressive thrombosis within the aneurysm sac while maintaining blood flow in the parent and branch vessels.1–3 As experience increases, however, it is becoming evident that branch vessel patency is not guaranteed.4,5 The Pipeline Embolization Device (PED; EV3, Irvine, CA) is approved by the US Food and Drug Administration for the treatment of internal carotid artery (ICA) aneurysms proximal to the posterior communicating artery (PCommA). In this treatment, the ophthalmic artery (OphA) is frequently covered. Accordingly, one of the theoretical complications of FD in the proximal ICA is blindness. Here we report a case of a proximal ICA aneurysm in which the OphA was occluded immediately following PED placement and the patient experienced no clinical sequelae.
Case report
The patient was a 45-year-old female with a past medical history of hypertension, smoking, and migraines, who presented with headaches and left-sided blurry vision and on non-invasive imaging was found to have bilateral ICA aneurysms. Conventional angiography revealed a small right ICA aneurysm and a larger (8.7 × 7.3 × 4.9 mm) left ICA/OphA aneurysm with a 5.3 mm neck (Figure 1). There was normal filling of the OphA and the choroidal blush (Figure 2). Both stent-assisted coiling and FD were considered as endovascular treatment options. The decision was made to proceed with FD given the large aneurysm size, relatively large neck size, and proximal supraclinoid ICA location. The patient was placed on daily Aspirin 325 mg and Plavix 75 mg 2 weeks prior to the procedure.
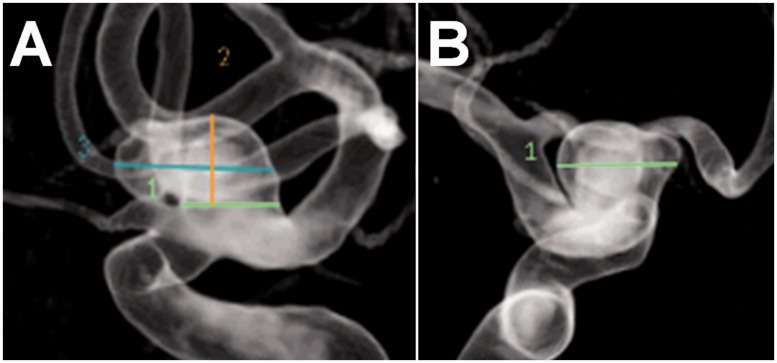
Figure 1.
Diagnostic angiogram, three-dimensional reconstructions, lateral (A) & AP (B). Left OphA aneurysm measuring 8.7 × 7.3 × 4.9 mm with a 5.3 mm neck. The OphA arises from the ICA just proximal to the aneurysm (A).
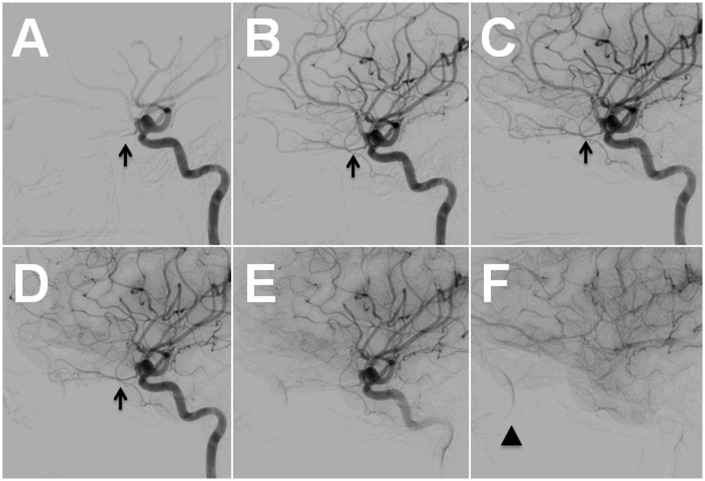
Figure 2.
Diagnostic angiogram, left ICA injection, lateral view. The OphA (arrow) can be traced from the arterial (A–E) to the venous phase (F) with good washout and good visualization of the choroidal blush (F; arrowhead).
Preoperatively, the patient was noted to be neurologically intact with 20/20 vision in the right eye and 20/40 vision in the left eye. On the day of the procedure, prior to intervention, it was noted that the OphA had delayed washout (Figure 3), which was new compared to the previous angiogram. The aneurysm was subsequently treated with two overlapping PEDs. After placement of the first PED it was noted that the aneurysm was no longer filling and the OphA continued to fill. After placement of the second PED it was noted that neither the aneurysm nor the OphA was filling (Figure 4). Intra-arterial Abciximab 5 mg was given to help prevent clot formation within the PED. The catheter was withdrawn into the common carotid artery (CCA) and an injection failed to demonstrate filling of the OphA from either the ICA or the external carotid artery (ECA) (Figure 5).
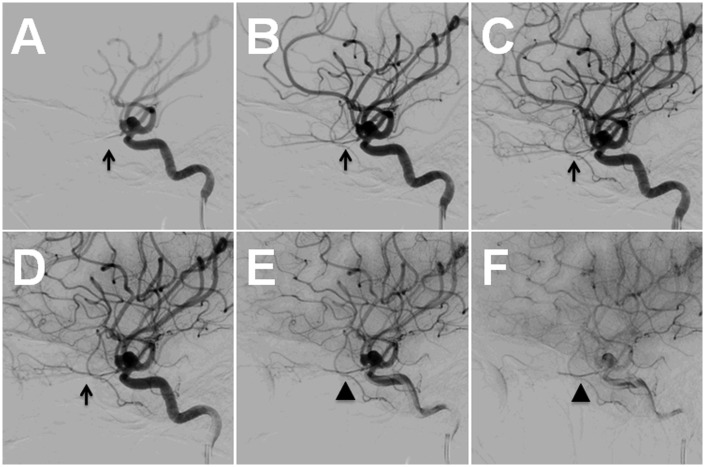
Figure 3.
Pre-treatment angiogram, left ICA injection, lateral view. The OphA (A–D; arrows) has delayed washout (E–F; arrowheads).
Figure 4.
Treatment angiogram, left ICA injection, lateral view. Pre-treatment (A). Following placement of the first PED, the aneurysm was no longer filling and the OphA continued filling (B). Following placement of the second PED, both the aneurysm and the OphA were completely occluded (C).
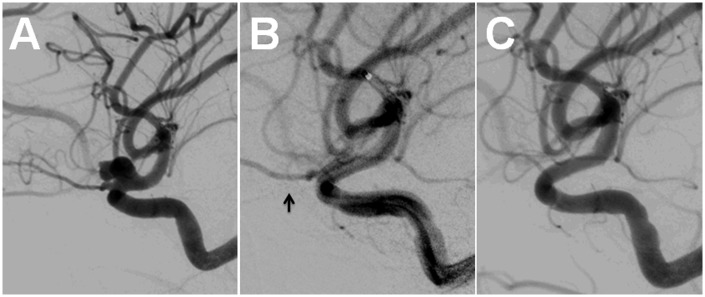
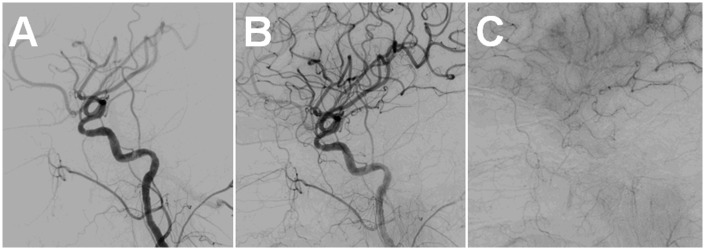
Figure 5.
Post-treatment angiogram, left CCA injection, lateral view. The OphA does not fill from either the ICA or the ECA (A, B). The choroidal blush is no longer seen (C).
Following the procedure, the patient reported stable vision and this was confirmed on examination. The following morning the patient reported improved vision in the left eye, although the examination remained the same. The patient was discharged on postoperative day 1. At the 3-week follow-up visit, the patient’s vision had remained objectively stable and subjectively improved compared to before intervention.
Discussion
As experience with FD increases, the fate of the OphA covered by a flow diverter is becoming more evident. Acute occlusion of the OphA is generally avoided during aneurysm intervention to prevent ischemia to the central retinal artery and blindness.6 Chronic occlusion is generally thought to be tolerated due to extensive ECA collaterals.4 The preclinical studies of FD models suggested that branch vessels covered by flow diverters remain patent over time.7
There are now a number of larger series that report OphA occlusion rates and clinical consequences. In the Budapest experience, 18 patients with 19 aneurysms were treated with FD.2 The OphA was covered in 17 cases, leading to one acute and two delayed occlusions. The acute occlusion lead to a “retinal branch occlusion” but the patient’s postoperative vision was not reported. The chronic occlusions were clinically silent.
Puffer et al. published a series of 19 patients with 20 aneurysms that were treated with FD.4 The OphA was covered in all cases and the study was designed specifically to understand the patency of the OphA following FD. Immediately after treatment, two patients experienced delayed anterograde filling of the OphA and one patient experienced retrograde filling via collaterals. At follow-up (average 8.7 months), only 13 OphAs had normal flow (68%). Two OphAs had slow flow and four were completely occluded. Of those that were occluded, two had good visualization of ECA collaterals and two did not. There were no changes in vision. There were no predictive anatomic or procedural factors (including number of PEDs placed) that were predictive of OphA compromise.
Malatesta et al. published a series of 28 patients with 35 aneurysms treated with FD.8 The exact number of OphAs covered was not reported, but there were 29 aneurysms in the proximal ICA. There were no acute occlusions and one clinically silent delayed occlusion.
Moon et al. published a series of 29 patients with 38 ICA/OphA aneurysms treated with FD.9 There were no acute occlusions and one delayed occlusion that was accompanied by good ECA collateral supply to the OphA and no clinical impact.
Sise et al. reported a single case of retinal artery occlusion 3 weeks following treatment of an ICA aneurysm with two PEDs.10 There was good visualization of the OphA following the procedure. The patient presented 3 weeks following the procedure with painless vision loss and an ophthalmologic examination consistent with retinal branch occlusion. Platelet inhibition was satisfactory. Although the patient refused angiography during that admission, follow-up angiography at 6 months revealed a patent OphA. The vision had stabilized at this time as well. The presumed mechanism was embolic in nature.
Branch artery occlusion during FD is clearly a concern. It is speculated that arteries with collateral supply, such as the OphA (which receives many collaterals from the ECA), may have a higher tendency to occlude over time. This collateral supply occurs via the supraorbital, infraorbital, anterior deep temporal, sphenopalatine, middle meningeal, and superficial temporal arteries.11 In comparison, end arteries, such as the anterior choroidal artery, may have a higher tendency to remain patent due to flow demand.4 The posterior communicating artery has been found to occlude over time in approximately 50% of cases treated with FD.5 This is likely due to its frequent anastamosis with the posterior circulation. No patients experienced clinical sequelae (but there were no fetal PCAs covered in this series). Branch occlusions during FD have also been reported in the posterior circulation and MCA.12
Non-visualization of OphA collaterals or a choroidal blush following the procedure was surprising and concerning. Osborn et al. reported that the choroidal blush should be visualized from all ICA and CCA injections.13 Vignaud et al. reported that direct angiography should be able to visualize some of the many ECA anastamoses.11 Most importantly, it has also been shown that the choroidal blush can be appreciated via a CCA injection during an ICA/OphA balloon test occlusion (BTO) and is present in approximately 85% of patients.6 Perhaps a selective ECA injection was necessary to demonstrate the choroidal blush in this case. Presumably, blood was reaching the retina by an alternative pathway that was not angiographically evident. In a review of central retinal artery occlusion, Varma et al. found that the majority of patients, but not all, experience blindness.14 The authors go on to describe collateral supply from the cilioretinal artery, which is not always present. This may explain our patient’s lack of visual changes.
There are a few possible explanations for the OphA occlusion in this case. The occlusion occurred after placement of the second PED, which was placed to achieve better aneurysm coverage. It is possible that this increased coverage was unnecessary and led to the occlusion. In retrospect, placement of a single PED may have been more appropriate in this case. Additionally, the artery may have already been compromised before PED placement, given the delayed washout (Figure 3), and was destined to occlude even with placement of a single PED. A BTO was not utilized in this case as it is not our practice, but may be an informative step prior to FD.6 Finally, we did not test the efficacy of the Aspirin and Plavix prescribed preoperatively, and a sub-therapeutic response could explain the acute thrombosis. One possible explanation of the patient’s improved vision is decreased mass effect from the aneurysm on the optic nerve.
Conclusions
Following FD, the OphA rarely occludes acutely, occasionally occludes over time (up to 21%), is frequently supplied by ECA collaterals, and usually does not result in clinical sequelae. This case is unique in that it demonstrates that an acute occlusion of the OphA without angiographic ECA collaterals can be tolerated clinically. Although we would certainly not advocate for a complete disregard to maintaining patency of the OphA, this phenomenon may be of interest to other interventionalists.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interest
Aman Patel is a consultant for the company Penumbra. This conflict had no bearing on this case.
References
- 1.Lylyk P, Miranda C, Ceratto R, et al. Curative endovascular reconstruction of cerebral aneurysms with the pipeline embolization device: The Buenos Aires experience. Neurosurgery 2009; 64: 632–642. [DOI] [PubMed] [Google Scholar]
- 2.Szikora I, Berentei Z, Kulcsar Z, et al. Treatment of intracranial aneurysms by functional reconstruction of the parent artery: The Budapest experience with the pipeline embolization device. Am J Neuroradiol 2010; 31: 1139–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nelson PK, Lylyk P, Szikora I, et al. The pipeline embolization device for the intracranial treatment of aneurysms trial. Am J Neuroradiol 2011; 32: 34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Puffer RC, Kallmes DF, Cloft HJ, et al. Patency of the ophthalmic artery after flow diversion treatment of paraclinoid aneurysms. J Neurosurg 2012; 116: 892–896. [DOI] [PubMed] [Google Scholar]
- 5.Brinjikji W, Lanzino G, Cloft HJ, et al. Patency of the posterior communicating artery after flow diversion treatment of internal carotid artery aneurysms. Clin Neurol Neurosurg 2014; 120: 84–88. [DOI] [PubMed] [Google Scholar]
- 6.Ahn JH, Cho YD, Kang HS, et al. Endovascular treatment of ophthalmic artery aneurysms: Assessing balloon test occlusion and preservation of vision in coil embolization. Am J Neuroradiol 2014; 35: 2146–2152. [DOI] [PMC free article] [PubMed]
- 7.Kallmes DF, Ding YH, Dai D, et al. A new endoluminal, flow-disrupting device for treatment of saccular aneurysms. Stroke 2007; 38: 2346–2352. [DOI] [PubMed] [Google Scholar]
- 8.Malatesta E, Nuzzi NP, Divenuto I, et al. Endovascular treatment of intracranial aneurysms with flow-diverter stents: Preliminary single-centre experience. Radiol Med 2013; 118: 971–983. [DOI] [PubMed] [Google Scholar]
- 9.Moon K, Albuquerque FC, Ducruet AF, et al. Treatment of ophthalmic segment carotid aneurysms using the pipeline embolization device: Clinical and angiographic follow-up. Neurol Res 2014; 36: 344–350. [DOI] [PubMed] [Google Scholar]
- 10.Sise AB, Osher JM, Kolsky MP, et al. Pipeline embolization device: A new source for embolic retinal vascular occlusion. J Neuroophthalmol 2013; 33: 373–376. [DOI] [PubMed] [Google Scholar]
- 11.Vignaud J, Hasso AN, Lasjaunias P, et al. Orbital vascular anatomy and embryology. Radiology 1974; 111: 617–626. [DOI] [PubMed] [Google Scholar]
- 12.Lall RR, Crobeddu E, Lanzino G, et al. Acute branch occlusion after Pipeline embolization of intracranial aneurysms. J Clin Neurosci 2014; 21: 668–672. [DOI] [PubMed] [Google Scholar]
- 13.Osborn AG, Thurman DJ, Van Dyk HJ. The angiographic ocular choroidal crescent: Distortion with intraorbital and remote intracranial pathology. Neuroradiology 1978; 15: 13–19. [DOI] [PubMed] [Google Scholar]
- 14.Varma DD, Cugati S, Lee AW, et al. A review of central retinal artery occlusion: Clinical presentation and management. Eye (Lond) 2013; 27: 688–697. [DOI] [PMC free article] [PubMed] [Google Scholar]