Abstract
Introduction
The technique of balloon remodeling allows the endovascular treatment of wide-neck intracranial aneurysms. For many years the only available devices were the Hyperform and the Hyperglide balloon catheters. Recently, other companies have developed newer devices, single or dual-lumen. We present our initial experience with the TransForm occlusion balloon catheter for the treatment of intracranial aneurysms.
Methods
We retrospectively analysed from our prospectively gathered aneurysm database all aneurysms that were treated with balloon remodelling using TransForm occlusion balloon catheters from January 2013 to February 2014. We assessed patient demographics, morphological features of the aneurysms, procedure feasibility, technical and clinical complications.
Results
Thirty-three patients harbouring 36 intracranial saccular aneurysms were treated during 33 procedures. Clinical finding were: 15 incidental discovery, 13 subarachnoid haemorrhage (SAH), five aneurysms with mass effect, one ruptured aneurysm with SAH and mass effect, one recanalisation and one intraparenchymal haematoma. Thirty-five aneurysms were in the anterior and one in the posterior circulation. Mean dome and neck size were, respectively, 5.8 mm and 3.6 mm. Twenty-three aneurysms were treated with TransForm C and 13 with TransForm SC. We had two procedural thromboembolic complications, without permanent clinical events. No early rebleeding occurred.
Conclusions
In our small series, the TransForm occlusion balloon catheter seems to be safe and effective for the treatment of intracranial aneurysms, in ruptured and unruptured cases.
Keywords: Intracranial aneurysm, balloon-assisted coiling, transForm
Introduction
Moret et al.1 first described the balloon-remodeling technique for the treatment of wide-neck intracranial aneurysms. Sluzewsky et al.2 found a higher complication rate of balloon remodeling compared to standard coiling, but recently many clinical series and literature reviews have proved the safety and effectiveness of this technique3–6 in ruptured and unruptured cases. In the last decade, most of the treatments of intracranial aneurysms with the remodeling technique were performed with the Hyperform and Hyperglide (Covidien/eV3, Irvine, CA, USA) single-lumen compliant or hyper compliant balloon catheters. Recently, other companies have developed single and dual-lumen balloon catheters for balloon-assisted coiling of intracranial aneurysms. Spiotta et al.7 reported the feasibility and safety of the coaxial dual-lumen Scepter C balloon catheter (Microvention, Tustin, California, USA), and Lazzaro et al.8 analysed some cases performed with the coaxial dual-lumen Ascent balloon catheter (Codman Neurovascular, Raynham, MA, USA). The aim of our study was to analyse the efficacy and safety of the TransForm occlusion balloon catheter (Stryker Neurovascular, Fremont, CA, USA) for the treatment of intracranial aneurysms. We report our preliminary experience with the use of the TransForm occlusion balloon catheter in sidewall and bifurcation intracranial aneurysms.
Materials and methods
Study design and patient selection
We retrospectively analysed from our prospectively gathered database all intracranial aneurysms treated with the TransForm occlusion balloon catheter from January 2013 to February 2014. All unruptured and ruptured aneurysms treated with this device were included in our study. All cases of unruptured aneurysms were previously discussed during our interventional meeting.
Device description
The Transform occlusion balloon catheter was designed for the treatment of intracranial aneurysms using the remodeling technique. It is a single-lumen, over-the-wire, compliant and super compliant balloon catheter compatible with 0.014-inch steerable microguidewires.
Endovascular procedure
The endovascular procedure was performed under general anaesthesia and full anticoagulation with heparin. In addition, all patients with no history of subarachnoid haemorrhage (SAH) were given intravenous aspirin 250 mg during the procedure. Three-dimensional images acquired with rotational angiography with a dedicated workstation (Xtra Vision, Interventional Tools, Philips Healthcare, The Netherlands) were used to choose the working projection. Angiographic images were acquired in anteroposterior, lateral and working projections before and immediately after the treatment. There were no exclusion criteria. The TransForm occlusion balloon catheter was navigated, when possible, with a J-shaped microguidewire to reduce the risk of distal perforation. The balloon catheter was inflated to stabilise the coil mass or the microcatheter. At the end of the procedure, the balloon catheter was inflated to retrieve the microcatheter used for coiling from the aneurysm. A contrast media/saline mixture of 50:50 was used to inflate the balloon catheter with a 1 cc syringe. A flat panel computed tomography scan (XperCT, Allura series, Philips Healthcare, The Netherlands) was performed at the end of every procedure.
Clinical events
Any clinical events appearing in the postoperative period were noted. Clinical assessment was performed before and after the endovascular treatment.
Data collection
Patient age and sex, aneurysm location, rupture status at presentation, clinical symptoms at presentation, degree of aneurysm occlusion, type of balloon catheters and microguidewires, technical and clinical complications were noted. Angiographic results were classified as described by Roy et al.9
Results
Patient and aneurysm characteristics
Thirty-three consecutive patients harboring 36 saccular intracranial aneurysms were treated with the TransForm occlusion balloon catheter during 33 procedures. We treated 11 men and 22 women with a mean age of 56 ± 13 years. Clinical findings were: 15/36 (41.7%) incidental discoveries; 15/36 (41.7%) acute bleedings (14 SAHs and one intraparenchymal haematoma); 5/36 (13.8%) aneurysms with mass effect and 1/36 (2.8%) recanalisation. The locations of the aneurysms were: 7/36 (19.4%) paraclinoidal; 7/36 (19.4%) posterior communicating artery; 10/36 (27.8%) middle cerebral artery (MCA); 8/36 (22.2%) anterior communicating artery (AcoA); 2/36 (5.6%) A1 segment; 1/36 (2.8%) carotid termination and 1/36 (2.8%) basilar tip. Mean dome size was: 5.8 ± 4.3 mm, mean neck size was: 3.6 ± 1.6 mm, mean width size was: 5.5 ± 3.2 mm, dome/neck ratio was <1.5 in 21/36 (58.3%) aneurysms, >1.5 in 15/36 (41.7%) aneurysms. Twenty-three aneurysms were treated with the TransForm compliant occlusion balloon catheter and 13 with the TransForm super compliant. Nineteen aneurysms were treated using the TransForm compliant 4×10, four with the TransForm compliant 4 × 15, nine with the TransForm super compliant 4×7 and four with the Transform super compliant 4×10. In 29 cases, the balloon catheter was used with the Transend microguidewire (Stryker Neurovascular, Fremont, CA, USA), in seven cases with the Synchro microguidewire (Stryker Neurovascular, Fremont, CA, USA). Patients, aneurysms and device characteristics are summarised in Table 1.
Table 1.
Patients, aneurysms and device characteristics.
| Patients | Aneurysms | Presentation | Dome/neck | Location | Transform type/size | Microguidewire |
|---|---|---|---|---|---|---|
| 1 | 1 | Incidental | 2.2/2.5 | VB | SC/4×7 | Synchro 0.014 in. |
| 2 | 2 | SAH | 6.0/6.0 | Paraclinoidal | C/4×10 | Transend 0.014 in. |
| 3 | 3 | Recurrence | 9.1/3.4 | MCA | SC/4×7 | Transend 0.014 in. |
| 4 | 4 | Mass effect | 2.8/3.1 | AcoA | SC/4×7 | Transend 0.014 in. |
| 5 | 5 | Incidental | 4.1/2.6 | MCA | C/4×10 | Transend 0.014 in. |
| 6 | 6 | SAH | 5.7/5.2 | A1 | C/4×10 | Synchro 0.014 in. |
| 7 | 7 | Incidental | 2.2/1.2 | AcoA | C/4×10 | Synchro 0.014 in. |
| 8 | Incidental | 6.4/3.2 | A1 | C/4×10 | Synchro 0.014 in. | |
| 8 | 9 | Incidental | 4.9/3.7 | MCA | SC/4×7 | Transend 0.014 in. |
| 9 | 10 | SAH | 4.7/2.9 | Pcom | SC/4×7 | Transend 0.014 in. |
| 10 | 11 | Mass effect | 7.6/4.2 | Pcom | SC/4×7 | Transend 0.014 in. |
| 11 | 12 | SAH | 15.1/5.3 | AcoA | C/4×10 | Transend 0.014 in. |
| 12 | 13 | Incidental | 6.3/4.5 | Carotid T | C/4×10 | Transend 0.014 in. |
| 13 | 14 | SAH | 2.4/2.3 | MCA | SC/4×10 | Transend 0.014 in. |
| 14 | 15 | Incidental | 3.9/1.9 | Paraclinoidal | C/4×10 | Transend 0.014 in. |
| 15 | 16 | Incidental | 4.0/3.0 | Pcom | C/4×15 | Transend 0.014 in. |
| 16 | 17 | Incidental | 3.4/2.4 | Paraclinoidal | SC/4×10 | Transend 0.014 in. |
| 17 | 18 | Hematoma | 4.4/3.2 | Paraclinoidal | SC/4×10 | Transend 0.014 in. |
| 18 | 19 | Mass effect | 16.6/4.9 | AcoA | SC/4×10 | Transend 0.014 in. |
| 19 | 20 | Incidental | 4.3/2.5 | MCA | C/4×10 | Transend 0.014 in. |
| 20 | 21 | Incidental | 10.7/8.4 | Paraclinoidal | C/4×15 | Transend 0.014 in. |
| 21 | 22 | SAH | 2.1/2.8 | Pcom | C/4×10 | Transend 0.014 in. |
| 22 | 23 | SAH | 2.8/2.9 | AcoA | SC/4×7 | Transend 0.014 in. |
| 23 | 24 | Mass effect | 18.5/8.7 | Paraclinoidal | C/4×15 | Transend 0.014 in. |
| 24 | 25 | SAH/mass effect | 3.7/3.9 | Pcom | C/4×10 | Transend 0.014 in. |
| 25 | 26 | Incidental | 6.7/3.5 | AcoA | C/4×10 | Synchro 0.014 in. |
| 26 | 27 | Incidental | 3.2/2.8 | MCA | C/4×10 | Transend 0.014 in. |
| 28 | Incidental | 6.3/3.9 | MCA | C/4×10 | Transend 0.014 in. | |
| 27 | 29 | Mass effect | 3.7/3.0 | Paraclinoidal | C/4×10 | Transend 0.014 in. |
| 28 | 30 | SAH | 16.2/6.0 | Pcom | C/4×15 | Transend 0.014 in. |
| 29 | 31 | SAH | 2.8/1.1 | MCA | C/4×10 | Transend 0.014 in. |
| 30 | 32 | SAH | 3.0/2.1 | AcoA | C/4×10 | Transend 0.014 in. |
| 31 | 33 | Incidental | 2.9/2.3 | MCA | C/4×10 | Transend 0.014 in. |
| 32 | 34 | SAH | 2.5/2.4 | MCA | SC/4×7 | Synchro 0.014 in. |
| 35 | SAH | 4.2/4.2 | AcoA | SC/4×7 | Synchro 0.014 in. | |
| 33 | 36 | SAH | 2.5/2.4 | Pcom | C/4×10 | Transend 0.014 in. |
SAH: subarachnoid haemorrhage; VB: vertebro-basilar; C: compliant; SC: supercompliant; MCA: middle cerebral artery; Pcom: posterior communicating artery; AcoA: anterior communicating artery; carotid T: carotid termination.
Feasibility
In all cases, the balloon-remodeling device was placed in a proper position and all the treatments were successful.
Safety
We did not experience any aneurismal or arterial procedural rupture. The balloon catheter inflated and deflated in a proper and rapid way, without leakage of contrast media in all cases except one treatment with a Transform 4×7 super compliant and a Synchro microguidewire; in this procedure, during the deployment of the first coil, the balloon deflated spontaneously; we resolved this problem by slightly turning and pushing forward the Synchro microguidewire. We had two procedural thromboembolic events related to the remodeling technique; the first was an occlusion of the inferior branch during the treatment of a ruptured MCA bifurcation aneurysm, which was promptly reopened with balloon angioplasty using the TransForm balloon, without distal migration of the clot or ischaemic complication, the second was a patient who presented after the procedure with a reversible dysarthria, diffusion-weighted imaging showed a small ischaemic complication in the genu of the corpus callosum. In another case we reported a coil protrusion in the parent artery at the end of the procedure during the treatment of an unruptured wide-neck carotid-ophthalmic aneurysm; the patient was left under 250 mg of aspirin once a day for 3 months without clinical complications (Figure 1). In one procedure, during the inflation to retrieve the microcatheter, the balloon catheter deflated spontaneously; when we retrieved it we found a small hole in its middle third.
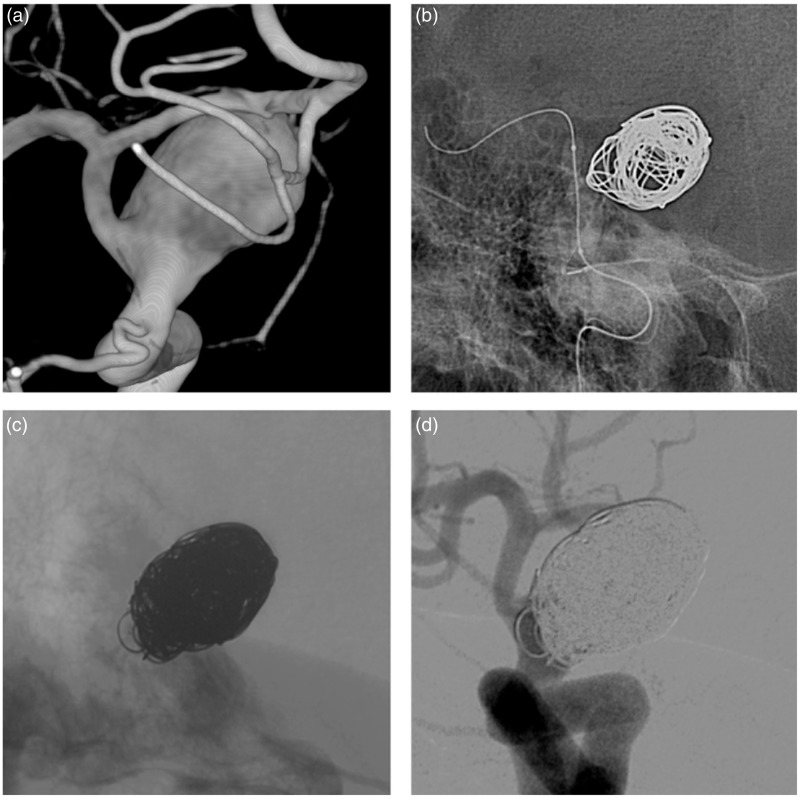
Figure 1.
(a) Three-dimensional imaging showing an unruptured paraclinoidal aneurysm. (b) Unsubtracted imaging showing the inflated Transform C 4×15 balloon catheter. (c) Unsubtracted imaging showing a coil protrusion into the parent artery. (d) Final control angiogram.
Efficacy
Angiographic final controls show a complete occlusion in 21/36 (58.3%) aneurysms, a neck remnant in 13/36 (36.1%) and a sac remnant in 2/36 (5.6%). Differences between ruptured and unruptured aneurysms are summarised in Table 2. We did not report any early rebleeding.
Table 2.
Angiographic final controls.
| A | B | C | Total | |
|---|---|---|---|---|
| Ruptured | 8 (53.3) | 6 (40.0) | 1(6.7) | 15 (41.7) |
| Unruptured | 13 (61.9) | 7 (33.3) | 1 (4.8) | 21 (58.3) |
| Total | 21 (58.3) | 13 (36.1) | 2 (5.6) | 36 (100.0) |
A: complete occlusion; B: neck remnant; C: sac remnant.
Data are expressed as n (percentage).
Discussion
We described our initial experience with the TransForm occlusion balloon catheter for the treatment of intracranial aneurysms. Our report is the first to analyse the feasibility, safety and efficacy of this new device. In our series, we treated ruptured and unruptured saccular aneurysms, with occlusion rates and technical complications comparable to recent literature.5
The remodeling technique was developed to allow the endovascular treatment of wide-neck intracranial aneurysms. The first devices were compliant latex balloons glued to the tip of a flow-dependent microcatheter. Later, compliant (Hyperglide, Covidien/eV3, Irvine, CA, USA) and hyper compliant (Hyperform, Covidien/eV3, Irvine, CA, USA) single-lumen balloon catheters were developed to treat wide-neck sidewall and complex bifurcation aneurysms. Recently, other companies have developed newer devices for balloon-assisted coiling: the single-lumen TransForm occlusion balloon catheter, the dual-lumen Scepter balloon catheter and the dual-lumen Ascent balloon catheter. These dual-lumen balloon catheters provide some advantages compared to single-lumen balloon catheters. They are compatible with all 0.014 inch microguidewires and 0.014 inch exchange microguidewires that can be removed and reshaped during the procedure. Furthermore, retrograde filling of blood product into the lumen does not prevent balloon deflation. The major drawback is the stiffness of the balloon catheter that could impair trackability in tortuous cerebrovascular anatomy.7,8 Concerning the profile of the distal tip of those catheters there are some differences; the smallest outer diameter is 2.1 F for the Scepter balloon catheter, 2.2 F for the Hyperglide, between 2.5 F and 3.0 F for the Hyperform, 2.7 F for the Transform and 2.9 F the Ascent. Distal tip length is 2 mm for the Hyperform balloon catheter, 4 mm for the Hyperglide, 3.25 mm for the TransForm, 5 mm for the Scepter and 3 mm for the Ascent. The distal tip of the Scepter balloon catheter can be formed with steam and all balloon catheters have a hydrophilic coating to improve trackability.
In our series, we successfully treated 36 consecutive sidewall and bifurcation intracranial aneurysms using the TransForm occlusion balloon catheter. This device, compatible with 0.014 inch steerable microguidewires, presented good trackability, also to access distal bifurcation aneurysms. This quality is probably due to the softness of the single-lumen device coupled with the stiffness of the 0.014 inch microguidewire. The balloon was used in 29 procedures with the Transend microguidewire without any technical problems. In seven cases, in particular for AcoA and MCA aneurysms with tortuous intracranial vascular anatomy, we used a Synchro microguidewire to navigate the balloon catheter in the bifurcation branches; in a case of ruptured AcoA aneurysm the Transform super compliant balloon slowly deflated during the deployment of the first coil. This problem was resolved by turning and pushing forward the Synchro microguidewire. This leakage was probably due to the irregular profile of the microguidewire tip, which does not perfectly obstruct the balloon catheter tip. Concerning stability during inflation and deflation, the balloon remained quite stable in all the procedures. The visibility with a mixed 50:50 contrast media/saline was optimal in carotid siphons and sufficient in distal bifurcations. The major drawback, compared to 0.010 inch compatible devices, is the stiffness of the system (balloon catheter and 0.014 inch microguidewire) that can modify the vascular anatomy, in particular in smaller and more distal arteries (Figure 2). In these cases we suggest performing an additional angiographic roadmap before coiling. Another hypothetical drawback is the risk of distal perforation, higher with the 0.014 inch microguidewire compared to the 0.010 inch.
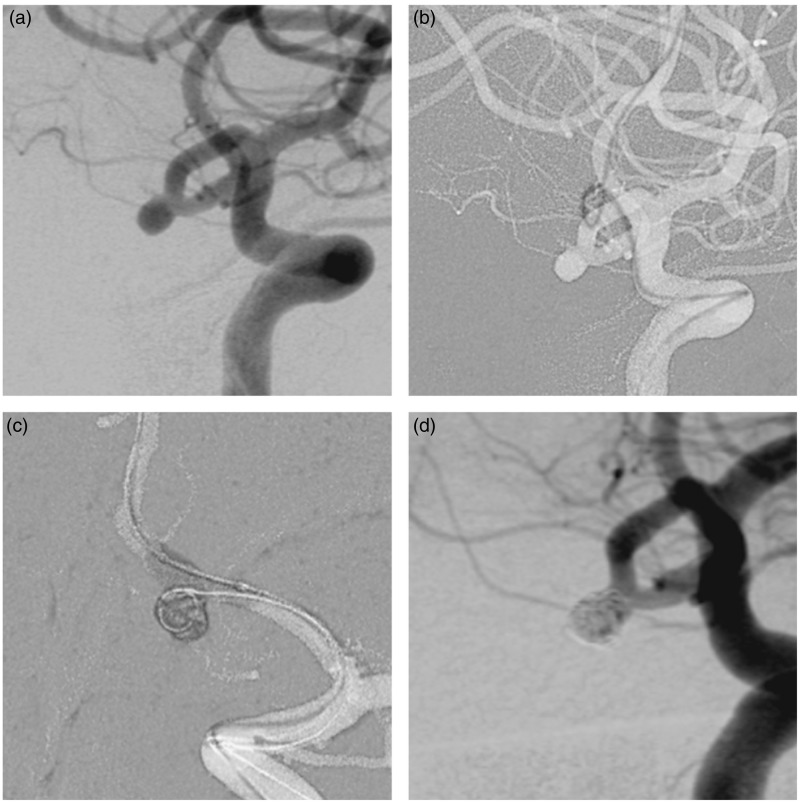
Figure 2.
(a) Initial subtracted diagnostic angiogram showing an unruptured anterior communicating artery aneurysm. (b) Roadmap imaging showing the vascular modifications related to the Transform balloon in the left anterior cerebral artery. (c) New working projection and balloon-assisted coiling. (d) Final control angiogram.
In our series we had some technical perioperative complications without permanent clinical events. We reported two thromboembolic complications, an acute occlusion of the inferior branch in a ruptured MCA bifurcation aneurysm and an ischaemic complication in the genu of the corpus callosum. Thromboembolic complications are frequent in balloon-assisted coiling, with a rate of 8.1% in a recent meta-analysis.4 In another procedure, we had a coil protrusion in the parent artery during the treatment of an unruptured wide-neck carotid-ophthalmic aneurysm. The blood flow was normal and we did not see any clot inside the parent artery, so we awoke the patient and prescribed 250 mg of aspirin once a day for 3 months for the prevention of ischaemic stroke. We reported a small perforation of the balloon catheter during the withdrawal of the microcatheter used for coiling. We do not have a clear explanation for this event, but it is likely that the balloon catheter was overinflated during preparation and testing, thus weakening the wall.
In our series we obtained a total or subtotal occlusion rate of 94.4%, similar results compared to those of previously reported series in the literature.4
This study presents some limitations, first of all it was a small single centre retrospective series, which did not directly compare 0.014 inch versus 0.010 inch balloons or single-lumen versus dual-lumen balloon catheters. Furthermore, we did not include any follow-up, but our goal was to analyse feasibility, safety and efficacy during treatment and hospitalization; long-term stability of the balloon remodelling technique was already well evaluated in larger series.3,10
Conclusion
Our preliminary results suggest that the Transform balloon catheter seems to be safe and effective for the treatment of intracranial aneurysms, in ruptured and unruptured cases. Larger observational studies are necessary to confirm our findings.
Ethics approval
The local ethics committee of the Rothschild Foundation Hospital approved the study.
Funding
Institutional grants were received from Covidien/eV3, Stryker Neurovascular, Microvention/Terumo, Balt.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Moret J, Cognard C, Weill A, et al. Reconstruction technic in the treatment of wide-neck intracranial aneurysms. Long-term angiographic and clinical results. Apropos of 56 cases. J Neuroradiol 1997; 24(1): 30–44. [PubMed] [Google Scholar]
- 2.Santillan A, Gobin YP, Mazura JC, et al. Balloon-assisted coil embolization of intracranial aneurysms is not associated with increased periprocedural complications. J Neurointerv Surg 2013; 5(Suppl. 3): iii56–61. doi: 10.1136/neurintsurg-2012-010351. [DOI] [PubMed] [Google Scholar]
- 3.Cekirge HS, Yavuz K, Geyik S, et al. HyperForm balloon remodeling in the endovascular treatment of anterior cerebral, middle cerebral, and anterior communicating artery aneurysms: clinical and angiographic follow-up results in 800 consecutive patients. J Neurosurg 2011; 114(4): 944–953. doi: 10.3171/2010.3.JNS081131. [DOI] [PubMed] [Google Scholar]
- 4.Shapiro M, Babb J, Becske T, et al. Safety and efficacy of adjunctive balloon remodeling during endovascular treatment of intracranial aneurysms: a literature review. Am J Neuroradiol 2008; 29(9): 1777–1781. doi: 10.3174/ajnr.A1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pierot L, Cognard C, Anxionnat R, et al. Remodeling technique for endovascular treatment of ruptured intracranial aneurysms had a higher rate of adequate postoperative occlusion than did conventional coil embolization with comparable safety. Radiology 2011; 258(2): 546–553. doi: 10.1148/radiol.10100894. [DOI] [PubMed] [Google Scholar]
- 6.Pierot L, Cognard C, Spelle L, et al. Safety and efficacy of balloon remodeling technique during endovascular treatment of intracranial aneurysms: critical review of the literature. Am J Neuroradiol 2012; 33(1): 12–15. doi: 10.3174/ajnr.A2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spiotta AM, Miranpuri A, Hawk H, et al. Balloon remodeling for aneurysm coil embolization with the coaxial lumen Scepter C balloon catheter: initial experience at a high volume center. J Neurointerv Surg 2013; 5(6): 5825 doi: 10.1136/neurintsurg-2012-010552. [DOI] [PubMed] [Google Scholar]
- 8.Lazzaro MA, Darkhabani Z, Zaidat OO, et al. Initial experience with the coaxial dual-lumen ascent balloon catheter for wide-neck aneurysm coil embolization. Front Neurol 2011; 2: 52 doi: 10.3389/fneur.2011.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roy D, Milot G, Raymond J. Endovascular treatment of unruptured aneurysms. Stroke 2001; 32(9): 1998–2004. doi: 10.1161/hs0901.095600. [DOI] [PubMed] [Google Scholar]
- 10.Chalouhi N, Starke RM, Koltz MT, et al. Stent-assisted coiling versus balloon remodeling of wide-neck aneurysms: comparison of angiographic outcomes. Am J Neuroradiol 2013; 34(10): 1987–1992. doi: 10.3174/ajnr.A3538. [DOI] [PMC free article] [PubMed] [Google Scholar]