Abstract
Background
Flow diverter stents represent a new endovascular tool to treat complex aneurysms, such as giant, large, wide-necked and fusiform. The highly dense mash of these stents reduces inflow and outflow inside the aneurysm, resulting in intra aneurysmal thrombosis and stent endothelialization.
Objectives
To present the results of treatment of intracranial aneurysms with flow diverter stents in a single center.
Methods
Retrospective review of 77 patients with 87 aneurysms treated using two different types of flow diverter stent, the Pipeline Embolization Device and SILK stent, between October 2010 and September 2013 in an interventional neuroradiology center.
Results
Flow diverter stent placement was successful in 98% of the lesions and resulted in an immediate major stasis within most of the treated aneurysms. The overall aneurysm occlusion rate at six months and 18 months was 80% and 84% respectively. Symptomatic complications occurred in 11 patients (14.3%) with morbidity in eight (10.4%) and mortality in three patients (3.9%).
Conclusion
Flow diversion is a promising technique for treatment of challenging intracranial aneurysms with acceptable morbidity. A high rate of complete occlusion for small large necked aneurysms, a low morbidity and mortality rate and no recanalization encourage their use in these aneurysms. Further studies accessing long-term aneurysm occlusion and recanalization are required.
Keywords: Aneurysm, endovascular, flow diverter stent, Pipeline, SILK
Introduction
Since the publication of the International Subarachnoid Aneurysm Trial (ISAT), management of intracranial aneurysms has changed significantly.1 Endovascular treatment with coiling of saccular aneurysm with a favorable neck has been acceptable as a safe and efficient procedure. However, complex aneurysms, such as large and giant ones, wide-necked and fusiform aneurysms, remains technically challenging for coiling, with a low occlusion and high recurrence rate of up to 80%.2,3
The advent of flow diverter stents (FDSs) brings a new endovascular tool for reconstructive treatment and vascular remodeling for these challenging aneurysms. Two proprieties of the FDS are new in treatment of aneurysms. The highly dense mash of the FDS reduces inflow and outflow on the aneurysm, resulting in intra aneurysmal thrombosis.4 The device works as a scaffold for strong neointimal proliferation resulting in remodeling the parent artery and curing the neck. The time course of the intra aneurysmal thrombosis remains unclear, as well as long-term occlusion recanalization rates. Many individual factors may influence complete occlusion, such as aneurysm size, location and morphology, parent vessel geometry, blood coagulation parameters, previously regular stent use as well as the type of flow diversion and resulting flow changes.5
The first FDS commercially available was the SILK stent (Balt Extrusion, Montmorency, France) in 2007, a braided device with 48 high-attenuation nickel and titanium alloy wires with four platinum markers.6 Later, the Pipeline Embolization Device (PED) (Ev3, Irvine, CA, USA) was developed, a cylindrical mesh device composed of 48-braided individual cobalt chromium and platinum strands.7 The device has about 30% to 35% metal surface area coverage when fully deployed. The safety of flow diversion stents and their reliability as a permanent cure are yet to be determined.
In this study, we present a single center series of 77 consecutive patients with 87 intracranial vascular lesions treated with SILK or PED, describing early results, clinical outcome and complications.
Materials and methods
Study design
We performed a retrospective review of patients treated using two different types of FDS, the PED and the SILK device, from October 2010 to September 2013 in a single interventional neuroradiology center (Hospital Beneficencia Portuguesa) in Sao Paulo, Brazil.
Indications for treatment with FDS were wide-necked aneurysms (neck > 4 mm) or a domus-to-neck ratio <2, large and giant aneurysms, high likelihood of failure with conventional endovascular or surgical techniques, remnants of aneurysms after surgical or endovascular treatment, partially thrombosed aneurysms, fusiform aneurysms, and dissected vessels. The decision between each FDS in individual cases was based on clinical and anatomical considerations. The SILK stent indications were: similar diameter of pre and post segment of the artery (<1 mm of diameter difference), parent artery diameter up to 5 mm and a high likelihood to redeploy the stent due to catheter instability. The PED indications were: parent artery with different diameter of pre and post segment (>1 mm of diameter difference), diameter of parent artery <5 mm and if stent shortening was not wanted.
Endovascular procedure
Procedures were performed on a biplane digital subtraction angiography (DSA) unit (Philips Integris BV 5000).
All procedures were carried out under general anesthesia, using regular techniques with a triaxial catheter system. Patients received a daily dose of 100 mg acetylsalicylic acid (ASA) and 75 mg of clopidogrel starting seven days prior to the treatment. Clopidogrel assays were checked on all patients and platelet aggregation was required to be lower than 30%. Four patients were found to be hyporesponders to clopidogrel and were given ticagrelor instead. Clopidogrel was maintained for the next six months, and thereafter 100 mg of ASA for 36 months.
Follow-up
Each patient was scheduled for a DSA after six months. DSA was repeated at 18 and 36 months in the case of no occlusion. After complete occlusion, angiographic image control was performed with magnetic resonance angiography (MRA) at 18 and 36 month after the treatment and then every three years. The DSA follow-up results were classified as complete or incomplete aneurysmal occlusion. The incomplete occlusion group was also divided into two groups: ≥95% occlusion group (near complete occlusion) and <95% occlusion group (residual aneurysm).
Results
Clinical presentation and aneurysm morphology
A total of 77 patients (19 men and 58 women) with 87 aneurysms were treated. The PED was used in 36 cases and the SILK device in 41 cases. The age of patients ranged from 18 to 82 years old (mean of 52.5 years). Table 1 shows the clinical presentation at admission.
Table 1.
Clinical presentation at admission.
| Clinical presentation | N = 87 |
|---|---|
| Incidentally | 31 (35%) |
| Non-SAH headache | 24 (27%) |
| Cranial nerve palsy | 18 (21%) |
| SAH | 11 (13%) |
| Symptomatic dissection | 3 (4%) |
SAH: subarachnoid hemorrhage.
The series comprises a total of 73 (84%) saccular aneurysms, eight (9%) fusiform, three (4%) arterial dissections, two (2%) pseudoaneurysms and one carotid segmental dysplasia (1%). Both pseudoaneurysms resulted from a carotid cavernous iatrogenic injury during an endoscopic pituitary tumor resection.
A total of 76 (87%) treated lesions were located in the anterior circulation and 11 (13%) were located in the posterior circulation. Among the eight fusiform aneurysms, six were located in the basilar artery, one in the cervical carotid segment and one in the carotid cavernous segment. Table 2 shows the location of the aneurysms.
Table 2.
Aneurysm locations.
| Aneurysm location | N = 87 |
|---|---|
| Ophthalmic segment | 39 |
| Superior hypophyseal artery | 13 |
| Cavernous segment | 10 |
| Basilar artery | 8 |
| Posterior communicating artery | 5 |
| Vertebral artery | 3 |
| Internal carotid bifurcation | 2 |
| Middle cerebral artery | 2 |
| Anterior choroidal artery | 2 |
| Carotid petrous segment | 1 |
| Carotid cervical segment | 1 |
| Anterior communicating artery | 1 |
Fifteen aneurysms had been treated previously: five with previous coiling; five with coiling and stenting; four with Onyx and one with surgical clipping. Twenty-two aneurysms (25%) were treated with coiling concomitant FDS deployment. The number of FDSs used ranged from one to four, with one stent used in 68 (88%) patients, two stents in eight (10%) and four stents in one (2%) patient.
The average diameter of 73 saccular aneurysms was 12.6 mm (range from 1.8 to 40.0 mm). Thirty aneurysms (41%) were small (<10 mm), 32 (44%) were large (10 to 25 mm) and 11 (15%) were giant (≥25 mm). The mean neck diameter of the aneurysm was 5.14 mm (range from 1.4 to 16.0 mm). The mean domus-to-neck ratio was 1.6 (range to 0.8 to 6.8). Both criteria (which classify the aneurysm as unsuitable for treatment with coiling alone) were found in 70 of 73 saccular aneurysms. The three lesions without these criteria were positioned in the carotid siphon making good coil packing unlikely due to instability of the microcatheter.
Follow-up
Immediate angiography following FDS deployment demonstrated major stasis of contrast material in 82 aneurysms (94%). Four aneurysms (5%) had no flow changes and one aneurysm (1%) had a complete occlusion – small, wide-neck, superior hypophyseal aneurysm (Figure 1).
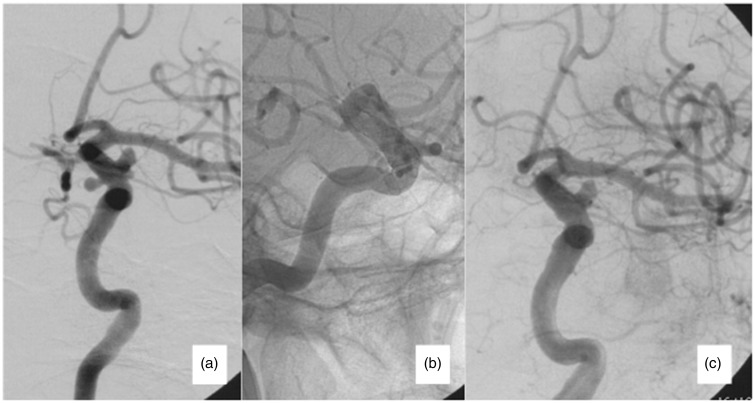
Figure 1.
Seventy-one year old female, presented with mild visual loss. (a) Initial angiography showing an ophthalmic segment and a superior hypophyseal aneurysm. (b) A 4.5 mm × 30 mm SILK device was applied, incorporating both aneurysms. (c) Immediate occlusion of the superior hypophyseal aneurysm was observed.
Table 3 shows the results for all lesions and also stratifies them by type and size. A six-month DSA control was available in 66 patients (86%) with 75 aneurysms (86%) and showed complete occlusion in 60 aneurysms (Figure 2) (80% occlusion rate), near complete occlusion in six lesions (8%) and residual aneurysm in nine (12%).
Table 3.
The results stratified by type of aneurysm.
| Type | Treated (n) | Angiographic control (n) | Complete occlusion (n) | Complete occlusion rate |
|---|---|---|---|---|
| All | 87 | 75 | 63 | 84% |
| All saccular | 73 | 67 | 59 | 88% |
| Small | 30 | 28 | 26 | 93% |
| Large | 32 | 29 | 26 | 90% |
| Giant | 11 | 10 | 7 | 70% |
| Previously treated saccular | 15 | 10 | 5 | 50% |
| Previously untreated | 58 | 57 | 54 | 95% |
| Small | 26 | 25 | 25 | 100% |
| Large | 25 | 25 | 24 | 96% |
| Giant | 7 | 7 | 5 | 71% |
| Fusiform | 8 | 4 | 1 | 25% |
| Pseudoaneurysm | 2 | 1 | 1 | 100% |
| Arterial dissection | 2 | 2 | 2 | 100% |
| Arterial dysplasia | 1 | 0 | – | – |
| Concomitant flow-diverter stent + coiling | 22 | 20 | 17 | 85% |
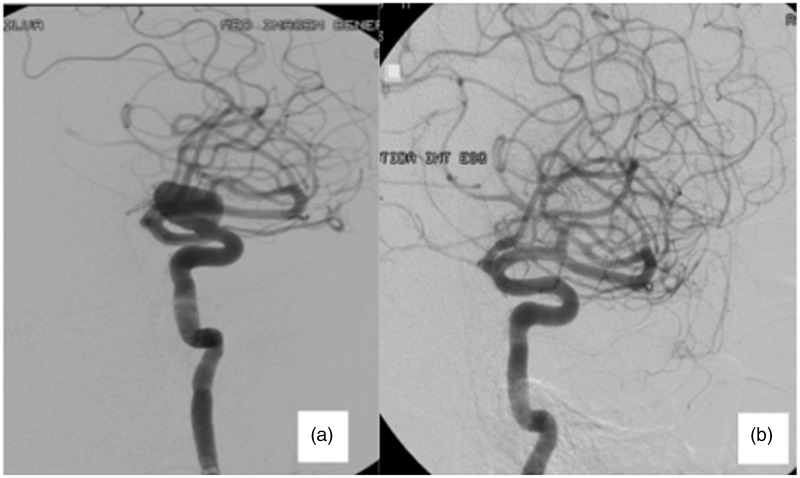
Figure 2.
Sixty-three year old male, presenting visual impairment. Pre-treatment (a) Angiography shows a 15.3 mm carotid ophthalmic aneurysm. (b) Six-month follow-up shows a complete occlusion of the aneurysm, preserving the ophthalmic artery’s patency. The patient’s visual impairment improved post treatment.
A second DSA follow-up was performed approximately 18 months (range from 7 to 22 months) after the procedure in 14 patients with 14 lesions. Nine of them were completely occluded aneurysms at the first DSA with no alteration at the second DSA, three were near complete (all saccular aneurysms – two small and one giant) and evolved to complete occlusion and two were classified as residual aneurysms (both were saccular – one small and the other one large) and remain with residual aneurysm. At 18 months post treatment control, 63 lesions were completely occluded (84% – overall rate of total occlusion).
A DSA follow-up was available in 10 of 15 saccular aneurysms previously treated, with five complete occlusions (50%). We observed 55 completely occluded aneurysms among 65 without previous treatment (85% occlusion rate).
In the saccular aneurysm group, DSA follow-up was available in 67 of the 73 aneurysms (92%), with 56 completely occluded lesions at six-month follow-up (84% occlusion rate) and 59 completely occluded lesions at 18-month follow-up (88% occlusion rate). The occlusion rate was 93% for small (26 of 28), 90% for large (26 of 29) and 70% for giant aneurysms (7 of 10) at 18-month follow-up. Considering the previous untreated saccular aneurysms, the occlusion rate was 95% (54 of 57), with 100% for small (25 of 25), 96% for large (24 of 25) and 71% for giant aneurysms (5 of 7) at 18-month follow-up – see Table 3.
Of eight fusiform aneurysms, four performed a six-month DSA control with one complete occlusion (25%). A DSA was also available in one of two pseudoaneurysms (with complete occlusion), in two dissecting aneurysms (complete occlusion on both lesions) and in the carotid dysplasia (incomplete occlusion).
Among the aneurysms treated concomitantly with FDS and coiling 20 performed a DSA control with 17 demonstrating a complete occlusion (85%).
Clinical outcome and complications
In Table 4, we present the clinical outcome, before and after treatment, according to the Modified Rankin Scale (mRS).
Table 4.
Modified Rankin Scale (mRS) pre-treatment, at discharge and six-month follow-up.
| mRS | Pre-treatment (n = 77) | Post-treatment (n = 77) | Six-month follow-up (n = 59) |
|---|---|---|---|
| 0 | 55 (71%) | 51 (66%) | 43 (72%) |
| 1 | 18 (23%) | 19 (24%) | 6 (10%) |
| 2 | 1 (1%) | 2 (2%) | 3 (5%) |
| 3 | 2 (2%) | 2 (2%) | 3 (5%) |
| 4 | 2 (2%) | 3 (4%) | 1 (2%) |
| 5 | 1 (1%) | 2 (2%) | 0 (0%) |
| 6 | 0 (0%) | 1 (1%) | 3 (5%) |
Symptomatic complications occurred in 11 patients (14.3%) with morbidity in eight patients (10.4%) and the mortality in three (3.9%). Hemorrhagic complications occurred in five patients, all of them with large or giant aneurysms, four in the anterior and one in the posterior circulation. The cause of bleeding was: secondary to ischemic event in one, probably due to hyperperfusion in one (Figure 3), rupture of other aneurysm (contralateral to the treated one) in one and unknown in two cases.
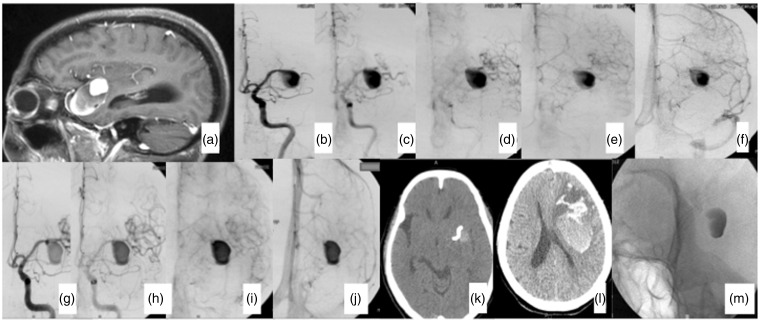
Figure 3.
Seventy-four year old female presenting with a long-term headache. (a) Magnetic resonance imaging showing a 40 mm left middle cerebral artery (MCA) partially thrombosed aneurysm. (b)–(f) Left internal carotid artery (ICA) pre treatment: a slow flow distally from the aneurysm is observed, compared with the anterior cerebral artery flow. (g)–(j) Left ICA post treatment. A significant change in the MCA flow distally from the aneurysm. (k) A computed tomography (CT) scan was performed after the procedure. The Pipeline Embolization Device and contrast material inside the aneurysm with no bleeding are observed. (l) Two days after procedure, CT demonstrating a large intraparenchymal hematoma far from the aneurysm. The hematoma was promptly drained; however, the patient died in the third day post procedure. (m) Radiography showing the stent and contrast inside the aneurysm.
Ischemic complication occurred in four patients and was related to internal carotid artery (ICA) and stent thrombosis in one case of large aneurysm (hemispheric infarct and dead) and perforator occlusion in three, all of them in basilar fusiform aneurysm.
Cranial nerve compression was the clinical presentation in 18 patients. After the procedure, we observed an improvement in 10 cases, including optic nerve, III, IV, V, VI and lower cranial nerves compression (Figure 2). No changes were observed in six patients and two patients got worse.
Transient motor deficit was observed in four patients due to a thromboembolic event. Those patients were treated with enoxaparin and volemic expansion, with a good outcome.
Discussion
The advent of FDS offers a new endovascular therapeutic modality for the treatment of challenging aneurysms such as large, giant and fusiform. Such aneurysms often present with mass effect and corresponding compression syndrome on the neighboring neural tissue. Aneurysm coiling has been the main endovascular treatment modality for the past 20 years. Aneurysm occlusion and pulsation reduction can only be achieved if completely sealed with coils; however, most of these lesions have a wide neck, making complete occlusion unlikely, even impossible.8 The use of Onyx HD (Micro Therapeutics, Inc., Irvine, CA, USA) reduces the mass effect in some cases, but can induce new symptoms and reach a complete rate of occlusion of 80% in large and 50% in giant aneurysms.9 FDS, as the name suggests, diverts the flow away from the aneurysm, reducing the shear stress on the aneurysm wall, promoting stasis and thrombosis. The extent of intra aneurysmal flow depends on the porosity and metal surface coverage.10
Angiographic findings
All lesions were successfully accessed in this series, and successful deployment of the devices was achieved in all but one procedure (retreatment of a giant wide-necked ophthalmic aneurysm previously treated with coil and stent. The microcatheter did not distally progress the stent). O’Kelly et al. also demonstrated failed distal progress of the microcatheter in two patients with wide-neck giant aneurysms.11 An internal carotid occlusion was performed after the patient passed a balloon test occlusion and tolerated the permanent vessel occlusion.
Immediately after the FDS deployment, a variable contrast stasis occurred in all lesions. Although this observation is expected, it is unlikely at this time complete aneurysm obliteration, considering the hemodynamic changes caused by FDSs12 (Figure 1).
At least one follow-up DSA was available in 66 patients (86%) with 75 lesions (86%). As expected from a series of this size, some patients were lost to follow-up for various reasons and nine patients (12%) did not complete the six-month period for the first follow-up DSA. We had an 80% and 84% complete occlusion rate after six-month and 18-month DSA, respectively. The occlusion rate in other series is extremely variable raging from 52% to 93%.4,11–18
Considering the near complete occlusion cases, the occlusion rate in our study is even higher (88%). Also, three aneurysms with near complete occlusion on the first DSA improved to complete occlusion in the 18-month follow-up DSA. O’Kelly et al. described a 65% complete occlusion and near complete occlusion after six-month follow-up and 83% at 15 months in 97 aneurysms after PED treatment.11 There are no available data about the rupture risk of a near complete occluded aneurysm treated with FDS.
The identification of aneurysmal or procedural features predisposing complete occlusion or persistent perfusion is not clear yet; however, there are some aspects that could influence the final outcome, including the aneurysm morphology, porosity of the FDS in the neck and presence of a branch rising from the aneurysm wall.
A complete occlusion rate improvement is observed depending on the type of aneurysm. If only saccular aneurysms are considered we had 84% complete occlusion rate for all lesions, 88% for all saccular aneurysms and 100% for small, not previously treated lesions. Nelson et al., in a multicenter trial, had a 93% aneurysmal occlusion on the six-month follow-up after treatment with PED of saccular aneurysms.14 Thromboembolic and hemorrhagic complications occurred only in large and giant lesions in our series. Complete occlusion occurred also in all pseudoaneurysms and dissections.
Our series includes 15 lesions treated previously with conventional techniques. At six-month DSA follow-up, 50% complete occlusion was observed in that group compared with 85% with no previous treatment. This observation is corroborated by McAuliffe et al., in an Australian multicenter prospective study.19 A six-month occlusion rate of 92% was achieved in the group with no previous treatment compared with 50% in the aneurysms previously treated with other endovascular techniques. The presence of a previously deployed stent may reduce the biological effect of a FDS, because the stent may affect the intimal changes that FDS is purposed to make.12
A six-month DSA follow-up was available in seven of 11 posterior circulation aneurysms, with an occlusion rate of 57% (four cases). Six patients had a fusiform aneurysm (four with a DSA control, presenting one complete occlusion) and three dissecting (both complete occlusion). These data suggest that the complete occlusion of fusiform aneurysm is more difficult with FDS. Chalouhi et al. presented 42% complete occlusion at six months in a small series of unruptured posterior circulation aneurysms. Besides a lower occlusion rate, there is substantial morbidity and mortality associated with the treatment of these lesions.20
Siddiqui et al., in a small series of seven patients with vertebro-basilar fusiform aneurysms, reported four deaths, one patient severely disabled, and only two patients with good outcome.18 Substantial morbidity and mortality associated with few options of treatment and the natural history brings up the use of FDS as a treatment option for fusiform aneurysm. However, there is not sufficient outcomes data showing FDS as a well defined safe procedure for the treatment of these aneurysms.21
Clinical outcome and complications
In our series, morbidity and mortality rate were 8% and 4% respectively. Other series demonstrated similar mortality and morbidity rates.4,11 The mechanism of intraparenchymal hemorrhage (IPH) after FDS treatment is not clear yet; however, there are some theories about the physiopathology aspect of this complication. Cruz et al.21 proposed a hypothesis that artery reconstruction with a FDS could reduce the local compliance of that vascular segment, changing the blood pressure waveform transmitted to the distal cerebral vasculature. This blood pressure waveform transmitted beyond the reconstructed segment might exhibit a higher systolic peak and a lower diastolic trough and this alteration could contribute to delayed post-procedural IPH.22 This theory could explain an IPH in two patients in our series without a clear cause. Although this is a valid hypothesis it does not fully explain these events because IPH rates are not uniform across multiple series.
In one of our patients presenting IPH post procedure (Figure 3), hyperperfusion was the probable mechanism. A very slow flow distally from the aneurysm is clearly observed on the pretreatment angiography. Immediately after PED deployment there is a significant increase in the middle cerebral artery (MCA) flow distally from the aneurysm. One patient had a hemorrhagic complication from an ischemic area after the treatment and another patient from a contralateral MCA aneurysm that had been clipped 12 years before and presented a residual aneurysm.
The IPH rate is variable raging from 0% to 8.5%.4,11,12,15,19,21 In recent publications, the overall incidence of IPH appears to be greater in patients treated with FDS than in patients treated with stent-assisted coil. A cohort of 284 patients treated with the Neuroform stent (Boston Scientific, Natick, MA, USA) showed a 1.1% (3/284) rate of spontaneous IPH after treatment.23 This rate is similar to the IPH rate due to double anti-aggregative treatment for secondary stroke prevention. These data suggest that post-procedural IPH might be a phenomenon that is most associated with FDS.21
Conclusion
Flow diversion is a promising technique for the treatment of intracranial aneurysms. The results are excellent for small saccular wide-necked aneurysms with near 100% complete occlusion rate and no complications. More challenging lesions, such as large and giant aneurysms, also have a very good result with a relatively low rate of complications.
The results for the challenging fusiform vertebro-basilar aneurysms, which have a very poor long term prognosis, are promising; however, the rate of ischemic events is still very high and mostly related to perforator occlusion.
There was no recanalization in this series and it seems that its occurrence is very rare with FDS. More follow-up time is needed to prove that.
Our study suggests efficacy and safety of FDS in the treatment of complex aneurysms. More studies are needed to further refine the possible indicators and the technical aspects of this treatment to improve clinical results.
Acknowledgements
LG certifies that all authors have participated sufficiently in the intellectual content, conception and design of this work or the analysis and interpretation of the data, as well as the writing of the manuscript, to take public responsibility for it and have agreed to have our names listed as contributors. Consent was obtained from the patients whose pictures are shown in this paper. The content of this manuscript, in part or in full, has not been published elsewhere in any form. LG certifies that this manuscript is a unique submission and is not being considered for publication with any other source in any medium.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors have no financial interest in this article. No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
References
- 1.Molyneux AJ, Kerr RS, Yu LM, et al. International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: A randomized comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet 2005; 366: 809–817. [DOI] [PubMed] [Google Scholar]
- 2.Sluzewski M, Menovsky T, van Rooij WJ, et al. Coiling of very large or giant cerebral aneurysms: Long-term clinical and serial angiographic results. Am J Neuroradiol 2003; 24: 257–262. [PMC free article] [PubMed] [Google Scholar]
- 3.Thornton J, Debrun GM, Aletich VA, et al. Follow up angiography of intracranial aneurysms treated with endovascular placement of Guglielmi detachable coils. Neurosurgery 2002; 50: 239–249. [DOI] [PubMed] [Google Scholar]
- 4.Fischer S, Vajda Z, Perez MA, et al. Pipeline embolization device (PED) for neurovascular reconstruction: Initial experience in the treatment of 101 intracranial aneurysms and dissections. Neuroradiology 2012; 54: 369–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kulcsar Z, Houdart E, Bonafe A, et al. Intra-aneurysmal thrombosis as a possible cause of delayed aneurysm rupture after flow-diversion treatment. Am J Neuroradiol 2011; 32: 20–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leonardi M, Dall’olio M, Princiotta C, et al. Treatment of carotid siphon aneurysms with a microcell stent. A case report. Interv Neuroradiol 2008; 14: 429–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fiorella D, Woo HH, Albuquerque FC, et al. Definitive reconstruction of circumferential, fusiform intracranial aneurysms with the Pipeline embolization device. Neurosurgery 2008; 62: 1115–1121. [DOI] [PubMed] [Google Scholar]
- 8.van Rooij WJ, Sluzewski M. Unruptured large and giant carotid artery aneurysms presenting with cranial nerve palsy: Comparison of clinical recovery after selective aneurysm coiling and therapeutic carotid artery occlusion. Am J Neuroradiol 2008; 29: 997–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piske RL, Kanashiro LH, Paschoal E, et al. Evaluation of Onyx HD-500 embolic system in the treatment of 84 wide-neck intracranial aneurysms. Neurosurgery 2009; 64: 865–875. [DOI] [PubMed] [Google Scholar]
- 10.Szikora I, Berentei Z, Kulcsar Z, et al. Treatment of intracranial aneurysms by functional reconstruction of the parent artery: The Budapest experience with the Pipeline Embolization Device. Am J Neuroradiol 2010; 31: 1139–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Kelly CJ, Spears J, Chow M, et al. Canadian experience with the Pipeline Embolization Device for repair of unruptured intracranial aneurysms. Am J Neuroradiol 2013; 34: 381–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lylyk P, Miranda C, Ceratto R, et al. Curative endovascular reconstruction of cerebral aneurysms with the Pipeline Embolization Device: the Buenos Aires experience. Neurosurgery 2009; 64: 632–642. [DOI] [PubMed] [Google Scholar]
- 13.Saatci I, Yavuz K, Ozer C, et al. Treatment of intracranial aneurysms using the Pipeline flow-diverter embolization device: A single-center experience with long-term follow-up results. Am J Neuroradiol 2012; 33: 1436–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nelson P, Lylyk I. Szikora, et al. The pipeline embolization device for the intracranial treatment of aneurysms trial. Am J Neuroradiol 2011; 32: 34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szikora I, Marosfo M, Salomvary B, et al. Resolution of mass effect and compression symptoms following endoluminal flow diversion for the treatment of intracranial aneurysms. Am J Neuroradiol 2013; 34: 935–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shankar JJ, Vandorpe R, Pickett G, et al. SILK flow diverter for treatment of intracranial aneurysms: Initial experience and cost analysis. J Neurointerv Surg 2013; 5(Suppl 3): iii1–iii11. [DOI] [PubMed]
- 17.Berge J, Biondi A, Machi P, et al. Flow-diverter SILK stent for the treatment of intracranial aneurysms: 1-year follow-up in a multicenter study. Am J Neuroradiol 2012; 33: 1150–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siddiqui AH, Abla AA, Kan P, et al. Panacea or problem: Flow diverters in the treatment of symptomatic large or giant fusiform vertebrobasilar aneurysms. J Neurosurg 2012; 116: 1258–1266. [DOI] [PubMed] [Google Scholar]
- 19.McAuliffe V, Wycoco H, Rice C, et al. Immediate and midterm results following treatment of unruptured intracranial aneurysms with the Pipeline Embolization Device. Am J Neuroradiol 2012; 33: 164–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chalouhi N, Tjoumakaris S, Dumont AS, et al. Treatment of posterior circulation aneurysms with the Pipeline Embolization Device. Neurosurgery 2013; 72: 883–889. [DOI] [PubMed] [Google Scholar]
- 21.Cruz JP, Chow M, O’Kelly C, et al. Delayed ipsilateral parenchymal hemorrhage following flow diversion for the treatment of anterior circulation aneurysms. Am J Neuroradiol 2012; 33: 603–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Velat GJ, Fargen KM, Lawson MF, et al. Delayed intraparenchymal hemorrhage following Pipeline Embolization Device treatment for a giant recanalized ophthalmic aneurysm. J Neurointerv Surg 2011; 4: e24. [DOI] [PubMed] [Google Scholar]
- 23.Diener HC, Bogousslavsky J, Brass LM, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischemic stroke or transient ischemic attack in high-risk patients (MATCH): Randomized, double-blind, placebo-controlled trial. Lancet 2004; 364: 331–337. [DOI] [PubMed] [Google Scholar]